Middle and Inferior Temporal Gyrus Gray Matter Volume Abnormalities in First-Episode Schizophrenia: An MRI Study
Abstract
Objective: Magnetic resonance imaging (MRI) studies of schizophrenia reveal temporal lobe structural brain abnormalities in the superior temporal gyrus and the amygdala-hippocampal complex. However, the middle and inferior temporal gyri have received little investigation, especially in first-episode schizophrenia. Method: High-spatial-resolution MRI was used to measure gray matter volume in the inferior, middle, and superior temporal gyri in 20 patients with first-episode schizophrenia, 20 patients with first-episode affective psychosis, and 23 healthy comparison subjects. Results: Gray matter volume in the middle temporal gyrus was smaller bilaterally in patients with first-episode schizophrenia than in comparison subjects and in patients with first-episode affective psychosis. Posterior gray matter volume in the inferior temporal gyrus was smaller bilaterally in both patient groups than in comparison subjects. Among the superior, middle, and inferior temporal gyri, the left posterior superior temporal gyrus gray matter in the schizophrenia group had the smallest volume, the greatest percentage difference, and the largest effect size in comparisons with healthy comparison subjects and with affective psychosis patients. Conclusions: Smaller gray matter volumes in the left and right middle temporal gyri and left posterior superior temporal gyrus were present in schizophrenia but not in affective psychosis at first hospitalization. In contrast, smaller bilateral posterior inferior temporal gyrus gray matter volume is present in both schizophrenia and affective psychosis at first hospitalization. These findings suggest that smaller gray matter volumes in the dorsal temporal lobe (superior and middle temporal gyri) may be specific to schizophrenia, whereas smaller posterior inferior temporal gyrus gray matter volumes may be related to pathology common to both schizophrenia and affective psychosis.
Abnormalities of the temporal lobe figure prominently in our understanding of the pathology of schizophrenia, especially in the superior temporal gyrus and the amygdala-hippocampal complex (1) . The middle and inferior temporal gyri, by contrast, have received far less attention. These gyri, located on the lateral surface of the temporal neocortex, are involved in cognitive functions such as language, visual perception, and memory (2 – 4) . Given this functional role, examining these gyri might contribute importantly to our understanding of the pathophysiology of schizophrenia.
The superior part of the superior temporal gyrus includes both the primary and secondary auditory sensory cortex (the Heschl gyrus and planum temporale). Recent functional magnetic resonance imaging studies have shown that the superior temporal gyrus is involved in early auditory processing common to speech and nonspeech stimuli (5) . The gray matter in the middle temporal gyrus and around the superior temporal sulcus (which separates the superior temporal gyrus from the middle temporal gyrus) is involved in processing the human voice (6) or audiovisual speech (7) , which are complex, socially relevant stimuli. The left posterior inferior temporal gyrus is probably involved in the visual word grapheme system and in semantic processing (2 , 5) . The middle and inferior temporal gyri are known to be importantly involved in both dorsal and ventral visual pathways (8) as well as in multimodal and higher sensory processing. Since functional deficits in language, memory (9) , and visual spatial perception (10) have all been reported in schizophrenia, gray matter abnormalities in the middle and inferior temporal gyri may be an important anatomical substrate.
In a postmortem study (11) , smaller bilateral inferior temporal gyrus gray matter volumes were noted in schizophrenia patients compared with control subjects, although the results fell short of statistical significance. Only a small number of in vivo morphometry studies of the middle and inferior temporal gyri in schizophrenia have been conducted, perhaps because these large regions have more complicated boundaries than other temporal lobe structures. Our laboratory has reported (12) significantly smaller volumes in the left middle temporal gyrus (13%) and bilaterally in the inferior temporal gyri (10%) in male chronic schizophrenia patients compared with healthy control subjects. In contrast, Goldstein et al. (13) , using an anatomic landmark-based semiautomated program, found no significant differences in middle or inferior temporal gyrus gray matter volume in chronic schizophrenia patients compared with control subjects.
