Sensory Gating Deficits Assessed by the P50 Event-Related Potential in Subjects With Schizotypal Personality Disorder
Abstract
OBJECTIVE: The schizophrenia spectrum includes individuals with schizophrenia, their relatives, and individuals with schizotypal personality disorder. Subjects in the schizophrenia spectrum have disorders of attention, cognition, and information processing. Attention and information processing can be assessed by testing suppression of the P50 event-related potential; the amplitude of the P50 wave is measured in response to each of two auditory clicks. In normal subjects, the P50 wave following the second click is suppressed, or “gated.” Schizophrenic patients and their relatives show less suppression of the second P50 wave. Deficits in P50 suppression have high heritability and show linkage to the alpha-7 subunit of the nicotinic cholinergic receptor gene in families with schizophrenia, suggesting that deficits in P50 suppression are trait markers for gating abnormalities in schizophrenia spectrum subjects. Although schizotypal subjects have been shown to have deficits in sensorimotor gating as measured by prepulse inhibition, to the authors’ knowledge P50 sensory gating in schizotypal personality disorder has yet to be reported. METHOD: P50 suppression in 26 subjects with schizotypal personality disorder and 23 normal subjects was assessed through auditory conditioning and testing. RESULTS: The subjects with schizotypal personality had significantly less P50 suppression than did the normal subjects. CONCLUSIONS: Subjects with schizotypal personality disorder may have trait-linked sensory gating deficits similar to those in patients with schizophrenia and their relatives. Because these subjects may manifest sensory gating deficits without overt psychotic symptoms, it is likely that these deficits represent a core cognitive dysfunction of the schizophrenia spectrum.
The schizophrenia spectrum of disorders includes schizophrenic patients and their relatives. The relatives are thought to have a genetic predisposition to schizophrenia but do not necessarily demonstrate full or any clinical manifestations of the disorder. Schizotypal personality disorder is phenomenologically, and perhaps genotypically, linked to schizophrenia and is also thought to be part of the schizophrenia spectrum (1). The DSM criteria for schizotypal personality disorder were developed on the basis of careful study of the symptoms that appear most commonly in affected relatives of schizophrenic patients and as prodromal symptoms of schizophrenia (2). The empirically based diagnostic criteria for schizotypal personality disorder continue to be refined with the goal of establishing criteria that identify individuals who are genotypically linked to persons with schizophrenia (3). Over the past two decades, family-genetic studies have determined that not only do relatives of schizophrenic patients have a higher than normal risk of schizotypal personality disorder (4–10) but relatives of subjects with schizotypal personality disorder have high rates of both schizophrenia (11, 12) and schizotypal personality disorder (13).
The study of vulnerability markers in clinically unaffected relatives of schizophrenic patients and subjects with schizotypal personality disorder has become increasingly important because it provides a means of assessing phenotypic traits of the schizophrenia spectrum that may not be grossly evident clinically (14). Subjects with schizotypal personality (and relatives of schizophrenic patients) do not have many of the confounding variables seen in schizophrenic patients because they are typically unmedicated, not overtly psychotic, and not chronically mentally ill. If vulnerability markers that are specific to these nonpsychotic schizophrenia spectrum disorders can be identified, it may be possible to “disentangle and isolate the contingent pathophysiological processes involved in schizophrenic disorders” (15).
Schizophrenia is commonly conceptualized as a disorder of attention, cognition, and information processing (16). Attention and information processing can be assessed psychophysiologically with measures such as the P50 event-related potential test of sensory gating, in which the amplitude of the P50 wave is measured in response to each of two auditory clicks (conditioning and test) (17). In normal subjects, the second P50 wave is suppressed, or “gated,” because of the inhibitory effects of the first click. Impaired suppression of the P50 wave has been identified as a vulnerability marker for the sensory gating deficits observed in schizophrenic patients and their relatives (18–20). In normal subjects, P50 suppression has been shown to have high heritability (21) and to be stable in repeated test sessions (22, 23). Schizophrenic patients (24–26) and their relatives (19, 20) have impaired suppression of the P50 wave in this test. Findings from animal testing of P50 gating implicate nicotinic cholinergic receptors in the inhibitory mechanism (27), and in genetic linkage studies of humans (14), these deficits have been linked to the alpha-7 subunit of the nicotinic cholinergic receptor gene.
