Large CSF Volume Not Attributable to Ventricular Volume in Schizotypal Personality Disorder
Abstract
OBJECTIVE: The purpose of this study was to determine whether schizotypal personality disorder, which has the same genetic diathesis as schizophrenia, manifests abnormalities in whole-brain and CSF volumes. METHOD: Sixteen right-handed and neuroleptic-naive men with schizotypal personality disorder were recruited from the community and were age-matched to 14 healthy comparison subjects. Magnetic resonance images were obtained from the subjects and automatically parcellated into CSF, gray matter, and white matter. Subsequent manual editing separated cortical from noncortical gray matter. Lateral ventricles and temporal horns were also delineated. RESULTS: The men with schizotypal personality disorder had larger CSF volumes than the comparison subjects; the difference was not attributable to larger lateral ventricles. The cortical gray matter was somewhat smaller in the men with schizotypal personality disorder, but the difference was not statistically significant. CONCLUSIONS: Consistent with many studies of schizophrenia, this examination of schizotypal personality disorder indicated abnormalities in brain CSF volumes.
One approach to obtaining insight into schizophrenia is to examine nonschizophrenic disorders that are in the schizophrenia spectrum. For example, data indicate that schizotypal personality disorder has the same genetic diathesis as schizophrenia; the risks of schizophrenia in a sibling of a proband with schizotypal personality disorder and a sibling of a proband with schizophrenia are the same (1–4). Schizotypal personality disorder is thus an ideal spectrum disorder to study because subjects with this disorder often have not been treated with neuroleptics. In addition, persons with schizotypal personality disorder have not experienced the possible complicating effects of a chronic illness, including hospitalization, nor the stress of a long-term illness, which may result in glucocorticoid-induced cell morphological changes (5, 6). Environmental richness and nutrition, which may be different in the chronically ill, have also been demonstrated to affect cellular morphology (5, 6). There is also the further possibility that, by examining similarities and differences of neuroanatomical abnormalities in schizotypal personality disorder and schizophrenia, one can not only observe endophenotypic similarities but also perhaps determine what additional features in schizophrenia relate to an overtly psychotic course and/or what features in schizotypal personality disorder may protect against overt psychosis.
Our initial examination of individual temporal lobe regions of interest in schizotypal personality disorder (7) demonstrated smaller volume of the left superior temporal gyrus gray matter than in comparison subjects and an abnormal parahippocampal asymmetry. However, we did not find the markedly abnormal volumes for the medial temporal lobe that are often noted for schizophrenia. These findings are compatible with the hypothesis that abnormalities of the left superior temporal gyrus are a consistent anatomic component of the schizophrenia spectrum disorders, as found in several studies of schizophrenia (8–14), which we have reviewed elsewhere (15–17). A study (18) of subjects with features of schizotypy (but not clinical schizotypal personality disorder) indicated a prefrontal volume deficit (involving a region and subject pool not evaluated by our group), suggesting that there may be an abnormality of the frontotemporal connection and/or that abnormalities in schizotypal personality disorder may be more widespread. These reports, however, suggest the possibilities that the abnormalities in schizotypal personality disorder may be more widespread than the temporal lobe and that any abnormalities in the frontal and temporal regions might also have an abnormality of the frontotemporal connection. As discussed in our reviews (15–17), one of the recurring issues in magnetic resonance imaging (MRI) studies of schizophrenia is the degree to which abnormalities are circumscribed or widespread throughout the brain. Thus, we decided to evaluate whether schizotypal personality disorder involves global anomalies in CSF, gray matter, cortical gray matter, and/or white matter. We here report larger than normal CSF volumes not attributable to large lateral ventricles.
