Visual Perception and Working Memory in Schizotypal Personality Disorder
Abstract
OBJECTIVE: Patients affected by schizophrenia show deficits in both visual perception and working memory. The authors tested early-stage vision and working memory in subjects with schizotypal personality disorder, which has been biologically associated with schizophrenia. METHOD: Eleven subjects who met DSM-III-R criteria for schizotypal personality disorder and 12 normal comparison subjects were evaluated. Performance thresholds were obtained for tests of visual discrimination and working memory. Both form and trajectory processing were evaluated for each task. RESULTS: Subjects with schizotypal personality disorder showed intact discrimination of form and trajectory but were impaired on working memory tasks. CONCLUSIONS: These data suggest that subjects with schizotypal personality disorder, unlike patients affected by schizophrenia, have relatively intact visual perception. Subjects with schizotypal personality disorder do show specific deficits on tasks of comparable difficulty when working memory demands are imposed. Schizotypal personality disorder may be associated with a more specific visual processing deficit than schizophrenia, possibly reflecting disruption of frontal lobe systems subserving visual working memory operations.
Patients with schizophrenia have been reported to show deficits in visual perception. These deficits may be particularly severe in tasks requiring the integration of information over brief time periods, such as discrimination of speed or trajectory (1–4) and temporally modulated (flickering) patterns (5, 6). These findings suggest that patients with schizophrenia may have a more pronounced deficit in neural systems involved in the processing of transient stimuli or information that requires sampling at a high temporal frequency. In the visual modality, such processing is often said to place demands on the transient channel, which may be subserved by the magnocellular system in the lateral geniculate nucleus (7) and the dorsal visual stream in the cortex (8). In contrast, the sustained channel of the visual system involved in the perception of form or object properties appears less affected by the disorder (1–5). Backward-masking deficits in schizophrenia have sometimes been interpreted to be indicative of transient channel abnormalities, although processing speed and short-term memory disturbances may also contribute to these deficits (9–14). These visual deficits are consistent with positron emission tomography and functional magnetic resonance imaging studies reporting hyperactivation of the visual cortex in response to photic stimulation in schizophrenia (15, 16) and neuropathological evidence of increased neuronal density in the occipital area 17 (17).
Visual short-term or working memory deficits have also been reported in schizophrenia (2, 14, 18, 19). Working memory tasks typically require maintaining, and sometimes transforming, a neural representation for a brief period of time in order to carry out a task (8). Visual working memory, like perceptual processing, may be subserved by distinct anatomical regions. In primates and humans, the prefrontal lobes have been implicated in the performance of visual delayed response tasks, with activation of the dorsolateral prefrontal cortex during spatial memory tasks and the inferior frontal cortex during pattern memory tasks (8, 20). On similar delayed spatial memory tasks, subjects with schizophrenia show marked impairments consistent with disturbances in prefrontal cortex working memory systems (18–20).
These findings raise the question of whether subjects with a DSM-III or DSM-IV diagnosis of schizotypal personality disorder might also show deficits in early-stage visual processing or working memory operations. Family studies (21–23) have shown higher rates of diagnosis of schizotypal personality disorder among relatives of patients with schizophrenia than among relatives of comparison subjects. Relatives of patients with schizophrenia are nearly seven times more likely to be diagnosed with schizotypal personality disorder than relatives of comparison subjects (21), and the lifetime risk for siblings of probands developing schizophrenia is the same whether probands were diagnosed with schizophrenia or schizotypal personality disorder (6.5% and 6.9%, respectively) (23).
Although schizotypal personality disorder shares genetic and psychological commonalities with schizophrenia, it is typically not associated with treatment by antipsychotic medication, chronic hospitalization, or lifestyle changes. Consequently, it can serve as a vehicle for testing whether specific information-processing disturbances in schizophrenia appear in a related disorder.
Disturbances of backward masking have been reported in unaffected siblings of patients with schizophrenia (24) and in subjects who showed deviant ratings on measures of psychosis proneness (25). Two studies (12, 26) have used subjects with a diagnosis of schizotypal personality disorder in backward-masking paradigms. Braff (12) reported that subjects with schizotypal personality disorder showed deficits on backward-masking tests compared with the performance of depressed patients. Cadenhead et al. (26) reported a trend (p=0.06) for subjects with schizotypal personality disorder to show a backward-masking deficit at 720 msec compared with normal subjects. These studies suggest that an examination of visual processing in schizotypal personality disorder is warranted, using tasks that vary in terms of their perceptual and working memory demands.
