Prospective Study of Risk Factors for Alzheimer’s Disease: Results at 7.5 Years
Abstract
OBJECTIVE: The primary goal of this study was to evaluate increased platelet membrane fluidity as a putative risk factor for Alzheimer’s disease and its relationship to the APOE ε4 genotype. METHOD: This report describes the results of a prospective, longitudinal study of 330 initially asymptomatic, first-degree relatives of probands with Alzheimer’s disease. RESULTS: Nine incident cases of Alzheimer’s disease were detected during the first 2,220 subject-years of the follow-up period. Age, increased platelet membrane fluidity, and the APOE ε4 allele made significant independent contributions to the risk of developing Alzheimer’s disease, while sex and years of education did not. Increased platelet membrane fluidity was associated with incident Alzheimer’s disease cases between the ages of 64 and 71, while the ε4 allele was associated with incident Alzheimer’s disease cases from age 64 until at least age 80. CONCLUSIONS: These results indicate that increased platelet membrane fluidity is not produced by the APOE ε4 allele. Instead, increased platelet membrane fluidity and the ε4 allele appear to make significant independent contributions to the risk of developing Alzheimer’s disease among the first-degree relatives of patients with this disorder. Moreover, the age ranges over which these risk factors operate appear to be different.
An association of increased platelet membrane fluidity, as reflected by the fluorescence anisotropy of 1,6-diphenyl-1,3,5-hexatriene (DPH) in labeled membranes, with Alzheimer’s disease was originally reported for clinical populations recruited in Boston and Pittsburgh (1, 2). This finding has been replicated by several research groups during the last decade, all of which recruited subjects from clinical populations in their respective locations on three continents (3–8). In aggregate, these published studies have included approximately 500 patients and control subjects and have incorporated strategies to control for the potential effects of age, sex, concurrent medical conditions, and medication exposure.
Available evidence indicates that increased platelet membrane fluidity is relatively specifically associated with Alzheimer’s disease among the mental disorders that produce dementia in late life (2, 3, 9) and that patients who manifest this characteristic may represent a clinically distinct subset of those with Alzheimer’s disease (2, 10–12). This membrane phenotype appears to be a stable trait that is vertically transmitted in families of patients with Alzheimer’s disease (13, 14). Complex segregation analysis suggests that increased platelet membrane fluidity is controlled by the inheritance of a Mendelian single major locus (15). At the cellular level, evidence from ultrastructural (5, 16), biochemical (5, 17, 18), and biophysical studies (6, 14) suggests that increased platelet membrane fluidity in Alzheimer’s disease results from the elaboration of an internal membrane compartment resembling endoplasmic reticulum that is functionally abnormal.
The association of the APOE ε4 allele with Alzheimer’s disease has been widely established (19–28). In addition to their association with both sporadic and familial forms of Alzheimer’s disease that have typical ages at onset, other similarities between the APOE and PMF loci have emerged. The APOE locus is the structural gene for apolipoprotein E (29), a cholesterol-carrying protein, while the increased platelet membrane fluidity phenotype arises, at least in part, from a reduction in platelet membrane cholesterol (17). Both the APOE and the PMF loci are autosomal genes, whose alleles are additively expressed in peripheral cells (15, 29). Individuals who are heterozygous for PMF alleles that confer increased or normal platelet membrane fluidity have fluidity values that are intermediate between those of either homozygous individual (15). Increased platelet membrane fluidity (2, 10, 30) and the number of APOE ε4 alleles (31, 32) have been reported to affect age at onset of prototypic forms of Alzheimer’s disease in most, but not all (4, 26, 33), patient populations. Both antemortem platelet membrane fluidity values (34) and the number of APOE ε4 alleles (26, 33, 35) appear to be correlated with the density of senile plaques in brain tissue obtained from the autopsies of patients with confirmed Alzheimer’s disease, with smaller or no associations with the density of neurofibrillary tangles. Finally, the ε4 allele has been reported to confer increased risk of developing Alzheimer’s disease-like dementia among elders with mild cognitive impairment (36) and of developing dementia, but not necessarily Alzheimer’s disease, among the participants in the Framingham study (37).