METHOD
Subjects
Subjects with schizotypal personality disorder were recruited from the community through newspaper advertisements. We received 473 responses, 170 of which were excluded because the respondents could not be reached, were left-handed, or were female. The remaining 303 subjects underwent telephone screening to determine whether or not they met the following inclusion criteria: 1) age between 18 and 55 years old; 2) English as the primary language; 3) no history of neurological disorder (including head trauma with loss of consciousness greater than 2 minutes), no history of ECT, no drug or alcohol dependence ever or abuse in the last year; and 4) no current use of psychotropic medications (including steroids). A total of 84 met these criteria and also positively answered at least three questions from the schizotypal personality disorder section of the Structured Clinical Interview for DSM-IV (SCID) (19). A psychiatrist (C.C.D.) or psychologist (M.M.V.) conducted a videotaped interview with these 84 subjects, using the SCID patient version (SCID-P) (20) and personality disorders version (SCID-II) (21). To establish interrater reliability for the diagnosis of schizotypal personality disorder, one-half of the videotapes were reviewed by a second psychologist (L.J.S.). The interrater reliability for the diagnosis of schizotypal personality disorder was high (kappa=0.89, N=25). Of the interviewed subjects, 30 met at least five of the nine DSM-IV criteria for a diagnosis of schizotypal personality disorder, but 14 did not complete the study because they were lost to follow-up (N=9), were unable to be scanned because of claustrophobia (N=3), or exceeded the weight limit of the MRI scanner (N=2). Therefore, the study group comprised 16 men with schizotypal personality disorder.
There were no differences in demographic characteristics (age, education, estimated IQ, parental or personal socioeconomic status) or in number of DSM-IV diagnostic criteria met between the subjects who did and did not complete the study. On the basis of the SCID-II interview, the following diagnoses or past diagnoses were also established: past major depression, N=2; dysthymia, N=1; panic disorder, N=1; past alcohol abuse, N=1; past polysubstance abuse, N=1; paranoid personality disorder, N=6; borderline personality disorder, N=5; passive-aggressive personality disorder, N=2; schizoid personality disorder, N=3; and narcissistic personality disorder, N=1. Four of the 16 subjects with schizotypal personality disorder had received marital, pastoral, or family counseling ranging from 2 weeks to less than 1 year, but none had received medication or individual treatment from a psychiatrist. The Scale for the Assessment of Positive Symptoms (SAPS) (22) and Scale for the Assessment of Negative Symptoms (SANS) (23) were also completed for these subjects. The mean SAPS score was 4.75 (SD=1.29), and the mean SANS score was 6.81 (SD=3.33); these measures did not correlate with MRI variables, perhaps because they were designed for more seriously ill subjects.
The comparison subjects were 14 healthy men recruited from the community. They met the additional requirements of no personal or family history of psychotic or bipolar illness and no personal history of personality disorder. They denied history of any developmental disorder. They were matched for age one-to-one with the subjects with schizotypal personality, although two comparison subjects were each matched to two schizotypal subjects. As shown in table 1, there was no statistically significant difference in parental socioeconomic status, but the comparison subjects did show a higher personal socioeconomic status. After a complete description of the study to the subjects, written informed consent was obtained.
MRI Procedures
The images were acquired on a 1.5-T MRI system (GE Medical Systems, Milwaukee). For the sagittal, localizer series the following acquisition variables were used: TR=600 msec, TE=19 msec, and 4.0-mm-thick slices with 1.0-mm skip. The axial double-echo spin-echo series, used to maximize information on CSF anatomy, had the following characteristics: 52 3.0-mm-thick interleaved slices, TR=3000 msec, and TE=30 and 80 msec. The coronal series, 124 contiguous slices with no skip, obtained by using a three-dimensional Fourier transform spoiled gradient/recalled echo (SPGR) acquisition, had the following characteristics: TR=35 msec, TE=5 msec, nutation angle=45º, and voxel size=0.975 × 0.975 × 1.5 mm) (figure 1A). For all images, the field of view was 24 cm and the matrix size was 256 × 256 (192 phase-encoding steps with zero filling).
Several steps were taken to process the images on workstations (SPARC2, Sun Microsystems, Mountain View, Calif.). First, a preprocessing filter was used to reduce noise while maintaining clear tissue class distinction (24). Next, T2 information from the double-echo spin-echo axial slices was registered with data from the SPGR images by reformatting the axial voxels to the voxel dimensions corresponding to those in the coronal SPGR images (25). Third, the intensity information from both the SPGR and T2 images was used in a fully automated segmentation program to classify tissue into gray matter, white matter, and CSF (as described in detail in reference 25). Wells’s iterative expectation-maximization algorithm initially estimates image intensity inhomogeneities and then uses these estimates to classify tissue on the basis of the same set of signal intensity characteristics for all subjects (25). Figure 1B illustrates the segmentation results. Next, we then summed the voxel volumes of gray and white matter, CSF, blood vessels, and unclassified voxels; the last type averaged less than 1 cm3 in all cases. Last, masks were automatically created to demarcate the outer extent of the intracranial contents, with the skull, scalp, and neck tissue removed. Minimal manual editing of the masks was required.
