Treatment of Major Depression With Nortriptyline and Paroxetine in Patients With Ischemic Heart Disease
Abstract
OBJECTIVE: This study compared the efficacy, tolerability, and safety of paroxetine and nortriptyline in depressed patients with ischemic heart disease. METHOD: After a 2-week, single-blind placebo lead-in phase, 81 outpatients with DSM-III-R-defined nonpsychotic unipolar major depression and ischemic heart disease were randomly assigned to double-blind treatment with paroxetine or nortriptyline for 6 weeks. Paroxetine was administered at a fixed-flexible dose of 20–30 mg/day. Nortriptyline dose was adjusted with the use of blood-level monitoring to reach a plasma concentration of 50–150 ng/ml. RESULTS: Twenty-seven of the 41 patients who started treatment with paroxetine and 29 of the 40 patients who started treatment with nortriptyline had an improvement of at least 50% in their Hamilton Depression Rating Scale scores. Significantly more patients taking nortriptyline discontinued treatment prematurely (35% versus 10%), and more patients taking nortriptyline had adverse events resulting in termination (25% versus 5%). CONCLUSIONS: Both treatments were efficacious. Sixty-three percent of all patients improved at least 50%, and of these, 90% met the criteria for remission. Paroxetine was better tolerated than nortriptyline and less likely to produce cardiovascular side effects.
Depression and heart disease have an important and complex interactive relationship (1). Depressed patients are at increased risk for myocardial infarction (2), and patients who have a myocardial infarction have a high incidence of depression (3). Perhaps most provocative is the finding that post-myocardial-infarction patients who became depressed had a fivefold increase in mortality during the 6 months after the infarction (3). The increase in mortality associated with depression was not accounted for by cardiovascular predictors. These data underscore the importance of finding a safe and effective treatment for depression in patients with heart disease and determining whether treatment of the depression will alter the course of ischemic heart disease.
Treatment of depressed patients with heart disease is prototypical of the challenge that confronts the clinician when treating depressed patients with comorbid medical conditions. The clinician needs to balance the expected efficacy of the medication with its potential adverse effects and the consequences of leaving the illness untreated. This is particularly important in patients with depression and cardiovascular disease because adverse events can be severe, but so are the effects of untreated depression on the course of cardiac disease.
What treatment is best? Roose et al. (4) have suggested that in melancholic patients, selective serotonin reuptake inhibitors (SSRIs) may be less effective than tricyclic antidepressants. Depressed patients with heart disease tend to be older and, as a result, are more likely to be melancholic. The same investigators, however, have pointed out the potential risk of tricyclic antidepressants in patients with ischemic heart disease (5). They argue that the Cardiac Arrhythmia Suppression Trials indicate that type I antiarrhythmic drugs increase the risk of sudden death after myocardial infarction, and because the tricyclic antidepressants have similar effects, they might also be expected to increase this risk. Determination of the safety and efficacy of antidepressant drugs in ischemic heart disease is thus imperative.
It has been assumed that SSRIs are safer agents for patients with heart disease. This assumption is based on the observation that SSRIs have little effect on the ECGs of depressed patients without heart disease. However, these drugs have seldom been tested directly in patients with ischemic heart disease. Roose et al. (6) examined the effect of fluoxetine in such patients and found that fluoxetine did not alter conduction or result in orthostatic hypotension, but the other serotonergic antidepressants have not been tested in these patients.
The current study aimed to test the safety and effectiveness of paroxetine in depressed patients with ischemic heart disease. The effects of paroxetine were compared with those of nortriptyline. Often the comparison drug in clinical trials of SSRIs has been a tertiary tricyclic antidepressant compound such as imipramine or amitriptyline, even though the tertiary tricyclic antidepressants are known to be associated with more side effects (7). For this study, we wished to compare paroxetine with the best available tricyclic antidepressant treatment. Nortriptyline was selected because of its established efficacy in patients with heart disease (4, 8) and its preferred side effect profile, including a lower incidence of orthostatic hypotension (9, 10).
Blood-level monitoring of nortriptyline is often used to maximize efficacy in clinical practice, since response rates are enhanced when adequate plasma concentrations are attained (11). For nortriptyline this relationship may be especially important because reduced effectiveness can result from plasma concentrations that are either too low and too high (11). This also has implications for research designs that involve the tricyclic antidepressants. One of the criticisms of prior tricyclic antidepressant-SSRI comparison studies is that the dose of the tricyclic antidepressant was not necessarily adjusted to achieve maximum effectiveness. Indeed, since the usual intent of studies with new drugs is to show comparable effectiveness with the tricyclic antidepressant comparator, there is little incentive to maximize response to the tricyclic antidepressant.