We investigated the performance of subjects with schizotypal personality disorder and normal comparison subjects on separate tests of visual discrimination and working memory. Form processing was used to probe the sustained or parvocellular channel, and motion processing was used to evaluate the transient or magnocellular channel. To more confidently interpret differential deficits, tasks were matched for difficulty by using a psychophysical staircase technique (27).
Method
Subjects
Eleven subjects who met DSM-III-R criteria for schizotypal personality disorder (six women, five men) and 12 normal comparison subjects (five women, seven men) were evaluated. All subjects were right-handed, and all were between 24 and 53 years of age. The mean age of the subjects with schizotypal personality disorder was 36.5 years (SD=8.7), which did not differ from the age of the comparison subjects (mean=35.7, SD=12.7) (t=0.01, df=21, p>0.99). Subjects with schizotypal personality disorder had completed a mean of 15.3 years (SD=1.1) of education, and the comparison subjects had completed 16.5 years (SD=1.5) of education (t=2.3, df=21, p=0.03). None of the subjects had a history of head trauma, ECT, substance or alcohol dependence (DSM-III-R criteria), or neurological illness. All subjects had normal or corrected-to-normal vision during testing. We also screened patients for contrast sensitivity deficits (6), described in detail later in this article.
Subjects with schizotypal personality disorder were recruited through newspaper advertisements for adults who 1) “believe they have ESP, clairvoyance, telepathy, or a ‘sixth sense’; sense the presence of others when alone; think others can feel your emotions” and 2) “are shy and uncomfortable around unfamiliar people or in close relationships.” All potential subjects were given the Structured Clinical Interview for DSM-III-R (28) to determine if they met diagnostic criteria for schizotypal personality disorder. No subject with schizotypal personality disorder met criteria for an axis I psychotic or affective disorder. The comparison group was recruited from newspaper advertisements and hospital staff. Potential comparison subjects were excluded if they had a history of psychiatric illness in a first-degree relative (see Voglmaier et al. [29] for a detailed description of the diagnostic procedures and reliability). After a complete description of the study was provided to all of the subjects, written informed consent was obtained.
Tests of Visual Processing
Contrast sensitivity screening
Contrast sensitivity is the inverse of the contrast threshold (the minimum difference in luminance or brightness needed to reliably detect a stimulus). Contrast sensitivity, which can be affected by retinal or lateral geniculate abnormalities, has been reported to be impaired in schizophrenia (6). Contrast sensitivity was evaluated for each eye by using the Freiberg Acuity Test (30), which varies the contrast of a Landolt C optotype on a cathode ray tube display. The Landolt C subtended 6.98˚ of visual angle, and the gap in the C subtended 1.40˚ of visual angle. On each trial, the gap in the C faced up, down, right, or left. The subject reported which direction the gap was facing. If the subject did not respond in 8 seconds, the response was scored incorrect and the next trial was presented. The contrast of the C and screen background was varied across 24 trials to arrive at threshold by using a maximum likelihood estimation algorithm (30). The log10 contrast sensitivity was used for statistical analysis.
Trajectory and form discrimination and recognition tests
These paradigms were designed to estimate thresholds for form and trajectory discrimination and working memory. For the discrimination tests, each trial consisted of the presentation of one stimulus for 2 seconds. For the working memory tests, two stimuli were successively presented with a 70-msec interstimulus interval and the subject was required to determine if the relevant feature in the two images was the same or different. Stimuli were presented on a cathode ray tube terminal in a darkened room at a viewing distance of 60 cm.