To our knowledge, P50 suppression has not been reported for schizotypal personality disorder. We previously reported (28) that individuals with schizotypal personality disorder have impaired prepulse inhibition of the startle response, a distinct although conceptually related operational model of sensorimotor gating. We hypothesized that subjects with schizotypal personality would have deficits in P50 suppression similar to those observed in schizophrenic patients and their relatives.
METHOD
Subjects
We included 23 normal subjects (12 men, 11 women) who had no history of axis I or II disorders, as assessed by the Structured Clinical Interview for DSM-IV Axis I Disorders—Non-Patient Edition (SCID-I/NP) (29) and the Structured Clinical Interview for DSM-IV Axis II Disorders (30), and no family history of psychiatric illness. The established interrater reliability for the SCID-I/NP in our laboratory is 0.98 (31). All of the normal subjects were recruited through newspaper advertisements and were excluded from participation if they reported a history of major medical or neurological disorders (seizures, head injury). None of the normal or schizotypal subjects reported substantial drug abuse in the past month. In addition, they were screened for current drug use through urine toxicology screening (no subject was excluded on this basis).
Twenty-six subjects with schizotypal personality disorder (14 men, 12 women) were recruited from the inpatient and outpatient facilities at the University of California, San Diego, Medical Center (N=3), at the La Jolla Veterans Affairs Medical Center (N=5), and at Balboa Naval Hospital, San Diego (N=1). Additional subjects with schizotypal personality disorder (N=14) were recruited by using newspaper advertisements for “people who had experiences with the paranormal,” “a UCSD ESP study,” and “people who are shy, have difficulty with trust and few friends.” The advertisements were designed to identify individuals with both the positive symptoms (magical thinking, perceptual abnormalities, ideas of reference) and negative symptoms (few friends, social anxiety) of schizotypal personality disorder. Additional schizotypal subjects (N=3) were identified through screening of potential normal subjects.
Before entry into the study, all subjects provided written informed consent after receiving an explanation of the study.
There was a significant difference in age between the normal subjects (mean=32.0 years, SD=9.8) and the subjects with schizotypal personality disorder (mean=38.6, SD=10.2) (t=–2.31, df=47, p<0.05). The two groups also differed in years of education (normal: mean=15.1, SD=2.3; schizotypal: mean=13.5, SD=2.3) (t=2.39, df=47, p<0.05).
All of the subjects with schizotypal personality disorder were assessed with the SCID-I/NP and with the Structured Interview for DSM-IV Personality (32). All subjects met the DSM-IV criteria for schizotypal personality disorder, and any subject with a history of a psychotic illness, current major depression, or major medical or neurological illness was excluded (N=3). Five of the 26 subjects were receiving psychotropic medication: two were receiving low-dose neuroleptics (thiothixene, 2 mg/day; perphenazine, 2 mg/day), two were receiving antidepressants, and one was receiving valproate and clonazepam.
All of the schizotypal subjects were queried regarding family history of psychiatric illness. Specifically, questions regarding a family history of psychiatric hospitalization, suicide, treatment with psychotropic medication, and nervous breakdown were asked in regard to first-, second-, and third-degree relatives. Subjects were then queried further regarding any identified family member to determine whether the identified symptoms and chronicity met the criteria for an axis I disorder. Of the 26 schizotypal subjects, 14 reported a family history of schizophrenia, paranoia, nervous breakdown, suicide, or chronic psychiatric hospitalization. Each subject was classified as having a “possible” or “probable” family history of schizophrenia on the basis of the information he or she was able to provide. A “probable” family history of schizophrenia was assigned to five subjects who had relatives diagnosed with schizophrenia or who described symptoms consistent with schizophrenia (delusions, hallucinations, withdrawal) that were chronic in nature. A “possible” family history of schizophrenia was assigned to nine individuals who were unable to provide sufficient information to make a psychiatric diagnosis for relatives who committed suicide or who returned to normal functioning after one brief episode of emotional disturbance requiring hospitalization. Because their relatives were not assessed and other family members were not interviewed, it was not possible to determine whether the subjects with schizotypal personality disorder had a definite family history of schizophrenia or other psychotic disorder. Seven other schizotypal subjects reported a family history of depression or anxiety disorders.