In the current study we attempted to maximize the effectiveness of the tricyclic; thus, nortriptyline was administered with the use of blood-level monitoring to achieve a desired blood level of the drug. To our knowledge, no previous comparison study of an SSRI and a tricyclic antidepressant has used plasma-level monitoring to ensure adequate tricyclic antidepressant treatment.
In medically ill patients, issues of safety and tolerability are as important as efficacy. For this reason, we emphasize an intent-to-treat analysis of effectiveness. This approach provides the clinician with a broader perspective on what happens to all patients beginning treatment (12).
METHOD
This was a 6-week, double-blind, parallel comparison of nortriptyline and paroxetine conducted at four sites. After the initial assessment, patients began a 2-week, single-blind placebo lead-in period. At the end of this period, patients still meeting entry criteria were randomly assigned to the 6-week active drug trial.
The subjects included male and female outpatients, 18 years of age and older, who met the DSM-III-R criteria for nonpsychotic unipolar major depression, who had a 17-item Hamilton Depression Rating Scale (13) score of 16 or more, and who provided written informed consent after the protocol had been fully explained and all questions had been answered. The patients also had ischemic heart disease, as evidenced by a previous myocardial infarction, coronary artery bypass graft, or angioplasty, or had stable angina, positive results on a stress test, or angiographic evidence of luminal narrowing of a major coronary artery. Patients with schizophrenia, bipolar disorder, or active substance abuse were excluded. Also excluded were patients with a myocardial infarction within the past 3 months, a baseline QTc interval of 460 msec or more, or unstable or crescendo angina and patients who were receiving type I antiarrhythmic medication or coumadin.
All subjects completed an initial psychiatric and medical assessment including a physical examination and laboratory assessments. The presence of the DSM-III-R symptoms of major depression was documented. Depressive symptoms were rated with the 17-item Hamilton Depression Rating Scale at baseline, after the 2-week lead-in phase (week 0), and weekly thereafter. Side effects were assessed with the treatment-emergent signs and symptoms method, in which each patient at each visit is asked a nonleading question about whether side effects have occurred.
Details of the cardiovascular assessment have been provided elsewhere (14). Briefly, supine and standing blood pressure and pulse were determined before treatment and weekly thereafter. An ECG and a continuous 24-hour ECG recording were performed at baseline, at the end of the lead-in period, after 2 weeks of drug treatment, and at the conclusion of the trial. A MUGA scan was performed during the lead-in phase to determine left ventricular function.
All subjects received placebo during the first 2 weeks. Lorazepam, up to 2 mg/day, was allowed for management of anxiety or insomnia. No other psychotropic drugs were administered. Patients receiving paroxetine were started on 20 mg/day, except for patients over 65 years of age, who started treatment with 10 mg/day, which was then raised to 20 mg/day after 1 week. After week 3, the treating clinician had the option of increasing the dose to 30 mg/day, if response up to that point was less than expected, or to 40 mg/day, if necessary for retaining the patient in the study. Patients receiving nortriptyline were started on a dose of 25 mg h.s. for 2 days, which was then raised to 50 mg h.s. Nortriptyline plasma concentrations were determined after week 1, week 2, and week 6. The week 1 concentration was reported to a third-party clinician who recommended dose adjustment to the treating clinician. Dose was adjusted to obtain a blood level between 50 and 150 ng/ml. Double-blind conditions were maintained with use of a “double dummy” method. All patients received two pill bottles, one for paroxetine to be taken in the morning and one for nortriptyline to be taken at bedtime. In each case, one drug was active and the other was a placebo. To maintain the blind condition, the third party adjusting nortriptyline doses also gave instructions to adjust the placebo nortriptyline.
Response was defined as a 50% or greater improvement in Hamilton depression scale score. Remission required a final Hamilton depression score of 8 or less. The intent-to-treat analysis of response and remission included in the denominator all patients who began treatment and carried forward the last Hamilton depression scale rating observed. Differences in the two study groups were assessed with t tests, chi-square analysis, or Fisher’s exact test when appropriate. Change in mean Hamilton depression scale scores was assessed with analysis of variance controlling for site.
RESULTS
Ninety-two subjects who met the study criteria were recruited. During the 2-week placebo lead-in phase, 11 patients were withdrawn. Eight patients were noncompliant with procedures, one abused alcohol, and two experienced cardiac changes (in one patient the QRS interval exceeded the entrance criteria, and one patient developed premature ventricular contractions).