In studies using voxel-based morphometry methods, findings of reduced volumes in the middle and inferior temporal gyri in schizophrenia have been inconsistent. One study reported reduced gray matter volume in the right middle temporal gyrus in schizophrenia patients compared with healthy control subjects (14) , but another reported reduced gray matter volume in the left middle temporal gyrus (15) . Most voxel-based morphometry studies, however, have reported no differences in the middle temporal gyrus between schizophrenia patients and healthy control subjects, and none have reported differences in the inferior temporal gyrus.
There are also many potential artifacts in MR images of the inferior temporal gyrus relative to other regions, including the susceptibility effect of air in the ear and the potential segmentation confound of the nearby tentorium cerebelli. Thus it may be more difficult to detect differences between groups in the inferior temporal gyrus when using automated methods, which may be more susceptible to artifacts than are manual measures.
The roles of the middle and inferior temporal gyri in the psychopathology and pathophysiology of schizophrenia are incompletely understood. It is not known to what extent abnormalities in these gyri are present at the beginning of the illness, nor whether such abnormalities are specific to schizophrenia. In this study, we sought to clarify these questions by analyzing temporal gyrus gray matter volumes in patients with first-episode psychosis; to determine the specificity of altered gray matter to schizophrenia, we included patients with both affective and schizophrenic psychoses. We analyzed high-resolution MR images with a manual segmentation method based on three-dimensional information to provide reliable segmentation. In an earlier study (16) , we reported that gray matter volume in the left posterior superior temporal gyrus was smaller in first-episode schizophrenia patients than in patients with affective psychosis and healthy comparison subjects. To determine whether the magnitude of the volume difference in the superior temporal gyrus gray matter differed from that of the middle or inferior temporal gyri, we compared the middle and inferior temporal gyri with the superior temporal gyrus. This study included 46 subjects from our previous study examining the superior temporal gyrus (16) and 17 new subjects—five patients with schizophrenia, four patients with affective psychosis, and eight healthy comparison subjects. Middle and inferior temporal gyrus volumes have not been reported previously for any subject in this study.
Method
Subjects
Twenty patients with first-episode schizophrenia (16 of them male) and 20 patients with first-episode affective psychosis (17 of them male), including 17 with bipolar disorder in a manic phase and three with major depressive (unipolar) disorder, were recruited from inpatient units at McLean Hospital, a private psychiatric hospital affiliated with Harvard Medical School. Twenty-three healthy comparison subjects (20 of them male) were recruited through newspaper advertisements. The statistical results reported below remained the same after exclusion of the female subjects and when the patients with unipolar depression were excluded from the affective psychosis group. Subjects were given a complete description of the study and provided written informed consent. The study was approved by the local institutional review boards.
The protocols for diagnosis and clinical evaluations have been described in detail elsewhere (16 , 17) . All subjects met the following criteria: age 18 to 55 years; IQ >75; right-handed (18) ; no history of seizures, head trauma with loss of consciousness, or neurological disorders; and no history of substance dependence within the past 5 years. For the comparison subjects, trained interviewers (D.F.S. and M.E.S.), using the Structured Clinical Interview for DSM-III-R or DSM-IV (SCID) and the SCID editions for nonpatients (SCID-NP, 19) and personality disorders (SCID-II, 20), ascertained that participants had no axis I or II disorders and that none of their first-degree relatives had axis I disorders. The same interviewers used the SCID and clinical information from the medical records to diagnose the patients in the schizophrenia and affective psychosis groups. Diagnoses were confirmed at a follow-up interview at least 6 months after the initial hospitalization whenever possible. Nineteen patients with schizophrenia, 17 patients with affective psychosis, and 23 comparison subjects were followed. Consistent with the literature (16 , 17 , 21) , first-episode psychosis was operationally defined as the first psychiatric hospitalization for psychosis.