The subjects with schizotypal personality disorder had a mean of 6.2 (SD=1.1) schizotypal symptoms and a mean of 1.4 (SD=1.5) other personality disorders. They had mean scores of 55.3 (SD=12.4) on the Global Assessment of Functioning Scale, 5.2 (SD=3.8) on the Scale for the Assessment of Negative Symptoms (33), 6.5 (SD=2.5) on the Scale for the Assessment of Positive Symptoms (34), and 33.0 (SD=10.8) on the Schizotypal Personality Questionnaire (35).
Measurement of P50 Event-Related Potential
The P50 sensory gating test used a signal generator and data acquisition system (San Diego Instruments, San Diego, Calif.) and amplifiers (Grass, West Warwick, R.I.) to record the EEG data according to our established methods (20, 24). The subject was seated in a comfortable recliner in a quiet, lighted room while wearing headphones for presentation of the auditory stimuli. The subject was instructed to relax, to keep his or her eyes open, and to focus on a fixation point. All subjects were monitored for signs of sleep by visual observation and EEG monitoring. When a subject was observed to be drowsy, the examiner would briefly interact with the subject. Eye movements were recorded by using electro-oculography (EOG) with Ag/AgCl electrodes placed at the outer canthus of the left eye and below the right eye. Electrodes were used at seven recording sites (Fz, Cz, Pz, F3, F4, C3, C4, according to the 10/20 system) with a forehead ground and referenced to linked earlobes. All electrode resistances were less than 5 kΩ . The stimuli were generated by means of computer-driven pulses with a 1-msec duration by using a signal generator and data acquisition system for the recording of EEG waveforms. To control background noise during stimulus presentation, 60-dB[A] broadband white noise was presented continuously throughout the session. The auditory clicks consisted of flat broadband (250 Hz to 50 kHz) square waves of 1-msec duration (rise time of 12–15 µsec) with an average resulting click of 89 dB[A]. The interpair interval was varied between 8 and 12 seconds in 1-second increments. The stimuli were 120 click pairs (stimulus 1 and stimulus 2) with a 500-msec interclick interval. The EEG responses were amplified and band-pass filtered with an analog filter of 0.01 to 300 Hz and no 60-Hz notch filter, at a sampling rate of 1000 Hz, for a total of 1000 msec (100 msec before to 400 msec after the stimuli with a 500-msec gap between stimulus 1 and stimulus 2). The P50 component was identified and quantified according to the established methods of Nagamoto et al. (26) and our laboratory (20, 24). The data were then digitally low-pass filtered at 100 Hz before artifact screening, to eliminate any residual electrical noise. After acquisition of the data, the EEG and EOG channels were screened for artifact, and trials containing artifact (plus or minus 50 µV EOG or EEG channel deflection) were not included in the waveform averaging. Artifact-free epochs were averaged and digitally band-pass filtered (5–50 Hz). The filter had 12-dB/octave high- and low-pass slopes similar in gain characteristics to those reported by Jerger (36) and is consistent with previously reported methods from our laboratory (37). The P50 component was identified as the most positive deflection 40 to 80 msec following stimulus presentation. The P50 amplitude is the absolute difference between the P50 peak and the preceding negative trough (24, 38). The data from the Cz site are reported because this is the best site for discriminating schizophrenic patients from normal subjects when using this electrode array (39). The percentage of P50 suppression was calculated by using the formula [1 – (stimulus 2 amplitude/stimulus 1 amplitude)] × 100. A minimum of –100% suppression (or 100% facilitation) was used to prevent outliers from disproportionately affecting the group means, consistent with the methods of Nagamoto et al. (38).
Data Analysis
The demographic and event-related potential data were assessed by using effect sizes, to accurately demonstrate group discrimination, and t tests. If homogeneity of variance assumptions were not met (Levene’s test), then separate variance t tests were performed. All t tests were two-tailed. To analyze the effects of age and gender on measures of event-related potentials, a univariate analysis of variance (ANOVA) was used.
RESULTS
As shown in table 1, the groups did not differ significantly in the amplitude or latency of the P50 wave in response to the first or second stimulus. Nevertheless, there was a significant difference in P50 suppression, as shown in table 1 and figure 1. Because the two groups differed significantly in age, age was used as a covariate in a univariate ANOVA that also assessed the effect of gender on P50 suppression. There was no significant gender, age, or interaction effect, and the group main effect approached significance only when the effects of gender and age were accounted for (F=3.69, df=1, 48, p=0.06).
