Sex Differences in Amphetamine-Induced Displacement of [ 18 F]Fallypride in Striatal and Extrastriatal Regions: A PET Study
Abstract
Objective: The authors examined gender differences in d -amphetamine-induced displacements of [ 18 F]fallypride in the striatal and extrastriatal brain regions and the correlations of these displacements with cognition and sensation seeking. Method: Six women and seven men underwent positron emission tomography (PET) with [ 18 F]fallypride before and after an oral dose of d -amphetamine. Percent displacements were calculated using regions of interest and parametric images of dopamine 2 (D 2 ) receptor binding potential. Results: Parametric images of dopamine release suggest that the female subjects had greater dopamine release than the male subjects in the right globus pallidus and right inferior frontal gyrus. Gender differences were observed in correlations of changes in cognition and sensation seeking with regional dopamine release. Conclusion: Findings revealed a greater dopamine release in women as well as gender differences in the relationship between regional dopamine release and sensation seeking and cognition.
Studies in animals suggest that estrogen significantly increases striatal dopamine synthesis, baseline dopamine release, d- amphetamine-induced dopamine release, and increased neuronal firing in substantia nigra (1) . Both postmortem and neuroimaging studies suggest that there is increased dopamine release in women (2 , 3) .
Relative to men, women have higher frontal cortical dopamine 2 (D 2 ) receptor levels (4) , a slower decline in striatal and cortical D 2 receptor levels with age (5 , 6) , and higher striatal dopamine transporter levels (7) . Previous studies report gender-related differences in dopaminergic neurotransmission (5 – 7) .
Dopaminergic neurotransmission plays an important role in mental disorders (8) , which show gender differences in their incidence, prevalence, clinical course, and treatment outcome (9) . Given the evidence of gender differences in animals and humans and the sensitivity of [ 18 F]fallypride to d -amphetamine-induced dopamine release (10) , we used positron emission tomography (PET) with [ 18 F]fallypride to evaluate whether there are gender differences in d -amphetamine-induced dopamine release and the relationship of dopamine release to cognition and sensation seeking in the striatal and extrastriatal brain regions.
Method
Thirteen healthy subjects (six women [mean age=24.8 years, range=21–29] and seven men [mean age=27.6 years, range=22–32]) without any history of psychiatric, neurological, or medical illness were recruited. Magnetic resonance imaging (MRI) scans were performed using a 1.5-T GE scanner. PET studies were performed using a GE Discovery LS PET scanner with a three-dimensional emission acquisition and a transmission attenuation correction. [ 18 F]Fallypride PET scans (5.0 mCi, specific activity greater than 3,000 Ci/mmol) were conducted prior to and 180 minutes following a 0.43 mg/kg oral dose of d -amphetamine. The Sensation Seeking Scale-Form V (11) was also administered. Prior to the initial PET study and 60 minutes after d -amphetamine administration, subjects began a 75-minute neuropsychological battery, including a continuous performance task, digit symbol coding and symbol search (12) , and spatial working memory (13) . Serial scans were obtained for 3.5 hours. Blood samples were collected to determine the plasma level of d -amphetamine. Serial PET scans were coregistered to each other and to thin section T 1 -weighted MRI scans (14) and reoriented to the anterior commissure-posterior commissure line. The regions of interest, caudate, putamen, ventral striatum, medial thalamus, amygdala, temporal cortex, and substantia nigra were delineated on MRI scans of the brain and transferred to the coregistered PET scans. Regional D 2 receptor binding potential and parametric images of D 2 receptor binding potential were calculated using the reference region method (15) . Percent displacement images were calculated on a pixel-by-pixel basis. Using an elastic deformation algorithm (16) , mean parametric images of [ 18 F]fallypride were also calculated. Correlations of changes in cognition and sensation seeking with parametric images of dopamine release were performed. Probability maps were calculated on a voxel-by-voxel basis using a two-tailed t test and corrected for multiple comparisons using the Forman method (17) . Only clusters with significance exceeding p<0.001 after correction for multiple comparisons were examined. (See Figure 1 .)