The median duration of psychotropic medication prior to the scan was 2.9 months (range=0.3–30.0) for the schizophrenia group and 0.7 months (range=0–19.8) for the affective psychosis group, with no significant differences between groups. Patients in the schizophrenia group and the affective psychosis group were variously receiving conventional antipsychotics (five patients and eight patients in the two groups, respectively), atypical antipsychotics (11 in each group), both conventional and atypical antipsychotics (two patients in the schizophrenia group), lithium (three patients and eight patients in the two groups, respectively), sodium valproate (four patients and five patients, respectively), only drugs other than antipsychotics and mood stabilizers (one patient in the affective psychosis group), no medication (one patient in the schizophrenia group). For one patient in the schizophrenia group who was also participating in an independent double-blind medication trial, medication status was unknown.
The socioeconomic status of all participants and of their parents was assessed with the Hollingshead two-factor index (22) . The Mini-Mental State Examination (MMSE) was performed with all participants to rule out any dementia or delirium. The information subscale of the WAIS-R was used to estimate general fund of information, and digit span subscales were used to test immediate and short-term memory, attention, and concentration. Demographic data are summarized in Table 1 .
The Brief Psychiatric Rating Scale (BPRS) (23) and the Global Assessment Scale (GAS) (24) were administered to all patients to evaluate severity of illness and general level of functioning.
MRI Acquisition and Processing
MR images were obtained on a 1.5-T General Electric scanner (GE Medical Systems, Milwaukee). Two MRI protocols were used. The first was a coronal series of contiguous spoiled gradient/recall acquisition images (repetition time=35 msec, echo time=5 msec, 1 repetition, 45° nutation angle, 24-cm field of view, number of excitations=1.0, matrix=256×256 [192 phase-encoding steps] ×124). Voxel dimensions were 0.9375×0.93751.5 mm. This protocol was used for delineating and measuring the middle and inferior temporal gyri. The second protocol was an axial series of contiguous double-echo (proton density and T 2 -weighted) images (repetition time=3,000 msec, echo time=30 and 80 msec, 24-cm field of view, interleaved acquisition with 3-mm slice thickness). Voxel dimensions were 0.9375×0.93753.0 mm. This protocol was used to evaluate total intracranial contents volume.
Presaturation of a slab inferior to the head was performed for both the axial and coronal acquisitions to reduce flow-related artifacts and to obtain low arterial signal intensity. The intensity information from spoiled gradient-recall acquisition and T 2 -weighted images was used in an automated segmentation program to classify tissue into gray matter, white matter, and CSF. Images were aligned by using the line between the anterior and posterior commissures and the sagittal sulcus to correct for head tilt; images were also resampled to make voxels isotropic (sides measured 0.9375 mm).
Definitions of Middle and Inferior Temporal Gyri
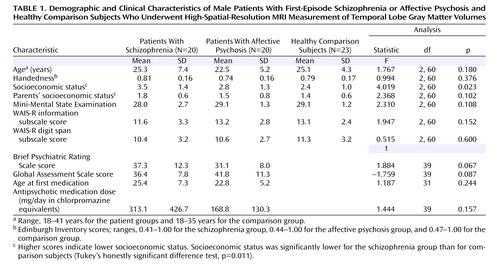
The middle and inferior temporal gyri were defined as in our previous study (12) . Figure 1 provides an overview of the region of interest subdivisions for each gyrus. Briefly, we used the following neuroanatomical landmarks: the middle temporal gyrus was divided from the superior temporal gyrus by the superior temporal sulcus; the inferior temporal sulcus was used to separate the middle and inferior temporal gyri; and the inferior temporal gyrus was divided from the occipitotemporal gyrus (fusiform gyrus) by the occipitotemporal sulcus. The anterior border of the middle and inferior temporal gyri was the most anterior slice in which the temporal stem could be seen. The posterior border was the anterior tip of the parieto-occipital sulcus.
a In the left-hand panel, the superior, middle, and inferior temporal gyri are shown on a coronal slice; the subject’s right is on the viewer’s left (radiologic convention). This slice is in the plane of the posterior superior temporal gyrus, the mid-middle temporal gyrus, and the middle inferior temporal gyrus, as illustrated in the right-hand panel. In the right-hand panel, the region of interest subdivisions in the left hemisphere are shown on a three-dimensional reconstruction.