The subjects with schizotypal personality disorder were divided into those who reported no family history of psychotic disorders (N=12), those with a possible family history of schizophrenia (N=9), and those with a probable family history of schizophrenia (N=5). Although there were no significant differences between these subgroups in P50 suppression (probably owing to reduced power from small groups), the subjects with a probable family history of schizophrenia appeared to have the worst suppression (mean=–6.5, SD=82.8), followed by those with a possible family history of schizophrenia (mean=21.6, SD=77.3) and those with no family history of psychotic disorders (mean=48.3, SD=65.2) (figure 1).
DISCUSSION
These findings confirm the hypothesis that subjects with schizotypal personality disorder have sensory gating deficits as assessed by a test of the P50 event-related potential. Because the P50 suppression test may identify an intermediate phenotypic marker for the sensory gating deficits of schizophrenia spectrum disorders (14), these data support the idea that subjects with schizotypal personality disorder are related genotypically to individuals with schizophrenia and their relatives. Additional studies are needed to determine whether the abnormalities in P50 suppression observed in schizotypal subjects are most prominent in individuals with a definite family history of schizophrenia. The present data are consistent with those from studies of schizotypal personality disorder that demonstrate deficits in sensorimotor gating and information processing as assessed by prepulse inhibition and habituation of the startle response (28). Prepulse inhibition and P50 suppression are both thought to be measures of inhibitory processes (40). People with schizotypal personality disorder have also been noted to have abnormalities in attention and executive functioning (41–46), suggesting that similar deficits observed in schizophrenic patients may represent a primary dysfunction related to core cognitive deficits rather than artifacts due to psychosis, medication, or generalized psychopathology.
It has long been noted that schizophrenic patients have a cognitive dysfunction that may be primary to the disorder (16, 47–49). Measures of cognitive functioning are better predictors of the clinical and functional outcomes of schizophrenic patients than are the more overt positive symptoms seen in the disorder (50, 51). Subjects with schizotypal personality disorder and clinically unaffected relatives of schizophrenic patients exhibit deficits in attention and information processing on a variety of measures without manifesting the more overt psychotic symptoms of schizophrenic patients (18, 20, 28, 52–55). Further work is needed to determine whether the information processing dysfunction in schizotypal subjects is related to their functional or clinical outcome. For example, it would be interesting to know whether schizotypal subjects with information processing abnormalities have a higher than normal risk for developing other cognitive and functional disabilities and ultimately schizophrenia. The idea of identifying markers for vulnerability to schizophrenia is intriguing but clearly needs to be developed further by means of a multifaceted approach that assesses hereditary, genetic, and outcome variables in persons with schizophrenia spectrum disorders, such as schizotypal personality disorder.
Received May 4, 1999; revision received Aug. 9, 1999; accepted Aug. 11, 1999. From the Department of Psychiatry, University of California, San Diego. Address reprints to Dr. Cadenhead, Department of Psychiatry, 0804, University of California, San Diego, 9500 Gilman Dr., La Jolla, CA 92093-0804; [email protected] (e-mail). Supported by NIMH grants MH-01124 and MH-42228, and the Department of Veterans Affairs (Mental Illness Research Education and Clinical Center, Veterans Integrated Service Network 22) supported preparation of this article. Dr. Geyer has an equity interest in San Diego Instruments, Inc.
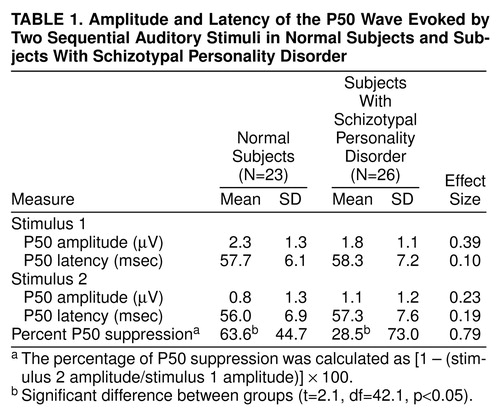 |
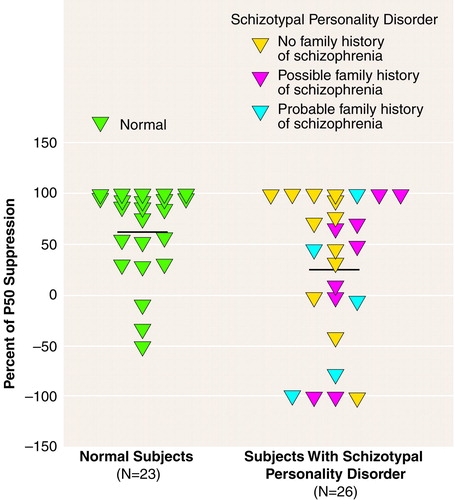
FIGURE 1. Individual Percentages of Suppression of P50 Wave Evoked by Second Auditory Stimulus in Normal Subjects and Subjects With Schizotypal Personality Disordera
aThe percentage of P50 suppression was calculated as [1 – (stimulus 2 amplitude/stimulus 1 amplitude)] × 100. The horizontal lines represent mean values.