This body of evidence raised the possibility that increased platelet membrane fluidity might be associated with an increased risk of developing Alzheimer’s disease, at least during the earlier part of the typical age at onset (65<onset≤75 years). To test this hypothesis, we embarked on a prospective, longitudinal study of 330 cognitively intact individuals between the ages of 40 and 75 years, who were first-degree relatives of probands with Alzheimer’s disease, all of whom were typed for platelet membrane fluidity at the time of recruitment. Subject selection was designed to include individuals who represented a wide age range so that the effects of an age-specific risk factor would be observable. By recruiting a cohort of individuals whose overall incidence rate of developing Alzheimer’s disease would be expected to be severalfold higher than that in a community sample by virtue of their family history of Alzheimer’s disease (38–42), we anticipated that the total number of subject-years of follow-up required to test our hypothesis would be practical. Transformed cell lines from these individuals also permitted us to explore the relationship of the APOE ε4 allele to the platelet membrane fluidity phenotype, as well as to determine whether the ε4 allele affected the risk of developing Alzheimer’s disease among these cognitively intact individuals. This report describes the results of this longitudinal study after 7.5 years of systematic follow-up.
METHOD
Recruitment of Alzheimer’s Disease High-Risk Cohort
The Alzheimer’s disease high-risk cohort consisted of 330 first-degree relatives of 189 demented probands who met the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (43) for possible (N=35), probable (N=103), or definite (N=6) Alzheimer’s disease or DSM-III-R criteria for primary degenerative dementia of the Alzheimer’s type (N=45) or both. Members of the cohort were between the ages of 40 and 75 and were cognitively intact at the time of recruitment, as determined by a review of medical and psychiatric history, a survey of current activities of daily living, a mental status examination, and the Mini-Mental State (44). Subjects were excluded if they had any clinical evidence of cognitive decline or impairment, a Mini-Mental State score of less than 27, or medical problems or were taking medications known to affect the determination of platelet membrane fluidity (2). For 303 of the 330 subjects, the Mini-Mental State was augmented by the more detailed Mattis Dementia Rating Scale (45), and all individuals tested had Mattis Dementia Rating Scale scores in the range expected for healthy elderly control subjects (135 or higher). All members of the cohort provided written informed consent for participation in the study, which was approved by the Biomedical Institutional Review Board of the University of Pittsburgh.
Longitudinal Surveillance Protocol
Semistructured telephone assessments were initiated on March 1, 1989, and were performed approximately annually by a mental health nurse with specialized experience in geriatrics who remained blind to the laboratory data. The telephone assessments included a review of the items on the Family History Form of Huff and colleagues (10, 42) and a survey of the subjects’ activities of daily living. Subjects and their identified “best” informants were encouraged to contact the research nurse if any evidence of a change in mental functioning occurred during the interim. To determine the sensitivity and specificity of the telephone assessments for the detection of individuals with newly emergent cognitive impairment, 202 members of the Alzheimer’s disease high-risk cohort were also evaluated by in-person interviews. The telephone assessments detected subjects whose Clinical Dementia Rating scores (46) exceeded 0 with a sensitivity of 82% and a specificity of 98%. These cross-sectional determinations seem likely to underestimate the sensitivity and specificity of the telephone assessments for identifying cases of progressive dementia when administered longitudinally over a period of years.
In-person assessments were performed when evidence suggestive of emergent cognitive dysfunction was reported during the telephone screen. If the presence of cognitive impairment suggested by the telephone screen was supported by a Mini-Mental State score of less than 27, a Mattis Dementia Rating Scale score of less than 135, a Clinical Dementia Rating score greater than 0, or other clinical evidence obtained during the in-person evaluation, the subject was referred for a complete diagnostic evaluation. If no such evidence was detected, the frequency of the periodic telephone follow-up was increased to approximately every 6 months. Consensus clinical diagnoses of Alzheimer’s disease among incident cases of dementia were established according to DSM-III-R criteria, as applied by two board-certified psychiatrists with added qualifications in geriatric psychiatry. Autopsies were performed by board-certified neuropathologists, and neuropathologic diagnoses were made according to generally accepted criteria (47–49).
Laboratory Methods
Platelets were qualitatively isolated from blood samples of fasting patients; blood samples were obtained at the time of recruitment (2, 16). Platelet membranes were prepared from coded samples, labeled with DPH, and were examined by fluorescence spectroscopy as previously described (2, 16). Increased platelet membrane fluidity was defined by a steady-state anisotropy value of less than 0.1920 at 37.0˚C for DPH-labeled membranes.