Results
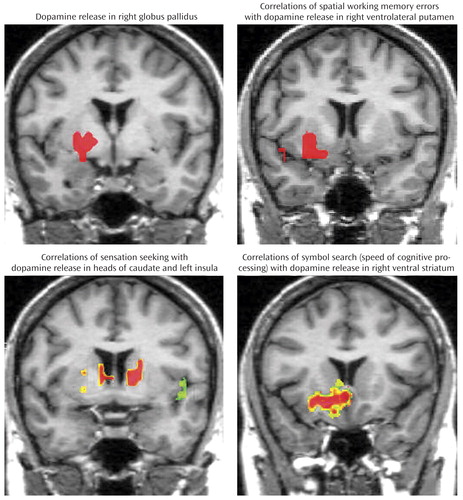
Parametric image analysis revealed significantly greater dopamine release in women in the right globus pallidus and right inferior frontal gyrus (p<0.001 [corrected for multiple comparisons]), while male subjects showed greater dopamine release in the dorsal striatum (p<0.1). Differences in greater dopamine release in women relative to men were also observed in the temporal and parietal cortices (p<0.1).
Examination of the correlations of sensation seeking with dopamine release in regions of interest demonstrated a striking gender difference in the left ventral striatum (men: r=0.898 [p=0.006]; women: r=−0.713). This difference was significant at the p<0.005 level, which was not corrected for multiple comparisons. Parametric image analysis demonstrated markedly different patterns of correlations between sensation seeking and d -amphetamine-induced dopamine release in male and female subjects, i.e., women but not men showed negative correlations with dopamine release in the heads of the caudate nuclei bilaterally (r=−0.84), the globus pallidus (r=−0.801), and the left anterior cingulate (r=−0.757, p<0.001 [corrected for multiple comparisons]). In contrast, male but not female subjects demonstrated significant clusters of negative correlations (p<0.001 [corrected for multiple comparisons]) in the left and right insular regions (r=−0.719 and −0.771, respectively), bilateral inferior temporal cortex (r=−0.710 on the left and −0.771 on the right), and the left lateral thalamus (r=−0.746). These differences in correlations were significant in the heads of the caudate nuclei bilaterally and the left insula.
Correlations of changes in spatial working memory errors with parametric images of dopamine release demonstrated a right ventral putamen cluster in male (r=−0.84, p<0.001 [corrected for multiple comparisons]) but not female subjects. Gender difference in the right ventral putamen correlations for spatial working memory was significant (p<0.001 [corrected for multiple comparisons]).
Changes in performance on the symbol search task was significantly correlated in male subjects with dopamine release in the ventral and lateral striatal bilaterally and right hippocampus (range: −0.741 to −0.829 [p<0.001]). Female subjects had positive correlation in the right ventral striatum (r=0.49). A significant gender difference in correlations was observed in the right ventral and lateral striatum (p<0.001 [corrected for multiple comparisons]). However, plasma levels of d -amphetamine were not significantly different in men and women (0.46 [SD=0.26] nM/ml and 0.45 [SD=0.23] nM/ml, respectively).
Discussion
While gender-related differences in dopaminergic neurotransmission have been reported in studies using PET and single photon emission computed tomography (SPECT), no previous studies of d -amphetamine-induced dopamine release, to our knowledge, have examined gender differences in humans. The greater dopamine release in the right globus pallidus and right inferior frontal gyrus in women is consistent with previous animal studies reporting greater d -amphetamine-induced dopamine release in women. These differences are not because of volumetric differences, since these structures are similar in size in both men and women (18) . In addition, gender differences in dopamine release were observed in cortical regions and other striatal regions, suggesting increased dopamine release in female subjects. Sensation-seeking behavior, spatial working memory, and attention and speed of cognitive processing are all believed to be modulated by dopaminergic neurotransmission (19) . Gender differences in the relationship of dopamine release to these behaviors suggest significant differences in regional dopaminergic function. The differences found in this study remained significant after correction for multiple comparisons for both within-image comparisons and multiple behavioral tests.