All tests described in this section used an adaptive staircase procedure (31) to determine the subject’s performance threshold for each task. In the adaptive staircase test, the subject’s ability to discriminate a stimulus was manipulated by varying the amount of noise in the stimulus over a series of trials (the staircase). Correct responses result in an increase in noise for the subsequent trial, making the discrimination harder; incorrect responses result in a decrease in noise, making the discrimination easier. After a series of trials, the subject’s performance gradually converges or oscillates around a threshold value, which can be estimated on the basis of the rule used to move up or down the staircase (31). In this study, the threshold was estimated for the percentage performance point of 0.707 by using the following up-down procedure: After two correct trials, the amount of noise was increased in the stimulus. After an incorrect response, the amount of noise was decreased. Noise was first introduced in increments of 10% and then, after the first error, in increments of 5%. After the fourth error, the increments were reduced to 1%. The threshold was calculated from the noise levels of the final four reversals on the 50-trial staircase and was used as the dependent variable for statistical tests. To minimize the role of processing speed in performance and to allow sufficient time for transfer of information into the working memory system, stimulus duration within all paradigms was 2000 msec. The order of test administration was random across subjects. Specific test descriptions follow.
For form discrimination, on each trial, a square with rounded corners or a circle was presented within a rectangular region in the center of the screen for 2 seconds, followed by a question mark to prompt a subject’s response (Figure 1). The subject was required to indicate which figure (square with rounded corners or circle) was presented by pressing a key. After the subject responded, the next trial began. The stimulus was presented in a rectangular image area containing 10,000 pixels (100¥100), which subtended 3.82˚ of visual angle. Visual noise was varied by increasing or decreasing the percentage of pixels that were randomly assigned the value of white or black.
The form working memory task required the subject to decide whether two successively presented stimuli were the same or different. On each of two presentations, either a square or circle would be presented using the stimulus parameters already described (Figure 1). The interval between the offset of the first stimulus and the onset of the second stimulus was 70 msec. After both stimuli were presented, the subject was prompted to press a key to indicate whether the figures on the two trials were the same or different.
For trajectory discrimination, a dynamic dot display was used for psychophysical measurement of motion thresholds (Figure 1). This form of testing motion perception has the virtue of stimulating motion-specific neural systems while minimizing familiar position cues (32). On each trial, a fixation point was displayed for 1 second followed by a 2-second display of 100 moving dots presented in a rectangle subtending 8.12˚ of visual angle. The subject was required to indicate whether the dots were moving to the left or right by pressing a key. The apparent velocity of the dots varied from 1.79˚ to 4.00˚ of visual angle per second. Initially, all dots were moving in one direction or the other, a condition often described as 100% correlation of trajectory. On subsequent trials, the number of dots moving randomly in the image (visual noise) was increased or decreased, depending on the subject’s performance. During the display of the moving dots, the dots that were moving in a correlated direction (left or right) would vary. At approximately 23-msec intervals, any dot that was moving in the target direction had a 25% probability of moving in a random direction on the next screen refresh, with a dot at a different location replacing it in the set of dots with a correlated trajectory.
For trajectory recognition memory, on each of two successive presentations, the field of moving dots was presented with an interstimulus interval of 70 msec. The trajectory of the correlated motion trajectory on each presentation was either upward or downward. The subject indicated whether the trajectories in the two presentations were the same or different.
Statistical Analysis
The contrast sensitivity measures were analyzed by using a mixed model analysis of variance (ANOVA) in which the between-group factor was diagnosis (subjects with schizotypical personality disorder versus comparison subjects) and the within-group factor was eye (right versus left). On the basis of previous studies of patients with schizophrenia (6), we predicted that contrast sensitivity would be worse in the subjects with schizotypal personality disorder than in the comparison subjects. The discrimination and working memory tests used a two-by-two-by-two mixed factorial design with two within‐group factors of visual feature (form versus motion) and task (discrimination versus memory) and one between‐group factor (schizotypal personality disorder versus comparison subjects). On the basis of previous studies of patients with schizophrenia (1–4), we predicted that subjects with schizotypal personality disorder would be worse on trajectory than on form perception, and that this deficit for motion processing would be worsened when working memory operations were required.
Results
Contrast Sensitivity
There were no significant differences between the subjects with schizotypal personality disorder and the comparison subjects on the contrast sensitivity measure. The mean contrast sensitivity for comparison subjects for the left eye was 1.75 (SD=0.13); for the right eye it was 1.77 (SD=0.14). For subjects with schizotypal personality disorder, the mean contrast sensitivity for the left eye was 1.67 (SD=0.16); for the right eye it was 1.74 (SD=0.12).