All editing was done blind to diagnosis. Interrater reliability for each region of interest was evaluated in six randomly selected cases assessed by three independent raters (N.K., T.O., and H.E-H.) blind to diagnosis. Intraclass correlation coefficients were 0.96 for the left middle temporal gyrus, 0.95 for the right middle temporal gyrus, 0.95 for the left inferior temporal gyrus, and 0.96 for the right inferior temporal gyrus.
Comparison of Middle, Inferior, and Superior Temporal Gyri
Superior temporal gyrus gray matter volume was measured for comparison of middle, inferior, and superior temporal gyri, using the same definition as in our previous study (16) . Briefly, the anterior border of the superior temporal gyrus is the same as for the middle and inferior temporal gyri. The posterior border of the superior temporal gyrus was the last slice showing the crux of the fornix. The superior temporal gyrus was subdivided into anterior and posterior regions at the first slice that included the mammillary body (see Figure 1 ). Interrater reliability was evaluated in four randomly selected cases assessed by two raters (Y.H. and N.K.). Intraclass correlation coefficients were 0.97 for the right anterior superior temporal gyrus, 0.99 for the left posterior superior temporal gyrus, and 0.99 for the right posterior superior temporal gyrus.
The middle and inferior temporal gyri were subdivided into anterior, middle, and posterior subregions at the same landmarks as the superior temporal gyrus. The first slice that included the mammillary body was used as the boundary for subdividing the structure into anterior and middle subdivisions, and the last slice showing the fornix along the crux of the fornix was used as the boundary for subdividing the structure into middle and posterior subdivisions. Anterior subdivisions were the same for all three gyri. Because the middle and inferior temporal gyri extend more posteriorly than the superior temporal gyrus, the posterior superior temporal gyrus corresponds to the mid-middle temporal gyrus and the inferior temporal gyrus (see Figure 1 ).
Statistical Analysis
Various statistical procedures were used to assess group differences in demographic and clinical variables and in region of interest volumes. The details are available in a data supplement that accompanies the online version of this article.
Results
There were no significant group differences in age or parental socioeconomic status. Patients with first-episode schizophrenia had significantly lower socioeconomic status overall than comparison subjects, which is consistent with reduced functioning due to the disorder (see Table 1 ). There were no significant differences between the patient groups in age at first medication, duration of medication, antipsychotic medication dose, BPRS total score, or GAS score, although schizophrenia patients tended to be slightly more symptomatic and impaired. One-way analysis of variance (ANOVA) showed no significant group differences in total intracranial contents volume. There was no significant group difference in relative length of the region of interest.
Volume of Middle and Inferior Temporal Gyri
Combined middle and inferior temporal gyrus volume differed between groups (F=10.542, df=2, 60, p<0.001), with a significant group-by-subregion interaction (F=2.624, df=2, 60, p=0.038). Follow-up post hoc tests revealed that schizophrenia patients had smaller middle and inferior temporal gyrus volumes than patients with an affective psychosis (p=0.012, effect size=1.01) and comparison subjects (p<0.001, effect size=1.33). There were no significant differences between the affective psychosis group and the comparison group (p=0.321, effect size=0.44). A table listing all volumes, effect sizes, and between-group comparisons is available in the data supplement that accompanies the online version of this article.