1. Siever LJ, Kalus OF, Keefe RS: The boundaries of schizophrenia. Psychiatr Clin North Am 1993; 16:217–244Crossref, Medline, Google Scholar
2. Spitzer RL, Endicott J, Gibbon M: Crossing the border into borderline personality and borderline schizophrenia: the development of criteria. Arch Gen Psychiatry 1979; 36:17–24Crossref, Medline, Google Scholar
3. Gunderson JG, Siever LJ, Spaulding E: The search for a schizotype: crossing the border again. Arch Gen Psychiatry 1983; 40:15–22Crossref, Medline, Google Scholar
4. Baron M, Gruen R, Rainer JD, Kane J, Asnis L, Lord S: A family study of schizophrenic and normal control probands: implications for the spectrum concept of schizophrenia. Am J Psychiatry 1985; 142:447–455Link, Google Scholar
5. Frangos E, Athanassenas G, Tsitourides S, Katsanou N, Alexandrakou P: Prevalence of DSM III schizophrenia among the first-degree relatives of schizophrenic probands. Acta Psychiatr Scand 1985; 72:382–386Crossref, Medline, Google Scholar
6. Gershon ES, DeLisi LE, Hamovit J, Nurnberger JI Jr, Maxwell ME, Schreiber J, Dauphinais D, Dingman CW II, Guroff JJ: A controlled family study of chronic psychoses: schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 1988; 45:328–336Crossref, Medline, Google Scholar
7. Lowing PA, Mirsky AF, Pereira R: The inheritance of schizophrenia spectrum disorders: a reanalysis of the Danish adoptee study data. Am J Psychiatry 1983; 140:1167–1171Google Scholar
8. Onstad S, Skre I, Edvardsen J, Torgersen S, Kringlen E: Mental disorders in first-degree relatives of schizophrenics. Acta Psychiatr Scand 1991; 83:463–467Crossref, Medline, Google Scholar
9. Kendler KS, Gruenberg AM, Kinney DK: Independent diagnoses of adoptees and relatives as defined by DSM-III in the provincial and national samples of the Danish adoption study of schizophrenia. Arch Gen Psychiatry 1994; 51:456–468Crossref, Medline, Google Scholar
10. Kety SS, Wender PH, Jacobsen LJ, Ingraham LJ, Jansson L, Faber B, Kinney D: Mental illness in the biological and adoptive relatives of schizophrenic adoptees: replication of the Copenhagen study in the rest of Denmark. Arch Gen Psychiatry 1994; 51:442–455Crossref, Medline, Google Scholar
11. Battaglia M, Gasperini M, Sciuto G, Scherillo P, Diaferia G, Bellodi L: Psychiatric disorders in the families of schizotypal subjects. Schizophr Bull 1991; 17:659–668Crossref, Medline, Google Scholar
12. Thaker G, Adami H, Moran M, Lahti A, Cassady S: Psychiatric illnesses in families of subjects with schizophrenia-spectrum personality disorders: high morbidity risks for unspecified functional psychoses and schizophrenia. Am J Psychiatry 1993; 150:66–71Link, Google Scholar
13. Siever LJ, Silverman JM, Horvath TB, Klar H, Coccaro E, Keefe RS, Pinkham L, Rinaldi P, Mohs RC, Davis KL: Increased morbid risk for schizophrenia-related disorders in relatives of schizotypal personality disordered patients. Arch Gen Psychiatry 1990; 47:634–640Crossref, Medline, Google Scholar
14. Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W: Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA 1997; 94:587–592Crossref, Medline, Google Scholar
15. Siever LJ: Brain structure/function and the dopamine system in schizotypal personality disorder, in Schizotypal Personality. Edited by Raine A, Lencz T, Mednick SA. New York, Cambridge University Press, 1995, pp 272–286Google Scholar
16. Braff DL: Psychophysiological and information processing approaches to schizophrenia, in Neurobiology of Mental Illness. Edited by Charney DS, Nestler EJ, Bunney BS. New York, Oxford University Press, 1999, pp 258–271Google Scholar
17. Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R: Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry 1982; 17:639–654Medline, Google Scholar