To separate cortical from noncortical gray matter, manual editing of each slice was done to exclude the cerebellum, brain stem, and gray matter of the basal ganglia and thalamus. The cortex of the medial temporal lobe regions (i.e., hippocampus and parahippocampal gyrus) was also included, as well as the amygdala. The latter was included since its reliable separation from the hippocampus was difficult. The interrater reliability for the separation of cortical from other tissue was high (intraclass correlation coefficient=0.995, N=3).
The lateral ventricles were identified and classified as such. On the slices in which the automated segmentation did not separate the left and right ventricles, left and right were manually defined by using the septum pellucidum. Because the temporal horns were small, manual editing of these structures was required.
Statistical Methods
A test of normality, the Shapiro-Wilk test, demonstrated that the data for CSF volume did not follow a normal distribution (Shapiro-Wilk statistic=0.92, df=30, p=0.03). When we separated the two groups, we found that it was the schizotypal group that was driving the statistic (Shapiro-Wilk statistic=0.86, df=16, p=0.02), not the comparison subjects (Shapiro-Wilk statistic=0.92, df=14, p=0.26). Rather than being due to a simple outlier or two, the sample probability density (e.g., histogram of values) of CSF volumes for schizotypal personality disorder had a nonnormal form that resembled a gamma distribution, type III in the Pearson nomenclature, and had a positive skew, i.e., with a “tail” to the right, toward higher values (see review of sampling probability densities in reference 26). Because of this nonnormality, all of the data were subsequently transformed by using the natural log, which produced distributions that were normally distributed. To correct for differences in brain size, the volume of each of the regions of interest was regressed on the volume of the intracranial contents. With this method each subject’s absolute value for each region of interest was regressed against each subject’s value for intracranial contents, and the residuals were used in subsequent analyses. The regression analysis controlled for the possibility of nonuniformity in the relationship between intracranial contents and the regions of interest within a particular subject group. However, while almost all laboratories correct for intracranial contents, there is some disagreement in the literature concerning which statistical procedure is most appropriate, although arguments favoring the regression method have been advanced (11).
A repeated measures analysis of variance (ANOVA) compared the two subject diagnostic groups on the within-subject measure of tissue class (gray, white, and CSF). The conservative Greenhouse-Geisser correction is reported, where appropriate. Planned contrasts consisting of Student’s t tests were subsequently performed. To determine whether a more refined segmentation of cortical gray matter, as opposed to total gray matter, altered results, the ANOVA and planned contrasts were repeated for cortical gray matter. We also performed t tests of ventricular volumes.
RESULTS
A repeated measures ANOVA comparing the subjects with schizotypal personality disorder and the comparison subjects on the three tissue classes (gray and white matter and CSF) revealed a statistically significant main effect of diagnosis (F=4.69, df=1, 28, p=0.04). There was no statistically significant main effect of tissue class (F=0.74, df=1.34, 56, p=0.43). There was, however, an interaction between diagnosis and tissue class (F=3.97, df=1.34, 56, p=0.04). When cortical gray matter, instead of total gray matter, was entered into the analysis, the results were not meaningfully changed: there was still a main effect of diagnosis (F=4.28, df=1.00, 28, p=0.05) and an interaction between diagnosis and tissue class (F=4.15, df=1.30, 56, p=0.04) but no main effect of tissue class (F=0.65, df=1.30, 56, p=0.47).
