5-HTTLPR Genotype-Specific Phenotype in Children and Adolescents With Autism
Abstract
Objective: The serotonin transporter gene (SLC6A4) is a strong autism candidate gene because of its association with anxiety, aggression and attention, and the effectiveness of selective serotonin reuptake inhibitors (SSRIs) in treating certain behavioral symptoms. In families with individuals with autism, several reports of biased transmission of both alleles (short, long) at the serotonin transporter gene promotor polymorphism (5-HTTLPR) locus of SLC6A4 now exist. The heterogeneity in these reports may be due to clinical heterogeneity. The authors hypothesized that 5-HTTLPR genotypes would be related to variation in specific symptoms in children with autism. Method: The authors explored whether variants of two functional polymorphisms of SLC6A4 (5-HTTLPR, intron 2 variable number tandem repeat [2 VNTR]) were related to behavioral characteristics measured by the Autism Diagnostic Interview-Revised and Autism Diagnostic Observation Schedule. Subjects (N=73, age 3–19 years old) met diagnostic criteria for autistic disorder based on both measures. Results: Evidence of genotype-phenotype interactions on the Autism Diagnostic Interview-Revised was found with the 5-HTTLPR short group of HTTLPR (S/L or S/S genotypes) being rated as more severe on the subdomain “failure to use nonverbal communication to regulate social interaction,” and the long group (L/L genotype) being more severe on the subdomain “stereotyped and repetitive motor mannerisms” and on an aggression measure. In contrast, on the Autism Diagnostic Observation Schedule, the long group was associated with greater severity on directed facial expressions and unusual sensory interests. There were no significant relationships between the intron 2 VNTR genotypes and subdomains or domains of symptoms on the Autism Diagnostic Interview-Revised or the Autism Diagnostic Observation Schedule. Conclusions: These findings provide initial support for genotype-specific phenotypes for 5-HTTLPR in autism based on ratings from the Autism Diagnostic Interview-Revised and Autism Diagnostic Observation Schedule.
Autism is a neurodevelopmental disorder characterized by qualitative impairments in three domains: reciprocal social interaction, communication, and repetitive behaviors and stereotyped behavior (DSM–IV). The etiology of autism is multifactorial with indications of strong, but complex, genetic influence. In relatives of individuals with autism, a high prevalence of characteristics and personality traits indicative of deficits in social, communicative, and repetitive behaviors coined the broad autism phenotype have been reported (for reviews see 1 , 2) . Estimates of concordance for autism among monozygotic twins is 64%–91%, which is greater than that among fraternal twins and siblings (0%–9%) (3 – 6) . The number of genes estimated to contribute to autism ranges from 2 to over 100 (for a review see 7). Independently, any individual gene probably makes a relatively small contribution to the likelihood of developing autism (8) .
The diagnosis of autism is often based on two highly validated and reliable instruments in addition to clinical judgment. One, the Autism Diagnostic Interview-Revised (9) , is a semistructured parent interview designed to assess children’s current and past behavior in the three domains: qualitative impairment in social interaction; qualitative impairment in communication; and restricted repetitive and stereotyped patterns of behavior, interests, and activities. Each domain is characterized in DSM–IV by a list of four items; children must exhibit a certain number of items within each domain for diagnosis. These DSM–IV items are captured by subdomains on the Autism Diagnostic Interview-Revised. The other instrument, the Autism Diagnostic Observation Schedule, is a standardized semistructured, instrument designed to assess the social, communication, and restricted and repetitive domains in an interview or play-like setting with the child (10) . Both instruments contain an algorithm with cutoff scores for autistic disorder based on select items; the Autism Diagnostic Observation Schedule algorithm does not include the repetitive domain because of the limited observation time.
Neither the Autism Diagnostic Interview-Revised nor the Autism Diagnostic Observation Schedule was designed as a rating scale. However, because of its established diagnostic validity, the Autism Diagnostic Interview-Revised has been used to rank behavioral severity within autism in behavioral (11) and genetic (12 – 14) research. Moreover, ratings on the Autism Diagnostic Interview-Revised vary less within sibling pairs compared with across sibling pairs in some studies (15 – 17) but not others (14 , 18) . Notably, family studies provide limited information about any particular gene because they measure the concordance of behavioral features among individuals (i.e., across all genes).