To determine the cause of the group-by-subregion interaction, follow-up three-factor ANOVAs were performed separately for each gyrus. Significant group gray matter volume differences were observed in the middle temporal gyrus (F=7.684, df=2, 60, p=0.001) without group-by-hemisphere or group-by-subregion interactions. Total volume of the middle temporal gyrus was 7.4% smaller in the schizophrenia group (7.7% on the left, 7.1% on the right) than in the affective psychosis group (p=0.009, effect size=0.96) and 8.5% smaller (7.1% on the left, 9.7% on the right) than in the comparison group (p=0.001, effect size=1.10) ( Figure 2 ). The right middle temporal gyrus was significantly larger than the left middle temporal gyrus in all three groups (F=58.627, df=2, 60, p<0.001).
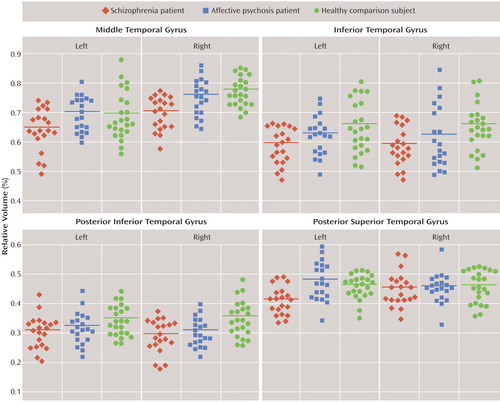
a Mean values are indicated by horizontal bars.
The inferior temporal gyrus showed significant group differences in gray matter volume (F=5.785, df=2, 60, p=0.005) with a significant group-by-subregion interaction (F=3.670, df=2, 60, p=0.007) (see Figure 2 ). Follow-up two-factor ANOVA further documented a significant group difference (F=8.128, df=2, 60, p=0.001) in the posterior inferior temporal gyrus without a significant group-by-hemisphere interaction. There were neither significant group differences nor group-by-hemisphere interactions in the other subregions. The posterior inferior temporal gyrus volume was 14.2% smaller (11.1% on the left, 17.3% on the right) in the schizophrenia group (p=0.001, effect size=1.12) and 10.3% smaller (7.3% on the left, 13.3% on the right) in the affective psychosis group (p=0.019, effect size=0.94) than in the comparison group. There were no significant differences between the schizophrenia group and the affective psychosis group (p=0.556, effect size=0.32). (For both the middle and inferior temporal gyri, see the table containing volume data and statistical analyses in the data supplement that accompanies the online version of this article.)
Comparison of Middle, Inferior, and Superior Temporal Gyri
Comparison between the superior, middle, and inferior temporal gyri revealed significant group differences in combined volumes (F=5.677, df=2, 60, p=0.006), with a significant group-by-hemisphere interaction (F=5.293, df=2, 60, p=0.008), a group-by-subregion interaction (F=3.332, df=2, 60, p=0.042), and a group-by-gyrus-by-hemisphere interaction (F=3.617, df=2, 60, p=0.008). To decompose the complex interactions, analyses between gyri were conducted separately for each hemisphere. Follow-up three-factor ANOVA revealed a significant group difference (F=10.951, df=2, 60, p<0.001) with a significant group-by-subregion interaction (F=4.277, df=2, 60, p=0.018) in the left hemisphere. There was no significant group difference or significant group-by-gyrus, group-by-subregion, or group-by-gyrus-by-subregion interaction in the right hemisphere.
Follow-up one-way ANOVA for each subregion in the left hemisphere revealed a significant group difference (F=8.731, df=2, 60, p<0.001) in the left posterior superior temporal gyrus (see Figure 2 ). There were no significant group differences in the other subregions. The left posterior superior temporal gyrus was 13.8% smaller in the schizophrenia group than in the affective psychosis group (p<0.001, effect size=1.16) and 10.5% smaller than in the comparison group (p=0.010, effect size=1.07).
Correlation With Clinical Measures
In the schizophrenia group only, age at first medication was negatively correlated with the gray matter volumes of the left middle temporal gyrus (r s =–0.527, p=0.036), left inferior temporal gyrus (r s =–0.568, p=0.022), and right middle temporal gyrus (r s =–0.534, p=0.033) ( Figure 3 ). Age was negatively correlated with left inferior temporal gyrus gray matter volume (r s =–0.499, p=0.025) in the schizophrenia group only. Region of interest volumes were not significantly correlated with duration of antipsychotic medication or medication dose in either of the patient groups. Region of interest volumes were also not significantly correlated with age in the comparison group.