Genomic DNA was isolated from lymphocytes or lymphoblasts by using minor modifications of standard methods (50, 51). Genotyping of the APOE locus was performed through use of polymerase chain reaction methodology and the oligonucleotide primers described by Hixson and Vernier (52). The radiolabeled products were digested with endonuclease HhaI, and the resulting DNA fragments were resolved by electrophoresis and visualized by autoradiography. Allele assignments were made as previously described (26, 30). Subtyping for platelet membrane fluidity and genotyping for APOE were performed by laboratory staff who were blind to sociodemographic and clinical information.
Statistical Analysis
The database for this project was frozen on Aug. 31, 1996. Continuous variables were compared through use of the t statistic. Categorical variables were compared with the chi-square statistic or Fisher’s exact test, as appropriate. Survival analysis employing the Kaplan-Meier product limit method and Cox proportional hazards models with covariates was performed by using the 1L and 2L programs from the BMDP Statistical Software package (1990 release).
RESULTS
A description of the Alzheimer’s disease high-risk cohort and the subgroups with increased and normal platelet membrane fluidity is provided in table 1. The cohort was composed almost entirely of Caucasians and included approximately equal proportions of men and women. The subjects had a mean age of 56.3 years at the time of entry into the longitudinal study and a mean age of 63.4 years at the time of their most recent follow-up assessment (effective Aug. 31, 1996; 7.5 years of follow-up). Consistent with the criteria for entry into the study, all subjects were cognitively intact as reflected by a mean Mini-Mental State score approaching 30. A total of 75 members (23.0%) of the cohort had increased platelet membrane fluidity. There were slightly more men than women in this subgroup; subjects in the subgroup were similar in mean age at entry and had completed a mean number of years of education that was similar to that of the subgroup with normal platelet membrane fluidity. The platelet membrane fluidity subgroups did not differ significantly with respect to their duration of follow-up or their cognitive performance as determined by mean Mini-Mental State scores.
As expected, the frequency of the APOE ε4 allele in the Alzheimer’s disease high-risk cohort, 0.27 (table 1), was midway between the frequencies previously reported for autopsy-confirmed cases of Alzheimer’s disease, 0.41, and elderly control subjects, 0.13, evaluated at our medical center. The ε4 allele frequencies, as well as the proportions of individuals who carried the ε4 allele, were indistinguishable between the platelet membrane fluidity subgroups. These findings provided no evidence of an association between the APOE ε4 allele and increased platelet membrane fluidity. As a result, they were evaluated as independent variables in subsequent analyses of risk factors for Alzheimer’s disease.
A total of 51 of the 183 probands with clinically diagnosed Alzheimer’s disease were autopsied during the course of this study, and the diagnosis was histopathologically confirmed in 49 (96.1%) of the 51 cases. Of the two autopsied probands who did not meet histopathologic criteria for Alzheimer’s disease, one exhibited striatonigral degeneration and the other exhibited neuronal loss without findings to support a more specific diagnosis. To date, a total of 32 members (9.7%) of the Alzheimer’s disease high-risk cohort have been lost through attrition from all sources. Nineteen of these died, four were relatives of the two autopsied probands who did not meet histopathologic criteria for definite Alzheimer’s disease, five could not be reached for follow-up, and four subjects declined to participate further.
A total of 12 incident cases of perceived memory impairment were detected by our surveillance protocol during the first 2,220 subject-years of this longitudinal study. Three of these cases reflected subjective reports of memory impairment that occurred in the context of depressive or anxiety states, were not associated with objective evidence of cognitive impairment as determined by cognitive test performance or functional decline, and resolved concurrently with the remission of the transient emotional disturbances. Two of these individuals have remained actively engaged in business and community affairs, while the third died unexpectedly. The remaining nine individuals revealed objective evidence of cognitive impairment and developed progressive dementias that resulted in their inability to function independently. In all nine cases, medical and laboratory evaluations supported the diagnosis of Alzheimer’s disease. Following the death of one of these individuals, for whom concurrent cerebrovascular disease was detected clinically, an autopsy was performed, and the diagnosis of Alzheimer’s disease with multiple infarcts was determined by neuropathologic examination of the brain.