18. Waldo MC, Carey G, Myles-Worsley M, Cawthra E, Adler LE, Nagamoto HT, Wender P, Byerley W, Plaetke R, Freedman R: Codistribution of a sensory gating deficit and schizophrenia in multi-affected families. Psychiatry Res 1991; 39:257–268Crossref, Medline, Google Scholar
19. Siegel C, Waldo M, Mizner G, Adler LE, Freedman R: Deficits in sensory gating in schizophrenic patients and their relatives: evidence obtained with auditory evoked responses. Arch Gen Psychiatry 1984; 41:607–612Crossref, Medline, Google Scholar
20. Clementz BA, Geyer MA, Braff DL: Poor P50 suppression among schizophrenia patients and their first-degree biological relatives. Am J Psychiatry 1998; 155:1691–1694Google Scholar
21. Young D, Waldo M, Rutledge JI, Freedman R: Heritability of inhibitory gating of the P50 auditory-evoked potential in monozygotic and dizygotic twins. Neuropsychobiology 1996; 33:113–117Crossref, Medline, Google Scholar
22. Boutros NN, Overall J, Zouridakis G: Test-retest reliability of the P50 mid-latency auditory evoked response. Psychiatry Res 1991; 39:181–192Crossref, Medline, Google Scholar
23. Smith DA, Boutros NN, Schwarkopf SB: Reliability of P50 auditory event-related potential indices of sensory gating. Psychophysiology 1994; 31:495–502Crossref, Medline, Google Scholar
24. Clementz BA, Geyer MA, Braff DL: P50 suppression among schizophrenia and normal comparison subjects: a methodological analysis. Biol Psychiatry 1997; 41:1035–1044Google Scholar
25. Judd LL, McAdams L, Budnick B, Braff DL: Sensory gating deficits in schizophrenia: new results. Am J Psychiatry 1992; 149:488–493Link, Google Scholar
26. Nagamoto HT, Adler LE, Waldo MC, Freedman R: Sensory gating in schizophrenics and normal controls: effects of changing stimulation interval. Biol Psychiatry 1989; 25:549–561Crossref, Medline, Google Scholar
27. Luntz-Leybman V, Bickford PC, Freedman R: Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res 1992; 587:130–136Crossref, Medline, Google Scholar
28. Cadenhead KS, Geyer MA, Braff DL: Impaired startle prepulse inhibition and habituation in patients with schizotypal personality disorder. Am J Psychiatry 1993; 150:1862–1867Google Scholar
29. First MB, Spitzer RL, Gibbon M, Williams JB: Structured Clinical Interview for DSM-IV Axis I Disorders—Non-Patient Edition (SCID-I/NP), version 2.0. New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
30. First MB, Gibbon M, Spitzer RL, Williams JB: Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II), version 2. New York, New York Psychiatric Institute, Biometrics Research, 1996Google Scholar
31. Perry W, Braff DL: A multimethod approach to assessing perseverations in schizophrenia patients. Schizophr Res 1998; 33:69–77Crossref, Medline, Google Scholar
32. Pfohl B, Blum N, Zimmerman M: Structured Interview for DSM-IV Personality: SIDP-IV. Iowa City, University of Iowa, Department of Psychiatry, 1995Google Scholar
33. Andreasen NC: Modified Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1984Google Scholar
34. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
35. Raine A: The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull 1991; 17:555–564Crossref, Medline, Google Scholar
36. Jerger J: Can age-related decline in speech understanding be explained by peripheral hearing loss? J Am Acad Audiol 1992; 3:33–38Google Scholar
37. Light GA, Malaspina D, Geyer MA, Luber BM, Coleman EA, Sackeim HA, Braff DL: Amphetamine disrupts P50 suppression in normal subjects. Biol Psychiatry 1999; 46:990–996Crossref, Medline, Google Scholar