In this article, we examine relations between two functional polymorphisms (serotonin transporter gene promotor polymorphism [5-HTTLPR], intron 2 variable number tandem repeat [2 VNTR]) of the serotonin transporter gene (SLC6A4) and behavior as measured by the Autism Diagnostic Interview-Revised and Autism Diagnostic Observation Schedule. Medications targeting the serotonin transporter reduce ritualistic and routine behaviors in some individuals with autism and associated disorders (19 , 20) . Further, seven of 12 genetic studies of children with autism report overtransmission of 5-HTTLPR from their parents (21) . Interestingly, which allele of 5-HTTLPR (i.e., short or long) is overtransmitted varies across studies. This leads to the question of whether variants of 5-HTTLPR influence which clinical signs are present in an individual with autism (8 , 22) .
Variants of SLC6A4 have been reported to influence the severity of autistic behavior. Using scores derived from the Autism Diagnostic Interview-Revised algorithm, Tordjman et al. (22) found increased severity on the Autism Diagnostic Interview-Revised combined social/communication domain and the conversation subdomain in subjects with a short 5-HTTLPR allele. Mulder et al. (23) found greater severity on a rigid-compulsive behavior factor derived from the Autism Diagnostic Interview-Revised to approximate behavioral phenotypes in subjects with two long intron 2 VNTR alleles. Sutcliffe et al. (24) also reported increased severity of this factor among amino acid coding variants at the SLC6A4 locus (lle425Leu, Leu550Val, Phe465Leu) (24) . Factor analytic studies of the Autism Diagnostic Interview-Revised, which include all Autism Diagnostic Interview-Revised items, have failed to reach consensus on its underlying factor structure (25 – 27) .
To examine associations between the serotonin transporter gene and phenotype in autism, previous studies derived clinical factors independent of genotype. In contrast, we began with genotype groups and then compared these groups on validated clinical measures; these clinical measures were the Autism Diagnostic Interview-Revised and Autism Diagnostic Observation Schedule. We focused on the Autism Diagnostic Interview-Revised and Autism Diagnostic Observation Schedule algorithms because they provide a minimal common data set across autism genetic studies; previous studies used nonalgorithm items when deriving factors. In this study, we explored genotype-specific phenotype by analyzing associations between two commonly studied variants in the serotonin transporter gene (5-HTTLPR and intron 2 VNTR) and Autism Diagnostic Interview-Revised and Autism Diagnostic Observation Schedule ratings in probands with autistic disorder. To quantify phenotype, scores from the Autism Diagnostic Interview-Revised and Autism Diagnostic Observation Schedule algorithms were used. On the Autism Diagnostic Interview-Revised, we predicted that genotype might influence phenotype as measured by domain or subdomain. Since the contribution of any single gene in a multifactorial disorder may be below the gross (i.e., domain) level, we tested every subdomain rather than the more conservative method of testing only subdomains within significant domains (15 , 22) .
Method
Subjects and Assessment
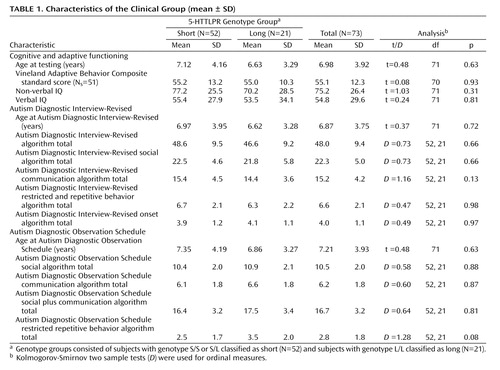
Subjects were a subset of a previous clinical group in which this gene was studied (28) . Recruitment, assessment, and inclusion criteria were the same as that outlined in Kim et al. (28) , but only one sibling was randomly selected from each affected sibling pair, and subjects 1) were only from the University of Chicago Developmental Disorders Clinic, 2) were at least 3 years old, 3) had sufficient blood or deoxyribonucleic acid (DNA) available for fine mapping studies, and 4) met Autism Diagnostic Interview-Revised and Autism Diagnostic Observation Schedule classification for autistic disorder. All subjects had a best estimate diagnosis of autistic disorder by a clinical psychologist and psychiatrist. Table 1 provides clinical characteristics of the study group. There were 73 subjects, 59 males and 14 females, between 3 and 19 years of age (mean=7 years). Subjects’ ethnicity was identified as follows: Caucasian, 60; African American, 5; Asian, 6; and Hispanic, 2. Parents were interviewed using the research version of the Autism Diagnostic Interview-Revised (29) . Subjects participated in the appropriate module (1 – 4) of the Autism Diagnostic Observation Schedule determined by language level (module 1=25, module 2=20, module 3=11, module 4=8) or the PL- Autism Diagnostic Observation Schedule (30) (N=8); the PL-Autism Diagnostic Observation Schedule was treated as module 1 for analyses. After complete description of the study to the parents, written informed consent was obtained. Assent was obtained from the children and adolescents.