Eighty-one patients were randomly assigned to treatment, 41 to paroxetine and 40 to nortriptyline (Table 1). The study group included 67 men and 14 women. Their mean age was 57.8 years (SD=–11.8, range=33–84). Seventy percent of the patients had recurrent depression. On average, their first episode had occurred 11.3 years (SD=–13.2) earlier. Sixty-four percent had had prior pharmacotherapy or ECT. On entry into the study, their mean Hamilton depression scale score was 22.6 (SD=5.0), and at week 0 it was 21.4 (SD=5.1). There were no significant differences between the two treatment groups on any of the demographic variables or on any of the variables related to their history of depression.
The cardiac history of the patients did not differ in the two groups (Table 2). Briefly, 67% had had a previous myocardial infarction, 36% had had bypass surgery, and 41% had had angioplasty. The mean left ventricular ejection fraction was 59%. In terms of their functional classification, 56% were in class I (asymptomatic), and 44% were in class II or III.
Sixty-three patients completed treatment. Reasons for discontinuation are shown in Table 3. Significantly more patients discontinued treatment with nortriptyline than with paroxetine.
Twenty-six (63%) of the paroxetine patients continued to take 20 mg/day of the drug to the end of the trial. The dose was increased to 30 mg/day for 10 patients. One patient received 40 mg/day during week 5, then returned to 30 mg/day. The mean final dose was 22 mg/day. The mean final dose of nortriptyline was 73 mg/day (range=50–125). Eighty-one percent of the 26 patients who continued to take nortriptyline achieved at week 6 a blood level between 50 and 150 ng/ml, the established therapeutic range for nortriptyline (11). The numbers of patients receiving lorazepam—11 of the paroxetine patients and 16 of the nortriptyline patients—did not differ significantly between the two groups.
Both drugs were efficacious. For patients completing treatment, the mean change in Hamilton depression scale score (Figure 1) was –13.6 (SD=7.5), or 64%, among the patients receiving paroxetine and –16.2 (SD=5.8), or 76%, among the patients receiving nortriptyline. If similar measures are determined for all patients who began treatment, by using the last observation carried forward, the mean decrease in Hamilton depression scale score was –12.7 (SD=–7.8), or 60%, for the paroxetine group and –13.1 (SD=7.4), or 61%, for the nortriptyline group. Neither difference was significant.
With use of 50% improvement in Hamilton depression scale score to define response, 27 (73%) of 37 paroxetine patients and 24 (92%) of 26 nortriptyline patients completed the trial and responded (χ2=2.55, df=1, p=0.07). For all patients starting treatment, response rates were 66% (N=27 of 41) with paroxetine and 73% (N=29 of 40) with nortriptyline (χ2=0.17, df=1, n.s.). Rates of remission, defined as a Hamilton depression score of 8 or less, were 68% (N=25 of 37) for patients completing treatment with paroxetine and 85% (N=22 of 26) for those completing treatment with nortriptyline (χ2=0.82, df=1, n.s.). For all patients starting treatment, 61% (N=25 of 41) of the paroxetine patients and 63% (N=25 of 40) of the nortriptyline patients had a remission, rates that are very similar.
A number of individual Hamilton depression scale items, including depressed mood, suicidality, agitation, retardation, anxiety, cognitive changes, and sleep, were examined. There were no differences between the two drugs in terms of their effects on these items.
More patients taking nortriptyline than patients taking paroxetine withdrew from the study (Table 3). Adverse events were more common in the patients taking nortriptyline than in those taking paroxetine, and this appeared to explain the difference in withdrawal.
The two adverse events resulting in discontinuation by patients taking paroxetine were diarrhea and angina. In the 10 patients taking nortriptyline who discontinued, three had noncardiac side effects (intractable constipation in two and persistent myoclonic jerks in one). The other seven had cardiovascular problems, including sinus tachycardia (>120 bpm) in four patients, severe angina associated with ST changes on ECG in one, and asymptomatic increase in ventricular ectopy meeting criteria for a proarrhythmic event in two. The rate of cardiovascular events leading to discontinuation was significantly higher with nortriptyline than with paroxetine (p<0.03, Fisher’s exact test, two-tailed).
DISCUSSION
Both treatments were effective in this group of moderately depressed patients with ischemic heart disease. Sixty-three percent of all patients beginning treatment had at least 50% improvement in Hamilton depression scale scores. In addition, more than 90% of those with 50% improvement met the criterion for remission. Although a treatment duration of 6 weeks might be challenged as too short, especially for older patients, the high rate of remission suggests that the length of treatment was adequate for most patients.