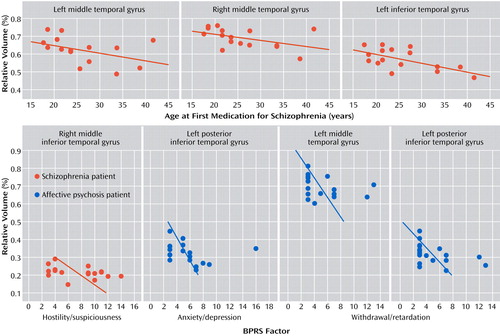
a Spearman’s rho was used for testing statistical significance. No significant correlations with age at first medication were observed for patients with affective psychosis.
In terms of correlations with BPRS clinical factors (see Figure 3 ), in the schizophrenia group only, the right middle inferior temporal gyrus gray matter volume was negatively correlated with the BPRS factor score for hostility/suspiciousness (r s =–0.483, p=0.036). In the affective psychosis group only, the factor score for withdrawal/retardation was negatively correlated with left total middle temporal gyrus gray matter volume (r s =–0.472, p=0.041) and left posterior inferior temporal gyrus gray matter volume (r s =–0.558, p=0.013), and the factor score for anxiety/depression was negatively correlated with left posterior inferior temporal gyrus gray matter volume (r s =–0.469, p=0.043).
Discussion
We measured middle and inferior temporal gyrus gray matter volumes from high-spatial-resolution MRI scans in patients with first-episode schizophrenia, patients with first-episode affective psychosis, and healthy comparison subjects. We found smaller middle temporal gyrus gray matter volumes bilaterally in the schizophrenia group but not in the affective psychosis group. In contrast, the posterior inferior temporal gyrus gray matter volume was smaller bilaterally in both patient groups than in the comparison group. Compared with these regions, the left posterior superior temporal gyrus gray matter volume in the schizophrenia group had the largest percentage difference and the largest effect sizes in comparisons with the affective psychosis group and the comparison group. The volume differences reported were not due to differences in anterior-posterior boundaries, since the number of slices did not differ among groups.
Only a small number of studies using voxel-based morphometry (14 , 15) have reported structural abnormalities of the middle or inferior temporal gyri in schizophrenia. In our earlier voxel-based morphometry study (25) , the only region that showed significantly less gray matter density was located within the left posterior superior temporal gyrus in patients with first-episode schizophrenia, and there were no significant differences in the middle and inferior temporal gyri. Manual segmentation methods thus offer advantages for detecting differences in the lateral and ventral surfaces of the temporal lobe in structural MRI studies.
The schizophrenia-specific smaller gray matter volumes in the dorsal temporal lobe neocortex (superior and middle temporal gyri) may be related to auditory and related abnormalities in higher cognitive function in schizophrenia, since this region is involved in auditory and related multimodal sensory processing as well as auditory short-term memory and language processing. In contrast, smaller inferior temporal gyrus gray matter volumes were observed bilaterally in both first-episode schizophrenia and first-episode affective psychosis. The latter disorder is associated with few reports of smaller neocortical regions of interest, although a noteworthy exception is the anterior pole of the temporal lobe ( 26 ; this reference includes a literature review), a region involved in emotion processing. A similar relationship may exist in the inferior temporal gyrus and fusiform gyrus, since these regions have reciprocal connections with the amygdala and orbitofrontal cortex, which are linked to affect processing and expression (27) . The correlations we observed between smaller inferior temporal gyrus gray matter volumes and several factors of the BPRS in the schizophrenia and affective psychosis groups are consistent with this hypothesis. In a postmortem study, Webster et al. (28) reported decreased glucocorticoid receptor mRNA levels in layer IV in the inferior temporal gyrus, possibly reflecting decreased neuronal density, in patients with schizophrenia, bipolar affective disorder, and depression without psychotic features compared with healthy comparison subjects; these findings are also suggestive of posterior inferior temporal gyrus gray matter abnormalities common to schizophrenia and affective psychosis.