DNA Analyses
Information on previously reported genotyping of this clinical group can be found in Kim et al. (28) . We focused on the 44-bp insertion/deletion polymorphism in the promoter region of the gene 5-HTTLPR. The analysis was repeated with the VNTR polymorphism in the second intron of the serotonin transporter gene with nine, 10, or 12 copies of the 17-bp repeat polymorphism; one subject was missing data for intron 2 VNTR genotype.
Statistical Analyses
The distributions of 5-HTTLPR and intron 2 VNTR genotypes were tested with χ 2 for Hardy-Weinberg equilibrium using the HWE program from the LINKUTIL package. Differences in clinical characteristics of genotype groups were compared using t tests for independent samples or Kolmogorov-Smirnov two sample tests ( Dm,n ) where applicable.
Group differences in phenotype based on the 5-HTTLPR and intron 2 VNTR alleles were analyzed using analysis of variance (ANOVA) with genotype group as the independent variable, total/domain/subdomain scores as dependent variables, and age and nonverbal IQ as covariates. Language level reported on the Autism Diagnostic Interview-Revised (phrase speech or single words/nonverbal) was entered as an independent variable for total and communication scores. Preliminary analyses revealed no main effect of sex.
Individual items were analyzed using a statistic for ordinal data, M2 , because the codes were intended to be qualitative (9 , 10) . The statistic M2 , referred to as Mahon’s χ 2 , has approximately a chi-squared distribution with df=1 for large groups (31) . Items were analyzed because 1) subdomain differences were suggestive of item differences and 2) subdomain could fail to capture genotype-specific phenotype. We predicted that subdomain scores would best capture the severity of an individual’s behavior and therefore the variability within groups. On the Autism Diagnostic Interview-Revised and Autism Diagnostic Observation Schedule, item scores range from 0 (behavior of type specified is not present) to 2 or 3 (behavior definitely present and meets criteria). For all analyses, scores were converted according to the algorithms (i.e., 3 to 2, 7 to 0, 8 to 0). Many Autism Diagnostic Interview-Revised items contain current and past (“most abnormal 4.0–5.0 years” or “ever”) ratings. The appropriate score was selected based on the subject’s age. On the Autism Diagnostic Interview-Revised, all 39 algorithm items were analyzed. In addition, aggression (the highest “ever” rating of self-injurious behavior, aggression toward caregivers/family and toward noncaregivers/nonfamily) and overactivity (“ever”) were also analyzed because of previous reports of relations with 5-HTTLPR. Some subjects were missing data for the latter items (N=5 and N=22, respectively) because they were not on the algorithm. On the Autism Diagnostic Observation Schedule, the 15 items that appear across modules were analyzed; responsive social smiling (module 1) was also analyzed because it related to a notable Autism Diagnostic Interview-Revised item. Associations among Autism Diagnostic Interview-Revised or Autism Diagnostic Observation Schedule scores were calculated using Spearman’s rho (r s ).
The alpha value was set at p≤0.05 for all analyses; p values presented are uncorrected for multiple comparisons in this exploratory set of analyses. Results are reported first for 5-HTTLPR and then for intron 2 VNTR.
Results
5-HTTLPR
For 5-HTTLPR, 37 probands were homozygous (S/S, L/L: 16, 21) and 36 were heterozygous (S/L). The genotype distribution was consistent with Hardy-Weinberg equilibrium (χ 2 =0.006, df=2, p=0.94). The S allele is typically considered dominant, so a combined short (S) genotype group (S/S, S/L) was compared with a long (L) group (L/L). Preliminary analysis confirmed that subjects with S/L genotype were more phenotypically similar to subjects with S/S genotype than subjects with L/L genotype; the pattern of findings reported remains the same with three groups.