Overall, the two drugs appeared very similar in their effectiveness. There was a suggestion of greater efficacy for nortriptyline in patients completing treatment (92%, versus 73% for paroxetine), but this in part reflected the higher dropout rate with nortriptyline. The actual numbers of patients who responded in the two groups, 27 and 29, were quite similar, and the numbers of patients with remission in the two groups, 25, were identical.
The results of the current study differ from those previously reported by one of us (S.R.) (4). In that study fluoxetine was not as effective as nortriptyline in depressed patients with heart disease. The subjects in that study, however, were severely depressed inpatients, and the majority were melancholic. In the current study, the subjects were moderately depressed and were outpatients. It is possible that differences in the effectiveness of tricyclic antidepressants and SSRIs are limited to more severely ill, melancholic patients. As in many other studies of depressed patients with heart disease, most of the patients in this study were men, and we would be cautious in generalizing from our data to conclusions about the treatment of depressed women with heart disease.
The primary difference between the two drugs was what happened to the patients for whom treatment was unsuccessful. For patients taking paroxetine, most nonresponders (N=10 of 14) completed treatment. For those taking nortriptyline, only two nonresponding patients completed treatment, but 35% (N=14) discontinued treatment early. Of these 14 patients, more than two-thirds (N=10) had an adverse event. Five patients taking nortriptyline who were judged responders at termination discontinued treatment early. Because most patients require continuation treatment for several months to prevent relapse, it is doubtful that brief treatment, resulting in early response but interrupted by adverse effects, can be considered successful.
Patients taking nortriptyline had significantly more cardiovascular adverse events. A detailed description of the cardiovascular findings has been previously reported (14). The most frequent adverse effect was tachycardia. For the entire study group taking nortriptyline, both the mean supine and the mean standing pulse rose 9 bpm at week 2. At 6 weeks the rise in pulse rate was essentially identical. Although most patients subjectively tolerated this change, an increased pulse rate may pose a serious long-term risk in patients with ischemic heart disease. The increased heart rate induced by a tricyclic antidepressant does not appear to accommodate with time (15, 16) and results in increased cardiac work. Other studies indicate that a prolonged increase in heart rate is associated with increased mortality in patients with ischemic heart disease (17–19). In this study paroxetine had no consistent significant effects on any of the pulse or blood pressure measures. One patient taking paroxetine experienced angina, but subsequent angiography indicated 90% occlusion of the circumflex artery; angioplasty was performed with good results. The patient continued to take paroxetine throughout.
The outcomes in this comparison are a good example of the value of considering intent-to-treat rates in the assessment of outcome. An overreliance on efficacy data might lead to the conclusion that nortriptyline is more effective. In fact, the actual numbers of patients responding or achieving remission were very similar for the two compounds. The primary difference in outcome was the greater number of patients who discontinued treatment with nortriptyline. This had the effect of reducing the denominator used in the determination of efficacy. An intent-to-treat approach to outcome provides a useful perspective for the clinician and the patient. It provides a simple estimate of the chance of response in all patients who start treatment. The efficacy rate, alternatively, requires two estimates: the chance of completing treatment and the chance of responding if the trial is completed.
To our knowledge, this is the first tricyclic antidepressant-SSRI comparison to adjust the tricyclic antidepressant dose to achieve a therapeutic plasma level. Although doing this added a layer of complexity to the study, it was feasible, and 81% of the patients achieved a plasma level within the established range.
This study did not address the question of whether antidepressant treatment will reduce mortality in depressed patients after myocardial infarction. It does suggest that depressed patients with ischemic heart disease can be treated effectively and safely, and it suggests that paroxetine is as effective as nortriptyline but less likely to produce serious side effects.
Received April 28, 1998; revision received Nov. 5, 1998; accepted Dec. 15, 1998. From the Department of Psychiatry, Yale University School of Medicine; the Department of Psychiatry, Vanderbilt University School of Medicine, Nashville, Tenn.; the Department of Psychiatry, University of Pittsburgh School of Medicine; the Department of Psychiatry, College of Physicians and Surgeons, Columbia University, New York; and the Department of Clinical Research and Development, SmithKline Beecham Pharmaceuticals, Collegeville, PA. Address reprint requests to Dr. Nelson, Yale-New Haven Hospital, 20 York St., New Haven, CT 06504. Supported in part by a grant from SmithKline Beecham Pharmaceuticals.