In this study, age at first medication was negatively correlated with region of interest volumes in the left and right middle temporal gyri and the left inferior temporal gyrus for the schizophrenia group only: the later the age at first medication, the smaller the volume. In our laboratory, Onitsuka et al. (12) reported a left-lateralized pattern of smaller middle temporal gyrus gray matter volume in patients with chronic schizophrenia. This raises the possibility that reduced gray matter volumes in untreated schizophrenia are progressive but might be ameliorated by medication in the middle and inferior temporal gyri, as we have reported for the left posterior superior temporal gyrus (29) and other laboratories have reported for other regions (30 , 31) . Given reductions observed in gray and white matter volumes in macaques after 1.5–2.3 years of antipsychotic treatment (32) , we emphasize the value of first-episode samples to avoid the potential confounding effect of medication. The duration of medication prior to scanning was brief in our patient groups, and we observed no correlation between volumes and medication duration or dose; moreover, the affective psychosis group exhibited a different pattern of smaller regions of interest than the schizophrenia group.
This study adds to the growing body of evidence that patients with first-episode illness show smaller gray matter volumes, with differential effects between patients with schizophrenia and those with affective psychosis. Smaller gray matter volumes of the left and right middle temporal gyri, the left and right posterior inferior temporal gyri, and the left posterior superior temporal gyrus were observed in patients with schizophrenia at the onset of their illness. Smaller gray matter volumes in the left and right posterior inferior temporal gyri were also evident at illness onset in patients with affective psychosis. Smaller superior and middle temporal gyrus gray matter volumes in the dorsal temporal neocortex may be specific to patients with schizophrenia. Smaller inferior temporal gyrus gray matter volume in the ventral temporal cortex may be related to pathology common to schizophrenia and affective psychoses. More research is needed to determine whether there are progressive middle and inferior temporal gyrus gray matter reductions through the course of illness, as has been observed for superior temporal gyrus gray matter (29) . Additional studies will also be needed to confirm the relationship between smaller inferior temporal gyrus volumes and clinical symptoms that we found in an exploratory analysis in this study. As we have previously noted, correlations between clinical symptoms and MRI volumes are often stronger later in the course of illness (29) , probably as a result of symptom instability at first hospitalization. The finding of bilaterally smaller inferior temporal gyrus volumes in both schizophrenia and affective psychosis, which has not been previously reported, suggests that abnormalities in this region are important in both types of psychosis. The dorsal to ventral gradient of left-lateralization of abnormalities in schizophrenia is also noteworthy and may correspond to a more lateralized anatomical substrate for language in the dorsal temporal lobe neocortex (12) .