Form and Trajectory Performance
The mean thresholds for each condition are shown in Figure 2. The threshold value indicates the amount of noise in the image required to reach a performance level of 70.7%. Higher threshold values indicate better performance. According to ANOVA, the interaction between group and memory demand was significant (F=7.13, df=1, 21, p=0.01), indicating that subjects with schizotypal personality disorder performed as well as comparison subjects on the discrimination tests but more poorly than comparison subjects on recognition memory tests using the same stimuli.
These group differences on recognition memory tests were confirmed by t tests for each condition: form recognition (t=2.1, df=21, p=0.05) and trajectory recognition (t=2.46, df=21, p=0.02) performance differed between groups, and form and trajectory discrimination did not. There was also a main effect of visual feature, with the noise threshold for form tests lower than for trajectory tests. There was an effect of memory (F=5.18, df=1, 12, p=0.03) and an interaction between visual feature and memory (F=6.80, df=1, 21, p=0.02). These indicated that adding a recognition requirement decreased the noise threshold for form processing but increased the threshold for trajectory processing. The better performance for trajectory working memory compared with discrimination performance by comparison subjects may represent a motion priming effect (33).
Discussion
Visual perception and working memory operations were evaluated in subjects with schizotypal personality disorder. These subjects did not differ from normal comparison subjects on tests of early-stage vision. However, subjects with schizotypal personality disorder did show deficits on both form and trajectory working memory tasks. These results indicate that although visual perception appears to be normal in schizotypal personality disorder, this disorder may be associated with a working memory deficit. This differs from the pattern of results found in patients with schizophrenia, who have both perceptual disturbances (1–6, 9) and visual working memory impairments (2, 18–20).
The finding of marked impairment in visual memory operations, with intact perceptual processing, in subjects with schizotypal personality disorder is similar to findings from other studies of subjects who have deviant scores on psychosis-proneness scales (34, 35). Silverstein et al. (34) used psychosis-proneness scales to identify subjects with anhedonia and found that these subjects showed normal performance on tests of perceptual organization. Park et al. (35) reported that undergraduate students who scored high on the Perceptual Aberration Scale performed less accurately on a visual delayed response task than students with low Perceptual Aberration Scale scores.
Comparison of the present study with studies using visual backward-masking paradigms in patients diagnosed with schizotypal personality disorder are of interest because backward-masking paradigms also test perceptual and visual short-term memory systems (12, 24, 26, 36, 37). However, these comparisons must be interpreted cautiously because the task requirements posed by the discrimination and recognition tasks used in the present study differ from those required by backward-masking tasks. The targets in backward-making paradigms are typically less than 25 msec in duration (e.g., references 12 and 26); this brief presentation places a premium on processing speed (12). In contrast, stimuli in both the discrimination and working memory conditions of the present study were presented for a long duration (2000 msec) to allow complete perceptual processing and transfer of information to working memory, which occurs within 300 msec after stimulus onset (36). Moreover, the subjects were required to compare the two stimuli to make a match versus nonmatch decision in the working memory task, rather than only identify the first stimulus, as is the case in a backward-masking paradigm.
Two studies using backward masking in subjects with schizotypal personality disorder are particularly relevant to the present study. Braff (12) compared subjects with schizotypal personality disorder (diagnosed according to DSM-III), patients with schizophrenia, and patients with depression. Subjects with schizotypal personality disorder showed deficits in backward masking compared with the performance of depressed patients at 300-msec interstimulus intervals but not at earlier interstimulus intervals (20, 60, and 120 msec). Patients with schizophrenia, in contrast, showed masking deficits both at 120-msec and 300-msec interstimulus intervals. Cadenhead et al. (26), using a similar paradigm, compared subjects with schizotypal personality disorder (diagnosed according to DSM-III-R) and a group of normal subjects and reported a trend for subjects with schizotypal personality disorder (p=0.06) to show a backward-masking deficit at 720 msec but not at earlier interstimulus intervals (60, 120, and 240 msec).