 |
 |
 |
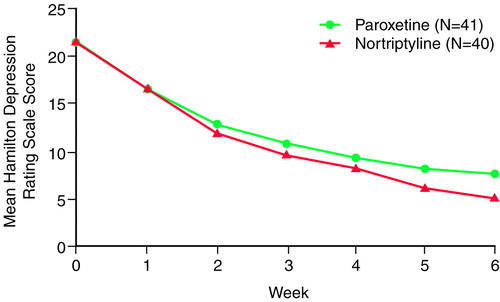
FIGURE 1. Hamilton Depression Rating Scale Scores of Patients With Heart Disease in a 6-Week Trial of Paroxetine and Nortriptyline
1. Glassman AH, Shapiro PA: Depression and the course of coronary artery disease. Am J Psychiatry 1998; 155:4–11Link, Google Scholar
2. Pratt LA, Ford DE, Crum RM, Armenian HK, Gallo JJ, Eaton WW: Depression, psychotropic medication, and risk of myocardial infarction. Circulation 1996; 94:3123–3129Google Scholar
3. Frazure-Smith N, Lesperance F, Talajic M: Depression following myocardial infarction: impact on 6-month survival. JAMA 1993; 270:1819–1825Google Scholar
4. Roose SP, Glassman AH, Attia E, Woodring S: Comparative efficacy of selective serotonin reuptake inhibitors and tricyclics in the treatment of melancholia. Am J Psychiatry 1994; 151:1735–1739Google Scholar
5. Glassman AH, Roose SP, Bigger JT Jr: The safety of tricyclic antidepressants in cardiac patients: risk/benefit reconsidered. JAMA 1993; 269:2673–2675Google Scholar
6. Roose SP, Glassman AH, Attia E, Woodring S, Giardina E-GV, Bigger JT Jr: Cardiovascular effects of fluoxetine in depressed patients with heart disease. Am J Psychiatry 1998; 155:660–665Link, Google Scholar
7. Ray WA, Griffin MR, Avorn J: Evaluating drugs after their approval for clinical use. N Engl J Med 1993; 329:2029–2032Google Scholar
8. Roose SP, Glassman AH, Giardina E-GV, Johnson LL, Walsh BT, Woodring S, Bigger JT Jr: Nortriptyline in depressed patients with left ventricular impairment. JAMA 1986; 256:3253–3257Google Scholar
9. Roose SP, Glassman AH, Siris SG, Walsh BT, Bruno RL, Wright LB: Comparison of imipramine- and nortriptyline-induced orthostatic hypotension: a meaningful difference. J Clin Psychopharmacol 1981; 1:316–319Crossref, Medline, Google Scholar
10. Thayssen P, Bjerre M, Kragh-Sorenson P, Moller M, Petersen OL, Kristensen CB, Gram LF: Cardiovascular effect of imipramine and nortriptyline in elderly patients. Psychopharmacology (Berl) 1981; 74:360–364Crossref, Medline, Google Scholar
11. American Psychiatric Association Task Force on the Use of Laboratory Tests in Psychiatry: Tricyclic antidepressants—blood level measurements and clinical outcome: an APA task force report. Am J Psychiatry 1985; 142:155–162Link, Google Scholar
12. Nelson JC: Importance of intent-to-treat response rates for the clinician. Psychiatr Annals 1996; 26:8–18Crossref, Google Scholar
13. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
14. Roose SP, Laghrissi-Thode F, Kennedy JS, Nelson JC, Bigger JT Jr, Pollock BG, Gaffney A, Narayan M, Finkel MS, McCafferty J, Gergel I: A comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. JAMA 1998; 279:287–291Crossref, Medline, Google Scholar
15. Rosenstein DL, Nelson JC: Heart rate during desipramine treatment as an indicator of beta1-adrenergic function (abstract). Biol Psychiatry 1991; 29(suppl):132AGoogle Scholar
16. Glassman AH, Bigger JT Jr: Cardiovascular effects of therapeutic doses of tricyclic antidepressants. Arch Gen Psychiatry 1981; 38:815–820Crossref, Medline, Google Scholar
17. Dyer AR, Persky V, Stamler J, Paul O, Shekelle RB, Berkson DM, Lepper M, Schoenberger JA, Lindberg HA: Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol 1980; 112:736–749Crossref, Medline, Google Scholar
18. Kannel WB, Kannel C, Paffenberger RS Jr, Cupples LA: Heart rate and cardiovascular mortality: the Framingham study. Am Heart J 1987; 113:1489–1494Google Scholar
19. Gillum RF, Makuc DM, Feldman JJ: Pulse rate, coronary heart disease, and death: the NHANES I epidemiologic follow-up study. Am Heart J 1991; 121:172–177Crossref, Medline, Google Scholar