1. Shenton ME, Dickey CC, Frumin M, McCarley RW: A review of MRI findings in schizophrenia. Schizophr Res 2001; 49:1–52Google Scholar
2. Price CJ: The anatomy of language: contributions from functional neuroimaging. J Anat 2000; 197(Pt 3):335–359Google Scholar
3. Noppeney U, Price CJ: Retrieval of visual, auditory, and abstract semantics. Neuroimage 2002; 15:917–926Google Scholar
4. Ojemann GA, Schoenfield-McNeill J, Corina DP: Anatomic subdivisions in human temporal cortical neuronal activity related to recent verbal memory. Nat Neurosci 2002; 5:64–71Google Scholar
5. Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, Possing ET: Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex 2000; 10:512–528Google Scholar
6. Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B: Voice-selective areas in human auditory cortex. Nature 2000; 403:309–312Google Scholar
7. Wright TM, Pelphrey KA, Allison T, McKeown MJ, McCarthy G: Polysensory interactions along lateral temporal regions evoked by audiovisual speech. Cereb Cortex 2003; 13:1034–1043Google Scholar
8. Sewards TV, Sewards MA: On the neural correlates of object recognition awareness: relationship to computational activities and activities mediating perceptual awareness. Conscious Cogn 2002; 11:51–77Google Scholar
9. Nestor PG, Akdag SJ, O’Donnell BF, Niznikiewicz M, Law S, Shenton ME, McCarley RW: Word recall in schizophrenia: a connectionist model. Am J Psychiatry 1998; 155:1685–1690Google Scholar
10. Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC: Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry 2002; 59:1011–1020Google Scholar
11. Highley JR, McDonald B, Walker MA, Esiri MM, Crow TJ: Schizophrenia and temporal lobe asymmetry: A post-mortem stereological study of tissue volume. Br J Psychiatry 1999; 175:127–134Google Scholar
12. Onitsuka T, Shenton ME, Salisbury DF, Dickey CC, Kasai K, Toner SK, Frumin M, Kikinis R, Jolesz FA, McCarley RW: Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am J Psychiatry 2004; 161:1603–1611Google Scholar
13. Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, Tourville J, Caviness VS Jr, Faraone SV, Tsuang MT: Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry 1999; 56:537–547Google Scholar
14. Wilke M, Kaufmann C, Grabner A, Putz B, Wetter TC, Auer DP: Gray matter changes and correlates of disease severity in schizophrenia: a statistical parametric mapping study. Neuroimage 2001; 13:814–824Google Scholar
15. Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM: Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage 2002; 17:880–889Google Scholar
16. Hirayasu Y, Shenton ME, Salisbury DF, Dickey CC, Fischer IA, Mazzoni P, Kisler T, Arakaki H, Kwon JS, Anderson JE, Yurgelun-Todd D, Tohen M, McCarley RW: Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry 1998; 155:1384–1391Google Scholar
17. Salisbury DF, Shenton ME, Sherwood AR, Fischer IA, Yurgelun-Todd DA, Tohen M, McCarley RW: First-episode schizophrenic psychosis differs from first-episode affective psychosis and controls in P300 amplitude over left temporal lobe. Arch Gen Psychiatry 1998; 55:173–180Google Scholar
18. Oldfield RC: The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971; 9:97–113Google Scholar
19. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Non-Patient Edition (SCID-NP, Version 1.0). Washington, DC, American Psychiatric Press, 1990Google Scholar
20. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Washington, DC, American Psychiatric Press, 1990Google Scholar
21. Lieberman JA, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D, Bilder R: Longitudinal study of brain morphology in first-episode schizophrenia. Biol Psychiatry 2001; 49:487–499Google Scholar
22. Hollingshead AB: Two Factor Index of Social Position. New Haven, Conn, Yale University Press, 1965Google Scholar
23. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Google Scholar
24. Endicott J, Spitzer RL, Fleiss JL, Cohen J: The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976; 33:766–771Google Scholar
25. Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA, McCarley RW: Voxel-based morphometric analysis of gray matter in first-episode schizophrenia. Neuroimage 2002; 17:1711–1719Google Scholar
26. Kasai K, Shenton ME, Salisbury DF, Onitsuka T, Toner SK, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW: Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. Arch Gen Psychiatry 2003; 60:1069–1077Google Scholar
27. Rolls ET, Stringer SM: A model of the interaction between mood and memory. Network 2001; 12:89–109Google Scholar
28. Webster MJ, Knable MB, O’Grady J, Orthmann J, Weickert CS: Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol Psychiatry 2002; 7:985–994Google Scholar
29. Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Lee CU, Ciszewski AA, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW: Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry 2003; 160:156–164Google Scholar
30. Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M: Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry 2003; 60:585–594Google Scholar
31. Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M: Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry 2005; 62:361–370Google Scholar
32. Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA: The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology 2005; 30:1649–1661Google Scholar