In summary, significant gender differences in regional dopamine release and the relationship of regional dopamine release to cognitive function and sensation seeking were observed. These differences are likely because of gonadal hormonal modulation of cerebral dopaminergic neurotransmission. To our knowledge, this is the first study to investigate gender-related differences in d -amphetamine-induced dopamine release in striatal and extrastriatal regions in humans. The results of this study, if confirmed, indicate the need to consider gender in studies of dopamine release and the need for further studies to consider the role of gender-related differences in dopaminergic neurotransmission in neuropsychiatric disorders.
1. Di Paolo T: Modulation of brain dopamine transmission by sex steroids. Rev Neurosci 1994; 5:27–42Google Scholar
2. Konradi C, Kornhuber J, Sofic E, Heckers S, Riederer P, Beckmann H: Variations of monoamines and their metabolites in the human brain putamen. Brain Res 1992; 579:285–290Google Scholar
3. Pohjalainen T, Rinne JO, Nagren K, Syvalahti E, Hietala J: Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry 1998; 155:768–773Google Scholar
4. Kaasinen V, Nagren K, Hietala J, Farde L, Rinne JO: Sex differences in extrastriatal dopamine-like receptors in the human brain. Am J Psychiatry 2001; 158:308–311Google Scholar
5. Wong DF, Broussolle EP, Wand G, Villemagne V, Dannals RF, Links JM, Zacur HA, Harris J, Naidu S, Braestrup C, Wagner HN, Gjedde A: In vivo measurement of dopamine receptors in human brain by positron emission tomography: age and sex differences. Ann NY Acad Sci 1988; 515:203–214Google Scholar
6. Kaasinen V, Kemppainen N, Nagren K, Helenius H, Kurki T, Rinne JO: Age-related loss of extrastriatal dopamine-like receptors in women. J Neurochem 2002; 81:1005–1010Google Scholar
7. Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, Maciejewski PK, O’Malley S, Innis RB: Sex differences in [ 123 I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse 2001; 41:275–284 Google Scholar
8. Jentsch JD, Roth RH, Taylor JR: Role for dopamine in the behavioral functions of the prefrontal corticostriatal system: implications for mental disorders and psychotropic drug action. Prog Brain Res 2000; 126:433–453Google Scholar
9. Hartung CM, Widiger TA: Gender differences in the diagnosis of mental disorders: conclusions and controversies of the DSM-IV. Psychol Bull 1998; 123:260–278Google Scholar
10. Riccardi P, Zald D, Li R, Park S, Ansari S, Dawant B, Anderson S, Woodard N, Schmidt D, Baldwin R, Kessler R: Sex differences in amphetamine induced displacement of [ 18 F] fallypride in striatal and extrastriatal regions in humans. J Nucl Med (in press) Google Scholar
11. Zuckerman M, Eysenck S, Eysenck HJ: Sensation seeking in England and America: cross-cultural, age, and sex comparisons. J Consult Clin Psychol 1978; 46:139–149Google Scholar
12. Wechsler D: Wechsler Adult Intelligence Scale, Third Edition. San Antonio, Tex.: Psychological Corporation, Harcourt Brace Jovanovich, 1997Google Scholar
13. Park S, Puschel J, Sauter BH, Rentsch M, Hell D: Spatial selective attention and inhibition in schizophrenia patients during acute psychosis and at 4-month follow-up. Biol Psychiatry 2002; 51:498–506Google Scholar
14. Maes F, Collignon A, Vandermuelen D, Marchal G, Suetens P: Multimodality image registration by maximization of mutual information. IEEE Trans Med Image 1997; 16:187–198Google Scholar
15. Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, Frackowiak RS: Comparison of methods for analysis of clinical [ 11 C]raclopride studies. J Cereb Blood Flow Metab 1996; 16:42–52 Google Scholar
16. Pluim JPW, Maintz JBA, Viergever MA: Mutual information matching in multiresolution context. Imag Vis Comput 2001; 19:45–52Google Scholar
17. Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC: Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995; 33:636–647Google Scholar
18. Gur RC, Gunning-Dixon FM, Turetsky BI, Bilker WB, Gur RE: Brain region and sex differences in age association with brain volume: a quantitative MRI study of healthy young adults. Am J Geriatr Psychiatry 2002; 10:72–80Google Scholar
19. Arnsten AF, Li BM: Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry 2005; 57:1377–1384Google Scholar