The S and L groups did not differ significantly with respect to gender (χ 2 =1.77, df=1, p=0.18), ethnicity (χ 2 =0.73, df=1, p=0.39), presence of phrase speech on the Autism Diagnostic Interview-Revised (χ 2 =0.67, df=1, p=0.41), or Autism Diagnostic Observation Schedule module (χ 2 =1.47, df=3, p=0.69). Table 1 shows mean age, nonverbal IQ, verbal IQ, language level, and Vineland Adaptive Behavior Composite standard score by group. Vineland domain standard scores did not significantly differ by group. Age was marginally correlated with verbal IQ (r=0.22, p=0.07) but not nonverbal IQ (r=–0.09, p=0.47).
Genotype-Phenotype Relations on the Autism Diagnostic Interview-Revised
There was no main effect of genotype on total or domain Autism Diagnostic Interview-Revised scores ( Table 1 ). Table 2 shows the effect of group, age, nonverbal IQ, and language level on total scores. Age was positively associated with each score, except onset. Nonverbal IQ was negatively associated with social domain scores.
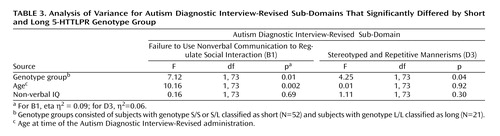
Two of the 12 subdomains had a main effect of genotype: “failure to use nonverbal behaviors to regulate social interaction” (abbreviated B1 in the Autism Diagnostic Interview-Revised) and “stereotyped and repetitive motor mannerisms” (abbreviated D3 in the Autism Diagnostic Interview-Revised). Figure 1 shows the percentage of probands receiving each subdomain score by genotype; Table 3 shows the effect of group, age, and nonverbal IQ. For B1, the S group had a higher mean score than the L group (4.69 [SD=1.39] and 3.71 [SD=1.55], respectively). For D3, the L group had a higher mean score than the S group (1.90 [SD=0.30] and 1.54 [SD=0.73], respectively). There was no significant association between B1 and D3.
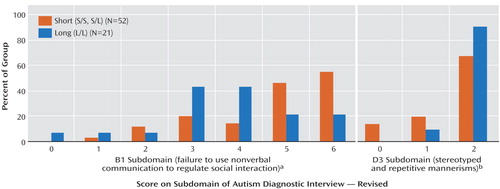
a Significant difference between genotype groups (F=7.12, df=1, 73, p=0.01).
b Significant difference between genotype groups (F=4.25, df=1, 73, p=0.04).
Exploratory analysis revealed these subdomains related to behavior that first caused parents’ concern about their children’s development. A lack of or abnormal socioemotional responsiveness was reported for 35% (N=18) of the S group, but only 14% (N=3) of the L group. Autistic-type behaviors, including hand-finger mannerisms and highly repetitious nonfunctional behaviors, were the primary concern for 24% (N=5) of the L group but only 2% (N=1) of the S group. Child’s age at which parents were concerned did not differ by group.
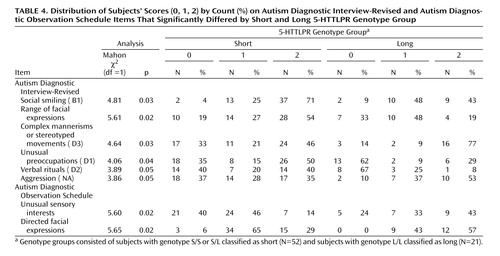
Comparison of the distribution of individual item scores by genotype revealed differences on six items ( Table 4 ). Within B1, the S group was more severe than the L group on “social smiling” and “range of facial expressions.” On the remaining B1 item, “direct gaze,” no subject in the S group received a 0 compared with three subjects in the L group. All items in B1 were positively associated with each other. The S group also scored more severely than the L group on “unusual preoccupations” and “verbal rituals.” D3 represents the higher score of two items, which were not significantly associated in this group; the L group was more severe than the S group on “other complex mannerisms or stereotyped body movements” but not on “hand and finger mannerisms”. The L group also scored more severely than the S group on the aggression item. Aggression was marginally related to “complex mannerisms” (r s =0.23, p=0.06). No other items, which differed by 5-HTTLPR genotype group, were significantly associated with each other. No significant difference between groups appeared on the overactivity item ( M2 =1.12, df=1, p=0.29).