The planned contrasts demonstrated that the CSF volumes were larger in the subjects with schizotypal personality disorder than in the comparison subjects (table 2 and figure 2). To determine whether the difference in CSF volume was driven by differences in the lateral ventricles, t tests were performed. There was no difference in the left or right lateral ventricle or in the left or right temporal horn. The range of the volumes of the left ventricle for the comparison subjects was 17.9 ml, whereas it was 13.7 ml for the subjects with schizotypal personality disorder. Two of the comparison subjects had large ventricular volumes. When these two subjects were removed from the analysis, the difference in ventricular volumes between the schizotypal and comparison subjects still lacked statistical significance. Subarachnoid CSF volume accounted for the difference in total CSF, as whole-brain CSF minus ventricular CSF remained significantly larger in the schizotypal group (t=2.69, df=28, p=0.01).
There was no difference between the two groups in overall intracranial contents (table 2). In addition, there was no difference between the comparison and schizotypal subjects in gray matter or in white matter. However, the subjects with schizotypal personality disorder had a smaller cortical gray matter volume, although the difference was not quite statistically significant (effect size=0.78, percentage difference=7.1%) (table 2 and figure 3). On the basis of this effect size, a power analysis suggests that fewer than 30 subjects per group would be needed to demonstrate a statistically significant difference in cortical gray matter volume.
DISCUSSION
In this study of whole-brain measures in subjects with schizotypal personality disorder, ANOVAs demonstrated a main effect of diagnosis and an interaction between diagnosis and the brain’s three main tissue classes. Follow-up planned comparisons showed larger CSF volume in the schizotypal subjects than in normal comparison subjects. As lateral ventricle size did not differ, this difference in CSF volume derived from other sources. In addition, there was also a nonsignificantly smaller (p=0.07) cortical gray matter volume with a moderate to large effect size (0.78), suggesting that an increase in the subject number would result in statistically significant differences between groups in total cortical gray matter.
These data suggest that the abnormalities in schizotypal personality disorder are not confined to the temporal lobe (7) but may be more widespread. As subarachnoid CSF accounts for the major portion of the nonventricular CSF (about 87% of whole-brain CSF) (9), we think it likely that this component is larger than normal, a finding consistent with results of a previous computerized tomography (CT) study (27). The most likely reason for a large CSF volume is a small volume of underlying gray or white matter. Our findings of no difference in white matter but smaller than normal cortical gray matter (although not statistically significant) suggest it may be primarily this latter component. Nonetheless, additional studies of gray matter volume in different cortical regions will be required to determine whether the larger CSF volume is accompanied by diffusely small gray matter volume or is better characterized by disproportionate abnormalities among regions, as has been true in the great majority of studies of schizophrenia (86%) (15–17).
In an important MRI study (28) examining the possible role of genetics in overall brain morphology, schizophrenic subjects and their siblings—10% of whom had schizotypal personality disorder—were shown to have smaller cortical gray matter volumes and larger sulcal CSF volumes. However, only the schizophrenic subjects had large lateral ventricles and concurrently small white matter volumes. The authors hypothesized that genetics contributed to the small cortical gray matter volume and that psychotic illness effects or “nonshared causative effects” contributed to the large ventricular volumes (28). Their finding is similar to ours: the subjects with schizotypal personality disorder showed a tendency for smaller cortical gray matter, no abnormality in ventricular volume, but a significantly larger than normal subarachnoid CSF volume.
Also, consistent with the preceding hypothesis, Buchsbaum and co-workers (29) showed no abnormality in lateral ventricle size in subjects with schizotypal personality disorder. However, they did find large temporal horns in those subjects. Possible reasons for the difference in temporal horn findings are their 1) use of a clinic population, 2) inclusion of both men and women, 3) inclusion of both right- and left-handed subjects, 4) use of axial images rather than coronal images, and 5) different tracing procedures. Indeed, only one study (30) showed high ventricle-brain ratios (VBRs) in subjects with schizotypal personality disorder, although these subjects may have VBRs intermediate between those of schizophrenic and comparison subjects (31) and larger VBRs than those of nonaffected siblings (32).
Overall, although there are differences in the technology used, populations sampled, and brain regions measured, there appears to be an emerging consensus from the literature. In terms of global measures, schizotypal personality disorder appears to be associated with large CSF volume and, possibly, small cortical gray matter volume. The exact area of the large CSF volume may differ among studies, and more work is needed to understand these differences.