If backward-masking deficits at interstimulus intervals less than 60 msec indicate a sensory-perceptual failure, and deficits at interstimulus intervals greater than 70 msec indicate abnormalities of attentional disengagement (24), then these findings suggest that subjects with schizotypal personality disorder show intact sensory-perceptual processing at short interstimulus intervals in backward-masking paradigms but disturbed attentional or working memory operations at long interstimulus intervals.
Studies using discrimination and working memory paradigms (delayed response or delayed match to sample) along with backward-masking paradigms in the same group of subjects with schizotypal personality disorder would be valuable in understanding the relationship between these different methods of characterizing visual deficits.
A question raised by our findings is what brain regions might underlie disturbances of visual working memory in schizotypal personality disorder. Imaging studies have implicated the prefrontal cortex in visual working memory (8, 20). In addition, frontal cortex abnormalities have frequently been reported in schizophrenia (e.g., references 17 and 38–41). Since schizotypal personality disorder and schizophrenia share similar visual working memory deficits, it would be of considerable interest to investigate frontal activity during visual working memory tasks in schizotypal personality disorder in terms of establishing neurobiological similarities between schizophrenia and schizotypal personality disorder.
Received April 22, 1999; revision received Nov. 9, 1999; accepted Dec. 20, 1999. From the Department of Psychiatry, Harvard Medical School and Brockton VA Medical Center, Brockton, Mass.; and the Psychology Department, Indiana University. Address reprint requests to Dr. O’Donnell, Psychology Department, Indiana University, Psychology Bldg. 150, Bloomington, IN 47405-1301; bodonnel@ indiana.edu (e-mail). Supported by NIMH grant MH-40799, the Department of Veterans Affairs Schizophrenia Center, and the Commonwealth of Massachusetts Research Center (Dr. McCarley); the National Alliance for Research on Schizophrenia and Depression (Drs. O’Donnell, Niznikiewicz, and McCarley); and NIMH Research Scientist Development Award MH-00746, NIMH First Award, and the Stanley Foundation (Dr. Shenton).
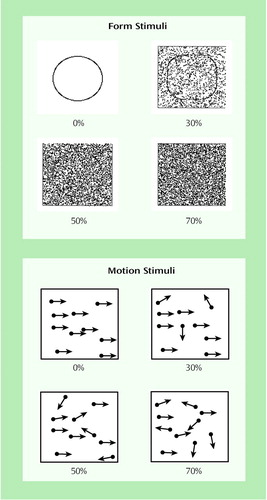
Figure 1. Illustrations of Form and Motion Stimuli Used in Form and Trajectory Processing Tests at Different Levels of Noisea
aFor the form tests, either a circle or a square with rounded corners was presented on each trial. The percentages indicate the proportion of pixels that were randomly assigned a value of black or white in the stimulus region. For the motion tests, a set of moving dots was plotted on the screen. The arrows represent the trajectory of each dot at different levels of noise. The percentages indicate the proportion of dots in the array that are moving randomly. For both tests, the percentage of noise was increased until the subject could no longer reliably identify the stimulus, which indicated the subject’s performance threshold.
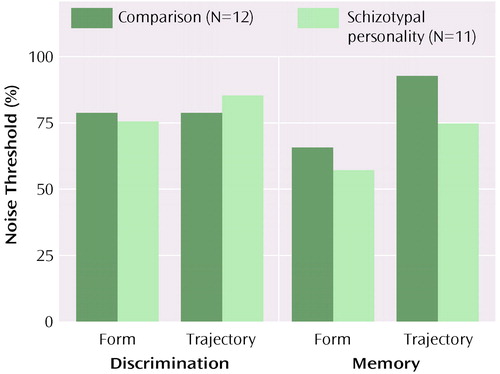
Figure 2. Noise Thresholds of Normal Comparison Subjects and Subjects With Schizotypal Personality Disorder and for Form and Trajectory Processing Tests During Discrimination and Working Memory Tasksa
aHigher noise thresholds indicate better performance. Mean values are presented. Subjects with schizotypal personality disorder showed a deficit during the working memory task but not during the discrimination task.