Genotype-Phenotype Relations on the Autism Diagnostic Observation Schedule
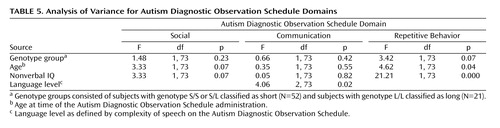
On the Autism Diagnostic Observation Schedule, there was no main effect of genotype on the sum of the cross module items ( Table 1 ). Table 5 shows the effect of group, age, nonverbal IQ, and language level on total scores.
Item analysis revealed that the L group scored more severely than the S group on “unusual sensory interests” and “directed facial expressions” ( Table 4 ). The later finding contrasts with the pattern on B1 of the Autism Diagnostic Interview-Revised. An item more similar to B1, responsive social smile, appears only in module 1 of the Autism Diagnostic Observation Schedule and the PL-Autism Diagnostic Observation Schedule. On this item, there was a tendency for the S group to be more severe than the L group (N=33, M2 =3.18, df=1, p=0.08). There was a marginal effect for the L group to be more severe on overactivity than the S group ( M2 =3.49, df=1, p=0.06), with 28% (N=6) of the L group receiving scores of 2 compared with 6% (N=3) of the S group. Notably, for hand and finger and other complex mannerisms, the L group was more severe than the S group, with only 38% of the L group receiving a 0 compared with 58% of the S group ( M2 =1.84, df=1, p=0.17). There were no significant differences on the other items: hand and finger/other mannerisms, aggression, self-injurious behavior, stereotyped speech, odd speech, immediate echolalia, overall level of language, gestures, unusual eye contact, shared enjoyment, quality of social overtures, and anxiety.
Intron 2 VNTR
For the intron 2 VNTR alleles, 30 probands were heterozygous (9/12, 10/12: 4, 26), and 43 were homozygous (10/10, 12/12: 4, 38). This distribution did not deviate from Hardy-Weinberg equilibrium (χ 2 =1.44, df=3, p=0.70). Probands were divided into groups collapsing the 9/12 and 10/12 into one group, referred to herein as 10/12. The groups (10/10, 10/12, 12/12) did not differ significantly with respect to any demographic factors, level of functioning, or Autism Diagnostic Observation Schedule module. No significant differences between groups were found on all analyses.
Discussion
Evidence for genotype specific phenotype was found for 5-HTTLPR, but not for intron 2 VNTR. These relationships could not be attributed to overall differences between the groups in total severity of autistic behavior as measured by Autism Diagnostic Interview-Revised, Autism Diagnostic Observation Schedule, nonverbal IQ, language level, adaptive functioning, or age. At the domain level, the groups did not differ significantly. By focusing on specific behaviors at the subdomain level, we found greater “failure to use nonverbal communication to regulate social interaction” in the S group, and increased severity in “stereotyped and repetitive mannerisms” in the L group.
Given the greater severity of the S group on using nonverbal communication to regulate social interaction (Autism Diagnostic Interview-Revised), the greater severity of the L group on directing facial expressions (Autism Diagnostic Observation Schedule) was surprising. Although the coding criteria for these items differ qualitatively, emphasizing initiation versus response, affect, and context, this alone seemed unlikely to account for the opposing pattern. Differences in parent report/observation could explain it. Alternatively, differences in past behavior (Autism Diagnostic Interview-Revised) compared with current behavior (Autism Diagnostic Observation Schedule) may account for the apparent discrepancy. For example, an interaction between development and genotype specific phenotype could exist such that the S group’s behavior improved; prospective longitudinal studies using an age homogenous group beginning in preschool could explore this possibility.