The nonnormal distribution of CSF volume demonstrated in this study may not be unusual as a skew toward the right (toward higher values) has also been reported in chronic schizophrenia (33). Thus, in the schizophrenia spectrum disorders, the skew toward higher values may reflect a severity factor affecting CSF volume. Although the reported CSF volume for this comparison group was not statistically different from a normal distribution, there are literature reports of a “tail” of higher CSF values in the general population (34), where mean CSF volume is usually between 130 and 140 ml (35, 36). Our mean value of 137 ml and our finding that two comparison subjects had CSF volumes 100 ml above the mean are consistent with these reports.
Moreover, the size of the lateral ventricles may also vary greatly in the general population. In a postmortem study of 183 grossly normal brains (37), the mean lateral ventricle size was 7 ml and the range was 2 to 39 ml, values that are consistent with our current findings of large lateral ventricles in two comparison subjects. No history of head trauma or developmental abnormalities was noted for either subject. (Removing these two normal subjects from the analysis had no effect on the absence of a statistically significant difference in lateral ventricle size between the schizotypal and comparison subjects.) The present data thus appear compatible with literature reports of variability of CSF and ventricle size in the general population and with a tendency for distributions to be skewed toward higher values.
Although there was no difference in total gray matter volume between the two groups, when total gray matter was more carefully delineated into cortical gray matter only, the subjects with schizotypal personality disorder showed nonsignificantly smaller volumes. In studies of schizophrenia, measurements of gray matter have yielded conflicting results: some studies have demonstrated a smaller than normal total gray matter volume (38–40) or cortical gray matter volume (39, 41, 42) and others have not (9, 10, 13, 43). From our review of such studies (15–17), however, the evidence suggests a nonuniform distribution of gray matter volume deficits in schizophrenia, i.e., that the deficits are greater in some areas than in others. One CT study (30) of subjects with personality disorders in the schizophrenia spectrum (the majority of whom had schizotypal personality disorder) used a visual scale to show “cortical atrophy” (greater sulcal prominence), which likely is compatible with our finding of larger than normal subarachnoid CSF volume.
The ability to generalize these findings to all persons with schizotypal personality disorder is limited by the relatively small number of subjects and the use of only male subjects. Future plans include the study of female subjects. Drawing subjects from the community may yield subjects less severely affected than in clinic groups but has the important advantage of finding subjects not yet exposed to neuroleptics or other psychotropic medications. In addition, these data do not address the issue of whether the abnormalities are neurodevelopmental and/or degenerative in nature, as this was not a longitudinal study designed to study changes over time.
The finding of larger than normal overall CSF volume and suggestive evidence for smaller cortical gray matter volume, taken together with previous findings of focal deficits in the temporal lobes of the same subjects, suggest that neuroanatomic abnormalities in schizotypal personality disorder may be present in widespread locations but may be greater in some regions than in others, not unlike what is seen in schizophrenia (15–17).
Received Jan. 27, 1999; revision received July 6, 1999; accepted July 9, 1999. From the Department of Psychiatry, Harvard Medical School at Brockton/West Roxbury VA Medical Center and Massachusetts Mental Health Center; and the Department of Radiology, Harvard Medical School at Brigham and Women’s Hospital, Boston. Address reprint requests to Dr. McCarley or Dr. Shenton, Psychiatry 116A, Brockton VA Medical Center, 940 Belmont St., Brockton, MA 02401; [email protected] or [email protected] (e-mail). Supported by NIMH grant MH-52807 to Dr. McCarley, a grant from the VA Center for Clinical and Basic Neuroscience Studies of Schizophrenia to Dr. McCarley, a VA Psychiatry Research/Neuroscience Fellowship to Dr. Dickey, and NIMH grants MH-01110 and MH-50747 to Dr. Shenton.
 |
 |
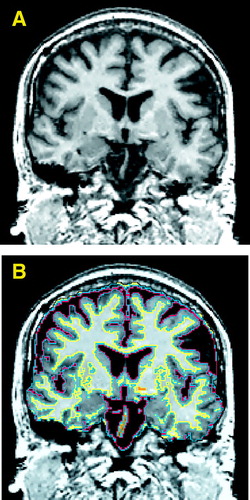
FIGURE 1. Coronal Gray-Scale Image of the Brain of a Man With Schizotypal Personality Disorder (A) and the Same Image With Automatically Segmented Tissue Types Overlaid (B)a
aFollowing radiological convention, the right side of the image is of the left side of the brain. In part B, CSF is outlined in red, gray matter in blue, white matter in yellow, and undefined tissue in orange.