38. Nagamoto HT, Adler LE, Waldo MC, Griffith J, Freedman R: Gating of auditory response in schizophrenics and normal controls: effects of recording site and stimulation interval on the P50 wave. Schizophr Res 1991; 4:31–40Crossref, Medline, Google Scholar
39. Clementz BA, Geyer MA, Braff DL: Multiple site evaluation of P50 suppression among schizophrenia and normal comparison subjects. Schizophr Res 1998; 30:71–80Crossref, Medline, Google Scholar
40. Cadenhead K, Braff DL: Neurophysiology of schizophrenia: attention, information processing, and inhibitory processes in schizophrenia, in Advances in the Neurobiology of Schizophrenia. Edited by den Boer JA, Westenberg HGM, van Praag HM. Chichester, UK, John Wiley & Sons, 1995, pp 329–342Google Scholar
41. Cadenhead KS, Perry W, Shafer K, Braff DL: Cognitive functions in schizotypal personality disordered subjects. Schizophr Res 1999; 37:123–132Crossref, Medline, Google Scholar
42. Lees Roitman SE, Cornblatt BA, Bergman A, Obuchowski M, Mitropoulou V, Keefe RSE, Silverman JM, Siever LJ: Attentional functioning in schizotypal personality disorder. Am J Psychiatry 1997; 154:655–660; correction, 154:1180Google Scholar
43. Lencz T, Raine A, Benishay DS, Mills S, Bird L: Neuropsychological abnormalities associated with schizotypal personality, in Schizotypal Personality. Edited by Raine A, Lencz T, Mednick SA. Cambridge, UK, Cambridge University Press, 1995, pp 289–328Google Scholar
44. Thaker GK, Moran M, Lahti A, Adami H, Tamminga CA, Schulz SC: Pilot studies of schizotypal subjects, in Schizophrenia Research: Advances in Neuropsychiatry and Psychopharmacology, vol 1. Edited by Tamminga CA, Schulz SC. New York, Raven Press, 1991, pp 201–208Google Scholar
45. Trestman RL, Keefe RSE, Mitropoulou V, Harvey PD, deVegvar ML, Lees-Roitman S, Davidson M, Aronson A, Silverman J, Siever LJ: Cognitive function and biological correlates of cognitive performance in schizotypal personality disorder. Psychiatry Res 1995; 59:127–136Crossref, Medline, Google Scholar
46. Voglmaier MM, Seidman LJ, Salisbury D, McCarley RW: Neuropsychological dysfunction in schizotypal personality disorder: a profile analysis. Biol Psychiatry 1997; 41:530–540Crossref, Medline, Google Scholar
47. Venables PH: The effect of auditory and visual stimulation on the skin potential response of schizophrenics. Brain 1960; 83:77–92Crossref, Medline, Google Scholar
48. Bleuler E: Dementia Praecox or the Group of Schizophrenias (1911). Translated by Zinkin J. New York, International Universities Press, 1964Google Scholar
49. McGhie A, Chapman J: Disorders of attention and perception in early schizophrenia. Br J Med Psychol 1961; 34:103–116Crossref, Medline, Google Scholar
50. Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321–330Google Scholar
51. Green M, Kern R, Braff D, Mintz J: Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the right stuff? Schizophr Bull (in press)Google Scholar
52. Cannon TD, Zorrilla LE, Shtasel D, Gur RE, Gur RC, Marco EJ, Moberg P, Price A: Neuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers. Arch Gen Psychiatry 1994; 51:651–666Crossref, Medline, Google Scholar
53. Clementz BA, Reid SA, McDowell JE, Cadenhead KS: Abnormality of smooth pursuit eye movement initiation: specificity to the schizophrenia spectrum? Psychophysiology 1995; 32:130–134Google Scholar
54. Cornblatt B, Obuchowski M: Update of high-risk research:1987–1997. Int Rev Psychiatry 1997; 9:437–447Google Scholar
55. Keefe RS, Silverman JM, Mohs RC, Siever LJ, Harvey PD, Friedman L, Roitman SE, DuPre RL, Smith CJ, Schmeidler J, Davis KL: Eye tracking, attention, and schizotypal symptoms in nonpsychotic relatives of patients with schizophrenia. Arch Gen Psychiatry 1997; 54:169–176Crossref, Medline, Google Scholar