These findings highlight the benefit of using both measures to explore genotype specific phenotype. Parallel findings were found across measures for social smiling and stereotyped and repetitive mannerisms. The Autism Diagnostic Observation Schedule may be too short to capture certain behaviors, such as aggression, or may not provide the appropriate context. It features summary items that appear individually on the Autism Diagnostic Interview-Revised (e.g., unusual preoccupation and verbal rituals), which may mask genotype specific phenotype. Yet, it is beneficial because an examiner may see a red flag, such as an unusual sensory behavior, that a parent may overlook. The use of both measures provides the opportunity to compare behavior at its most severe (Autism Diagnostic Interview-Revised) to its current level (Autism Diagnostic Observation Schedule). If development or environment influences genotype-phenotype relationships, the likelihood of seeing group differences on the Autism Diagnostic Observation Schedule compared with the Autism Diagnostic Interview-Revised increases.
The Autism Diagnostic Interview-Revised is frequently used in research groups and is readily available for large populations of genotyped probands. The role of heterogeneity across these groups should not be underestimated. Deriving factors from within an individual clinical group will influence the identification of genotype specific phenotype. In a group in which the short allele is overtransmitted, there will be an overrepresentation of traits associated with short allele containing genotypes. For example, the current clinical group may have even more limited power to detect genotype specific phenotype associated with the long/long genotype.
Group recruitment may affect studies of SLC6A4 in autism. For example, recruiting from a center running a clinical trial for medication aimed at reducing aggression may increase the number of subjects with the L genotype, whereas drawing from an early intervention program targeted at improving social skills may increase the number of subjects with the S genotypes. In our clinical group, there was an indication that group recruitment could relate to behavior; parents’ first behavioral concerns related to later group differences in behavior and genotype specific phenotype. Early signs may be useful in studying heterogeneity across different groups of subjects with autistic spectrum disorders. Another issue with ascertainment is inclusion criteria. In this study, only probands who met Autism Diagnostic Interview-Revised and Autism Diagnostic Observation Schedule criteria for autistic disorder and had a best estimate diagnosis of autistic disorder were included. Including subjects on the autism spectrum may affect the likelihood of finding genotype specific phenotype because the range of severity of behaviors would increase.
In nonautistic groups, both alleles of 5-HTTLPR have been related to characteristics associated with autism. Increased prevalence of attention deficit/hyperactivity disorder (32) and repetitive rituals in obsessive-compulsive disorder (33) have been found among the homozygous long genotype. Anxiety (34) , harm avoidance (35) , neuroticism (36) , shyness (37 , 38) , low social orientation scores (39) , recurrent overt physical violence (40) , and minimal responses to facial expressions in typically developing children (38) have been associated with the short allele in some but not all studies. The diversity of characteristics associated with this variant may contribute to the heterogeneity of autism. However, because of different gene-gene and gene-environment interactions, these genotype-specific phenotypes may not generalize to nonautistic groups.
Our inference about genotype-specific phenotype is limited by our group size. These results need to be replicated in a larger group. We predict the S group to be associated with increased severity on items related to using nonverbal behavior to regulate social interaction, as measured by B1 on the Autism Diagnostic Interview-Revised, and the L group to be associated with increased severity on stereotyped and repetitive mannerisms, as measured by D3 on the Autism Diagnostic Interview-Revised. If the findings reported in this study are replicated, this approach to genotype specific phenotype may contribute to understanding the role of 5-HTTLPR genotypes in the complex pathophysiology of autism. Finally, differences in phenotype within and across autism genetic studies may have contributed to heterogeneity of findings for 5-HTTLPR transmission (21) .