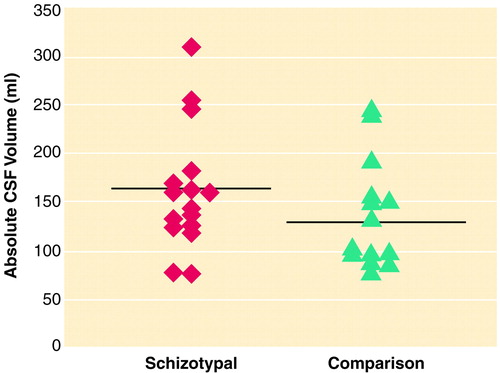
FIGURE 2. Individual Absolute CSF Brain Volumes for 16 Men With Schizotypal Personality Disorder and 14 Healthy Comparison Subjectsa
aHorizontal lines indicate mean values.
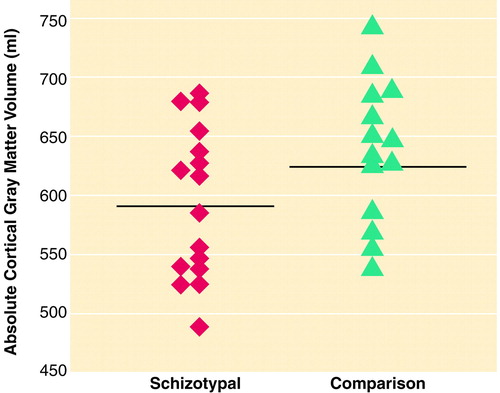
FIGURE 3. Individual Absolute Cortical Gray Matter Volumes for 16 Men With Schizotypal Personality Disorder and 14 Healthy Comparison Subjectsa
aHorizontal lines indicate mean values.
1. Kety SS, Rosenthal D, Wender PH, Schulsinger F: The types and prevalence of mental illness in the biological and adoptive families of adopted schizophrenics, in Second Research Conference of the Foundation’s Fund for Research in Psychiatry. Edited by Rosenthal D, Kety SS. Elmsford, NY, Pergamon Press, 1967, pp 345–362Google Scholar
2. Siever LJ, Silverman JM, Horvath TB, Klar H, Coccaro E, Keefe RS, Pinkham L, Rinaldi P, Mohs RC, Davis KL: Increased morbid risk for schizophrenia-related disorders in relatives of schizotypal personality disordered patients. Arch Gen Psychiatry 1990; 47:634–640Crossref, Medline, Google Scholar
3. Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D: The Roscommon family study, I: methods, diagnosis of probands, and risk of schizophrenia in relatives. Arch Gen Psychiatry 1993; 50:527–540Crossref, Medline, Google Scholar
4. Kendler KS, Walsh D: Schizotypal personality disorder in parents and the risk for schizophrenia in siblings. Schizophr Bull 1995; 21:47–52Crossref, Medline, Google Scholar
5. McEwen B, Gould E, Sakai R: The vulnerability of the hippocampus to protective and destructive effects of glucocorticoids in relation to stress. Br J Psychiatry 1992; 150(suppl 15):18–24Google Scholar
6. McEwen BS, Magarinos AM: Stress effects on morphology and function of the hippocampus. Ann NY Acad Sci 1997; 821:271–284Crossref, Medline, Google Scholar
7. Dickey C, McCarley R, Voglmaier M, Niznikiewicz M, Seidman L, Hirayasu Y, Fischer I, Teh E, Van Rhoads R, Jakab M, Kikinis R, Jolesz F, Shenton M: Schizotypal personality disorder and MRI abnormalities of the temporal lobe. Biol Psychiatry 1999; 45:1393–1402Google Scholar
8. Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE: Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry 1990; 147:1457–1462Google Scholar
9. Shenton ME, Kikinis R, Jolesz FA, Pollack SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604–612Crossref, Medline, Google Scholar
10. Schlaepfer TE, Harris GJ, Tien AY, Peng LW, Lee S, Federman EB, Chase GA, Barta PE, Pearlson GD: Decreased regional cortical gray matter volume in schizophrenia. Am J Psychiatry 1994; 151:842–848Link, Google Scholar
11. Zipursky R, Marsh L, Lim K, DeMent S, Shear P, Sullivan E, Murphy G, Csernansky J, Pfefferbaum A: Volumetric MRI assessment of temporal lobe structures in schizophrenia. Biol Psychiatry 1994; 35:501–516Crossref, Medline, Google Scholar
12. Menon RR, Barta PE, Aylward EH, Richards SS, Vaughn DD, Tien AY, Harris GJ, Pearlson GD: Posterior superior temporal gyrus in schizophrenia: grey matter changes and clinical correlates. Schizophr Res 1995; 16:127–135Crossref, Medline, Google Scholar