1. Chen Y, Palafox GP, Nakayama K, Levy DL, Matthysse S, Holzman PS: Motion perception in schizophrenia. Arch Gen Psychiatry 1999; 56:149–154Crossref, Medline, Google Scholar
2. O’Donnell BF, Swearer JM, Smith LT, Nestor PG, Shenton ME, McCarley RW: Selective deficits in visual perception and recognition in schizophrenia. Am J Psychiatry 1996; 153:687–692Link, Google Scholar
3. Stuve TA, Friedman L, Jesberger JA, Gilmore GC, Strauss ME, Meltzer HY: The relationship between smooth pursuit performance, motion perception and sustained visual attention in patients with schizophrenia and normal controls. Psychol Med 1997; 27:143–152Crossref, Medline, Google Scholar
4. Wertheim AH, van Gelder P, Lautin A, Peselow E, Cohen N: High thresholds for movement perception in schizophrenia may indicate abnormal extraneous noise levels of central vestibular activity. Biol Psychiatry 1985; 20:1197–1210Google Scholar
5. Schwartz BD, McGinn T, Winstead DK: Disordered spatiotemporal processing in schizophrenics. Biol Psychiatry 1987; 22:688–698Crossref, Medline, Google Scholar
6. Slaghuis WL: Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J Abnorm Psychol 1998; 107:49–62Crossref, Medline, Google Scholar
7. Livingstone MS, Hubel DH: Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J Neurosci 1987; 7:3416–3468Google Scholar
8. Ungerleider LG, Courtney SM, Haxby JV: A neural system for human visual working memory. Proc Natl Acad Sci USA 1998; 95:883–890Crossref, Medline, Google Scholar
9. Saccuzzo DP, Schubert DL: Backward masking as a measure of slow processing in schizophrenia and spectrum disorders. J Abnorm Psychol 1981; 90:305–312Crossref, Medline, Google Scholar
10. Cadenhead KS, Serper Y, Braff DL: Transient versus sustained visual channels in the visual backward masking deficits of schizophrenia patients. Biol Psychiatry 1998; 48:132–138Crossref, Google Scholar
11. Green MF, Nuechterlein KH, Mintz J: Backward masking in schizophrenia and mania, II: specifying the visual channels. Arch Gen Psychiatry 1994; 51:945–951Crossref, Medline, Google Scholar
12. Braff DL: Impaired speed of information processing in nonmedicated schizotypal patients. Schizophr Bull 1981; 7:499–508Crossref, Medline, Google Scholar
13. Rund BR: Backward-masking performance in chronic and nonchronic schizophrenics, affectively disturbed patients, and normal control subjects. J Abnorm Psychol 1993; 102:74–81Crossref, Medline, Google Scholar
14. Rabinowicz EF, Opler LA, Owen DR, Knight RA: Dot enumeration perceptual organization task (DEPOT): evidence for a short-term visual memory deficit in schizophrenia. J Abnorm Psychol 1996; 105:336–348Crossref, Medline, Google Scholar
15. Renshaw PF, Yurgelun-Todd DA, Cohen BM: Greater hemodynamic response to photic stimulation in schizophrenic patients: an echo planar MRI study. Am J Psychiatry 1994; 151:1493–1495Google Scholar
16. Taylor SF, Tandon R, Koeppe RA: PET study of greater visual activation in schizophrenia. Am J Psychiatry 1997; 154:1296–1298Google Scholar
17. Selemon LD, Rajkowska G, Goldman-Rakic PS: Abnormally high neuronal density in the schizophrenic cortex. Arch Gen Psychiatry 1995; 52:805–818Crossref, Medline, Google Scholar
18. Carter CS, Robertson L, Thomas N, Chaderjian M, Kraft L, O’Shora-Celaya L: Spatial working memory deficits and their relationship to negative symptoms in unmedicated schizophrenic patients. Biol Psychiatry 1996; 41:930–932Crossref, Google Scholar
19. Park S, Holzman PS: Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry 1992; 49:975–982Crossref, Medline, Google Scholar
20. Goldman-Rakic PS, Selemon LD: Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull 1997; 23:437–458Crossref, Medline, Google Scholar
21. Baron M, Gruen R, Rainer JD, Kane J, Asnis L, Lord S: A family study of schizophrenic and normal control probands: implications for the spectrum concept of schizophrenia. Am J Psychiatry 1985; 142:442–445Google Scholar