1. Bailey A, Palferman S, Heavey L, Le Couteur A: Autism: the phenotype in relatives. J Autism Dev Disord 1998; 28:369–392Google Scholar
2. Murphy M, Bolton PF, Pickles A, Fombonne E, Piven J, Rutter M: Personality traits of the relatives of autistic probands. Psychol Med 2000; 30:1411–1424Google Scholar
3. Smalley SL, Asarnow RF, Spence MA: Autism and genetics: a decade of research. Arch Gen Psychiatry 1988; 45:953–961Google Scholar
4. Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, Bailey A, Rutter M: A case-control family history study of autism. J Child Psychol Psychiatry 1994; 35:877–900Google Scholar
5. Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M: Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med 1995; 25:63–78Google Scholar
6. Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, Jakobsson G, Bohman M: A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiat 1989; 30:405–416Google Scholar
7. Veenstra-VanderWeele J, Cook EH: Molecular genetics of autism spectrum disorder. Mol Psychiatry 2004; 9:819–832Google Scholar
8. Veenstra-Vanderweele J, Cook E Jr, Lombroso PJ: Genetics of childhood disorders: xlvi. autism, part 5: genetics of autism. J Am Acad Child Adolesc Psychiatry 2003; 42:116–118Google Scholar
9. Lord C, Rutter M, Le Couteur A: Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24:659–685Google Scholar
10. Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M: The Autism Diagnostic Observation Schedule-Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000; 30:205–223Google Scholar
11. Ozonoff S, Cook I, Coon H, Dawson G, Joseph RM, Klin A, McMahon WM, Minshew N, Munson JA, Pennington BF, Rogers SJ, Spence MA, Tager-Flusberg H, Volkmar FR, Wrathall D: Performance on Cambridge neuropsychological test automated battery subtests sensitive to frontal lobe function in people with autistic disorder: evidence from the Collaborative Programs of Excellence in Autism Network. J Autism Dev Disord 2004; 34:139–150Google Scholar
12. Hollander E, King A, Delaney K, Smith CJ, Silverman JM: Obsessive-compulsive behaviors in parents of multiplex autism families. Psychiatry Res 2003; 117:11–16Google Scholar
13. Nurmi EL, Dowd M, Tadevosyan-Leyfer O, Haines JL, Folstein SE, Sutcliffe JS: Exploratory subsetting of autism families based on savant skills improves evidence of genetic linkage to 15q11-q13. J Am Acad Child Adolesc Psychiatry 2003; 42:856–863Google Scholar
14. Spiker D, Lotspeich L, Dimiceli S, Myers R, Risch N: Behavioral phenotypic variation in autism multiplex families: evidence for a continuous severity gradient. Am J Med Genet B Neuropsychiatr Genet 2002; 114:129–136Google Scholar
15. Silverman J, Smith C, Schmeidler J, Hollander E, Lawlor B, Fitzgerald M, Buxbaum J, Delaney K, Galvin P, Autism Genetic Research Exchange Consortium: Symptom domains in autism and related conditions: Evidence for familiality. Am J Med Genet B Neuropsychiatr Genet 2002; 114:64–73Google Scholar
16. Kolevzon A, Smith CJ, Schmeidler J, Buxbaum JD, Silverman JM: Familial symptom domains in monozygotic siblings with autism. Am J Med Genet B Neuropsychiatr Genet 2004; 129:76–81Google Scholar
17. Spiker D, Lotspeich L, Kraemer HC, Hallmayer J, McMahon W, Petersen PB, Nicholas P, Pingree C, Wiese-Slater S, Chiotti C, Wong DL, Dimicelli S, Ritvo E, Cavalli-Sforza LL, Ciaranello RD: Genetics of autism: characteristics of affected and unaffected children from 37 multiplex families. Am J Med Genet 1994; 54:27–35Google Scholar
18. Cuccaro ML, Shao Y, Bass MP, Abramson RK, Ravan SA, Wright HH, Wolpert CM, Donnelly SL, Pericak-Vance MA: Behavioral comparisons in autistic individuals from multiplex and singleton families. J Autism Dev Disord 2003; 33:87–91Google Scholar
19. Gordon C, State R, Nelson J, Hamburger S, Rapoport J: A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Arch Gen Psychiatry 1993; 50:441–447Google Scholar
20. McDougle C, Naylor S, Cohen D, Volkmar F, Heninger G, Price L: A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry 1996; 53:1001–1008Google Scholar
21. Devlin B, Cook E, Coon H, Dawson G, Grigorenko E, McMahon W, Minshew N, Pauls D, Smith M, Spence M, Rodier P, Stodgell C, Network CG, Schellenberg G: Autism and the serotonin transporter: the long and short of it. Mol Psychiatry 2005; 10:1110–1116Google Scholar