13. Hajek M, Huonker R, Boehle C, Volz H-P, Nowak H, Sauer H: Abnormalities of auditory evoked magnetic fields and structural changes in the left hemisphere of male schizophrenics—a magnetoencephalographic-magnetic resonance imaging study. Biol Psychiatry 1997; 42:609–616Crossref, Medline, Google Scholar
14. Sullivan E, Mathalon D, Lim K, Marsh L, Pfefferbaum A: Patterns of regional cortical dysmorphology distinguishing schizophrenia and chronic alcoholism. Biol Psychiatry 1998; 43:118–131Crossref, Medline, Google Scholar
15. Shenton M, Wible C, McCarley R: Review of magnetic resonance imaging studies of brain abnormalities in schizophrenia, in Brain Imaging in Clinical Psychiatry. Edited by Krishnan K, Doraiswamy P. New York, Marcel Dekker, 1997, pp 297–380Google Scholar
16. McCarley R, Wible C, Frumin M, Hirayasu Y, Levitt J, Fischer I, Shenton M: MRI anatomy of schizophrenia. Biol Psychiatry 1999; 45:1099–1119Google Scholar
17. Shenton ME, Frumin M, McCarley RW, Maier S, Westin CF, Fischer IA, Dickey CC, Kikinis R: MR morphometric findings in schizophrenia, in Psychiatric Neuroimaging Strategies: Research and Clinical Applications. Edited by Dougherty D, Rauch S, Rosenbaum J. Washington, DC, American Psychiatric Press (in press)Google Scholar
18. Raine A, Sheard C, Reynolds GP, Lencz T: Pre-frontal structural and functional deficits associated with individual differences in schizotypal personality. Schizophr Res 1992; 7:237–247Crossref, Medline, Google Scholar
19. Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-IV (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
20. First MB, Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-IV—Patient Edition (SCID-P). Washington, DC, American Psychiatric Press, 1995Google Scholar
21. First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin L: Structured Clinical Interview for DSM-IV Personality Disorders (SCID-II): Interview and Questionnaire. Washington, DC, American Psychiatric Press, 1997Google Scholar
22. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
23. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1981Google Scholar
24. Gerig G, Kubler O, Kikinis R, Jolesz F: Non-linear anisotropic filtering of MRI data. IEEE Trans Med Imaging 1992; 11:221–232Crossref, Medline, Google Scholar
25. Wells W, Viola P, Atsumi H, Nakajimi S, Kikinis R: Multi-modal volume registration by maximization of mutual information. Med Image Anal 1996; 1:35–51Crossref, Medline, Google Scholar
26. Chakravarti I, Laha R, Roy J: Handbook of Methods of Applied Statistics, vol 1: Techniques of Computation, Descriptive Methods, and Statistical Inference. New York, John Wiley & Sons, 1968Google Scholar
27. Cannon TD, Mednick SA, Parnas J, Schulsinger F, Praestholm J, Vestergaard A: Developmental brain abnormalities in the offspring of schizophrenic mothers, II: structural brain characteristics of schizophrenia and schizotypal personality disorder. Arch Gen Psychiatry 1994; 51:955–962Crossref, Medline, Google Scholar
28. Cannon T, van Erp T, Huttunen M, Lonnqvist J, Salonen O, Valanne L, Poutanen V-P, Standerskjold-Nordenstam C-G, Gur R, Yan M: Regional gray matter, white matter, and cerebrospinal fluid distribution in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry 1998; 55:1084–1091Google Scholar