22. Kety SS, Wender PH, Jacobsen F, Ingraham LJ, Jansson L, Faber B, Kinney D: Mental illness in the biological and adoptive relatives of schizophrenic adoptees: replication of the Copenhagen study in the rest of Denmark. Arch Gen Psychiatry 1994; 51:442–455Crossref, Medline, Google Scholar
23. Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D: The Roscommon Family Study, I: methods, diagnosis of probands and risk of schizophrenia in relatives. Arch Gen Psychiatry 1993; 50:527–540Crossref, Medline, Google Scholar
24. Green MF, Nuechterlein KH, Breitmeyer B: Backward masking performance in unaffected siblings of schizophrenic patients. Arch Gen Psychiatry 1997; 54:465–472Crossref, Medline, Google Scholar
25. Merritt RD, Balogh DW: Backward masking as a function of spatial frequency: a comparison of MMPI-identified schizotypics and control subjects. J Nerv Ment Dis 1990; 178:186–193Crossref, Medline, Google Scholar
26. Cadenhead KS, Perry W, Braff DL: The relationship of information-processing deficits and clinical symptoms in schizotypal personality disorder. Biol Psychiatry 1996; 40:853–858Crossref, Medline, Google Scholar
27. Chapman LJ, Chapman JP: The measurement of differential deficit. J Psychiatr Res 1973; 14:303–311Crossref, Google Scholar
28. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R, Version 1.0 (SCID). Washington, DC, American Psychiatric Press, 1990Google Scholar
29. Voglmaier MM, Seidman LJ, Salisbury D, McCarley RW: Neuropsychological dysfunction in schizotypal personality disorder: a profile analysis. Biol Psychiatry 1997; 41:530–540Crossref, Medline, Google Scholar
30. Bach M: The “Frieburg Acuity Test”—automatic measurement of visual acuity. Optom Vis Sci 1996; 73:49–53Crossref, Medline, Google Scholar
31. Levitt H: Transformed up-down methods in psychoacoustics. J Acoust Soc Am 1971; 49:467–477Crossref, Medline, Google Scholar
32. Newsome WT, Pare EB: A selective impairment of motion perception following lesions of the middle temporal visual area (MT). J Neurosci 1988; 8:2201–2211Google Scholar
33. Pinkus A, Pantle A: Probing visual motion signals with a priming paradigm. Vision Res 1997; 37:541–552Crossref, Medline, Google Scholar
34. Silverstein SM, Raulin ML, Pristach EA, Pomerantz JR: Perceptual organization and schizotypy. J Abnorm Psychol 1992; 101:265–270Crossref, Medline, Google Scholar
35. Park S, Holzman P, Lenzenweger M: Individual differences in spatial working memory in relation to schizotypy. J Abnorm Psychol 1995; 104:355–363Crossref, Medline, Google Scholar
36. Rolls ET, Tovè£ MJ, Panzeri S: The neurophysiology of backward visual masking: information analysis. J Cogn Neurosci 1999; 11:300–311Crossref, Medline, Google Scholar
37. Knight RA: Specifying cognitive deficiencies in premorbid schizophrenics. Prog Exp Pers Psychopathol Res 1992; 15:252–289Medline, Google Scholar
38. Pettegrew JW, Keshavan MS, Panchalingam K, Strychor S, Kaplan DB, Gretta MG, Allen M: Alterations in brain high-energy phosphate and membrane phospholipid metabolism in first-episode, drug-naive schizophrenics. Arch Gen Psychiatry 1991; 48:563–568Crossref, Medline, Google Scholar
39. Ragland JD, Gur RC, Glahn DC, Censits DM, Smith RJ, Lazarev MG, Alavi A, Gur RE: Frontotemporal cerebral blood flow change during executive and declarative memory tasks in schizophrenia: a positron emission tomography study. Neuropsychology 1998; 12:399–413Crossref, Medline, Google Scholar
40. Weinberger DR, Berman KF, Zec RF: Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia, I: regional cerebral blood flow evidence. Arch Gen Psychiatry 1986; 43:114–124Crossref, Medline, Google Scholar
41. Andreasen NC: A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry 1999; 56:781–787Crossref, Medline, Google Scholar