22. Tordjman S, Gutneckt L, Carlier M, Spitz E, Antoine C, Slama F, Cohen D, Ferrari P, Roubertoux P, Anderson G: Role of the serotonin transporter in the behavioral expression of autism. Mol Psychiatry 2001; 6:434–439Google Scholar
23. Mulder EJ, Anderson GM, Kema IP, Brugman AM, Ketelaars CE, de Bildt A, van Lang ND, den Boer JA, Minderaa RB: Serotonin transporter intron 2 polymorphism associated with rigid-compulsive behaviors in Dutch individuals with pervasive developmental disorder. Am J Med Genet B Neuropsychiatr Genet 2005; 133:93–96Google Scholar
24. Sutcliffe J, Delahanty R, Prasad H, McCauley J, Han Q, Jinag L, Li C, Folstein S, Blakely R: Allelic heterogeneity at the serotonin transporter gene (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet 2005; 77:265–279Google Scholar
25. Constantino JN, Gruber CP, Davis S, Hayes S, Passanante N, Przybeck T: The factor structure of autistic traits. J Child Psychol Psychiatry 2004; 45:719–726Google Scholar
26. Szatmari P, Merette C, Bryson SE, Thivierge J, Roy MA, Cayer M, Maziade M: Quantifying dimensions in autism: a factor-analytic study. J Am Acad Child Adolesc Psychiatry 2002; 41:467–474Google Scholar
27. Tadevosyan-Leyfer O, Dowd M, Mankoski R, Winklosky B, Putnam S, McGrath L, Tager-Flusberg H, Folstein SE: A principal components analysis of the Autism Diagnostic Interview-Revised. J Am Acad Child Adolesc Psychiatry 2003; 42:864–872Google Scholar
28. Kim S-J, Cox N, Courchesne R, Lord C, Corsello C, Akshoomoff N, Guter S, Leventhal B, Courchesne E, Cook E: Transmission disequilibrium mapping in the serotonin transporter gene (SLC6A4) region in autistic disorder. Mol Psychiatry 2002; 7:278–288Google Scholar
29. Rutter ML, Lord C, Le Couteur A: Autism Diagnostic Interview-R Research, 1995Google Scholar
30. DiLavore P, Lord C, Rutter M: Pre-linguistic Autism Diagnostic Observation Schedule (PL-ADOS). J Autism Dev Disord 1995; 25:355–379Google Scholar
31. Agresti A: An Introduction to Categorical Data Analysis. New York, John Wiley and, Inc., 1996Google Scholar
32. Zoroglu SS, Erdal ME, Alasehirli B, Erdal N, Sivasli E, Tutkun H, Savas HA, Herken H: Significance of serotonin transporter gene 5-HTTLPR and variable number of tandem repeat polymorphism in attention deficit hyperactivity disorder. Neuropsychobiology 2002; 45:176–181Google Scholar
33. Cavallini MC, Di Bella D, Siliprandi F, Malchiodi F, Bellodi L: Exploratory factor analysis of obsessive-compulsive patients and association with 5-HTTLPR polymorphism. Am J Med Genet 2002; 114:347–353Google Scholar
34. Lesch K-P, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL: Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996; 274:1527–1531Google Scholar
35. Munafo MR, Clark T, Flint J: Does measurement instrument moderate the association between the serotonin transporter gene and anxiety-related personality traits? A meta-analysis. Mol Psychiatry 2005; 10:415–419Google Scholar
36. Sen S, Villafuerte S, Nesse R, Stoltenberg SF, Hopcian J, Gleiberman L, Weder A, Burmeister M: Serotonin transporter and gabaa alpha 6 receptor variants are associated with neuroticism. Biol Psychiatry 2004; 55:244–249Google Scholar
37. Arbelle S, Benjamin J, Golin M, Kremer I, Belmaker RH, Ebstein RP: Relation of shyness in grade school children to the genotype for the long form of the serotonin transporter promoter region polymorphism. Am J Psychiatry 2003; 160:671–676Google Scholar
38. Battaglia M, Ogliari A, Zanoni A, Citterio A, Pozzoli U, Giorda R, Maffei C, Marino C: Influence of the serotonin transporter promoter gene and shyness on children’s cerebral responses to facial expressions. Arch Gen Psychiatry 2005; 62:85–94Google Scholar
39. Retz W, Thome J, Blocher D, Baader M, Ro-sler M: Association of attention deficit hyperactivity disorder-related psychopathology and personality traits with the serotonin transporter promoter region polymorphism. Neurosci Lett 2002; 319:133–136Google Scholar
40. Retz W, Retz-Junginger P, Supprian T, Thome J, Rosler M: Association of serotonin transporter promoter gene polymorphism with violence: relation with personality disorders, impulsivity, and childhood adhd psychopathology. Behav Sci Law 2004; 22:415–425Google Scholar