29. Buchsbaum M, Yang S, Hazlett E, Siegel B, Germans M, Haznedar M, O’Flaithbheartaigh S, Wei T, Silverman J, Siever L: Ventricular volume and asymmetry in schizotypal personality disorder and schizophrenia assessed with magnetic resonance imaging. Schizophr Res 1997; 27:45–53Crossref, Medline, Google Scholar
30. Cazzullo C, Vita A, Giobbio G, Dieci M, Saccheti E: Cerebral structural abnormalities in schizophreniform disorder and in schizophrenia spectrum personality disorders, in Schizophrenia Research: Advances in Neuropsychiatry and Psychopharmacology, vol 1. Edited by Tamminga C, Schultz S. New York, Raven Press, 1991, pp 209–217Google Scholar
31. Siever LJ, Rotter M, Losonczy M, Guo SL, Mitropoulou V, Trestman R, Apter S, Zemishlany Z, Silverman J, Horvath TB: Lateral ventricular enlargement in schizotypal personality disorder. Psychiatry Res 1995; 57:109–118Crossref, Medline, Google Scholar
32. Silverman J, Smith C, Guo S, Mohs R, Siever L, Davis K: Lateral ventricular enlargement in schizophrenic probands and their siblings with schizophrenia-related disorders. Biol Psychiatry 1998; 43:97–106Crossref, Medline, Google Scholar
33. Flaum M, Swayze VW II, O’Leary DS, Yuh WTC, Ehrhardt JC, Arndt SV, Andreasen NC: Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am J Psychiatry 1995; 152:704–714Link, Google Scholar
34. DeLisi L, Stritzke P, Riordan H, Holan V, Boccio A, Kushner M, McClelland J, Van Eyl O, Anand A: The timing of brain morphological changes in schizophrenia and their relationship to clinical outcome. Biol Psychiatry 1992; 31:241–254Crossref, Medline, Google Scholar
35. Carpenter M: Core Text of Neuroanatomy, 4th ed. Baltimore, Williams & Wilkins, 1991, p 10Google Scholar
36. Afifi A, Bergman R: Functional Neuroanatomy Text and Atlas. New York, McGraw-Hill, 1998, p 566Google Scholar
37. Deck MDF: The lateral ventricles, in Radiology of the Skull and Brain, vol 4: Cisterns and Ventricles. Edited by Newton T, Potts D. St Louis, CV Mosby, 1978, pp 3489–3587Google Scholar
38. Zipursky R, Lim K, Sullivan E, Brown B, Pfefferbaum A: Widespread cerebral gray matter volume deficits in schizophrenia. Arch Gen Psychiatry 1992; 49:195–205Crossref, Medline, Google Scholar
39. Harvey I, Ron M, Du Boulay G, Wicks D, Lewis S, Murray R: Reduction of cortical volume in schizophrenia on magnetic resonance imaging. Psychol Med 1993; 23:591–604Crossref, Medline, Google Scholar
40. Woods B, Yurgelun-Todd D, Goldstein J, Seidman L, Tsuang M: MRI brain abnormalities in chronic schizophrenia: one process or more? Biol Psychiatry 1996; 40:585–596Google Scholar
41. Sullivan E, Shear P, Lim K, Zipursky R, Pfefferbaum A: Cognitive and motor impairments are related to gray matter volume deficits in schizophrenia. Biol Psychiatry 1996; 39:234–240Crossref, Medline, Google Scholar
42. Zipursky R, Seeman M, Bury A, Langevin R, Wortzman G, Katz R: Deficits in gray matter volume are present in schizophrenia but not bipolar disorder. Schizophr Res 1997; 26:85–92Crossref, Medline, Google Scholar
43. Breier A, Buchanan R, Elkashef A, Munson R, Kirkpatrick B, Gellad F: Brain morphology and schizophrenia: a magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch Gen Psychiatry 1992; 1992:921–926Crossref, Google Scholar