The age-specific cumulative incidence rates of Alzheimer’s disease for the platelet membrane fluidity subgroups are presented in figure 1. Increased platelet membrane fluidity was associated with a 5- to 10-year reduction in the age at onset of Alzheimer’s disease (Mantel-Cox statistic=16.01, df=1, p=0.0001; Breslow statistic=35.87, df=1, p<0.0001). Similarly, the mean age at onset of dementia occurred approximately 5 years earlier for the five incident Alzheimer’s disease cases with increased platelet membrane fluidity (mean=67.2 years, SD=2.6) than for those with normal platelet membrane fluidity (mean=72.5 years, SD=3.3) (t=2.7, df=7, p=0.03). The effect of this risk factor was restricted to incident Alzheimer’s disease cases in which symptomatic onsets occurred between the ages of 64 and 71.
The age-specific cumulative incidence rates of Alzheimer’s disease for the APOE ε4 carriers and noncarriers are presented in figure 2. As with increased platelet membrane fluidity, the ε4 allele was associated with a significant increase in the age-specific cumulative incidence of Alzheimer’s disease in the high-risk cohort (Mantel-Cox statistic=6.23, df=1, p=0.01; Breslow statistic=5.44, df=1, p=0.02). In contrast to the findings for increased platelet membrane fluidity, the ε4 allele was associated with an increased incidence of Alzheimer’s disease over a wider age range, from 64 until at least 80 years of age.
A best-fitting multivariate Cox proportional hazards model was developed to determine the independent effects of having increased platelet membrane fluidity and carrying an APOE ε4 allele on the risk of developing Alzheimer’s disease, with control for the potential effects of age, sex, and education (global χ2=25.57, df=5, p=0.0001). As shown in table 2, age, platelet membrane fluidity subtype, and the ε4 genotype all made significant independent contributions to the prediction of Alzheimer’s disease in this population, with risk ratios of 1.2, 6.7, and 11.4, respectively. Although gender has sometimes been reported to be associated with greater risk for Alzheimer’s disease (for review see reference 53), sex did not emerge as a significant risk factor for Alzheimer’s disease in the high-risk cohort during the first 2,220 subject-years of follow-up. Similarly, years of education did not make a significant contribution to the prediction of Alzheimer’s disease, although limited educational experience has been reported to be a risk factor for Alzheimer’s disease in some (54, 55) but not all (56, 57) epidemiologic studies.
DISCUSSION
Increased platelet membrane fluidity and the inheritance of an APOE ε4 allele emerged as significant independent risk factors for Alzheimer’s disease in our high-risk cohort during the first 2,220 subject-years of follow-up. Both factors appeared to be associated with substantial increases in risk, as reflected by risk ratios of 6.7 and 11.4, respectively. The risk ratio of 6.7 associated with increased platelet membrane fluidity is similar to the 7.7 value observed by the end of the first 5 years of follow-up (30). The risk ratio of 11.4 associated with carrying an APOE ε4 allele among the subjects in our study was approximately 2.5 times the corresponding value of 4.4 reported from a prospective study of elderly subjects with mild cognitive impairment (36) and approximately three times the corresponding value of 3.7 for ε4/ε3 heterozygous individuals enrolled in the Framingham study (37). These results suggest that the relative risk of developing Alzheimer’s disease associated with carrying an APOE ε4 allele may be greater among the first-degree relatives of patients with Alzheimer’s disease, who may carry additional Alzheimer’s disease risk alleles, than among elderly subjects with mild cognitive impairment or individuals chosen at random from the community.
Age at onset of dementia has emerged as an important descriptive variable for classifying genes that influence the development of Alzheimer’s disease (34). Although they are uncommon, the majority of cases of Alzheimer’s disease that produce symptoms before the age of 60 appear to arise from highly penetrant, autosomal dominant genetic lesions. These include mutations in the structural genes for the amyloid precursor protein located on chromosome 21 (58–61), presenilin located on chromosome 14 (62–70), and presenilin 2 located on chromosome 1 (71–73), as well as trisomy 21 (74). The development of more typical, later-onset forms of Alzheimer’s disease appears to be influenced by at least four genes including APOE(20, 21, 31, 75), an anonymous risk locus in the vicinity of the centromere on chromosome 12 (76), an anonymous risk locus on the long arm of the X chromosome (77), and an anonymous locus that controls approximately 80% of the variance in platelet membrane fluidity (15). Evidence from a recent genome survey employing batched genotyping methods for typing pools of DNA from autopsied Alzheimer’s disease cases and control subjects suggests that there may be several dozen chromosomal loci that influence the risk of developing Alzheimer’s disease (78). Mutations in selected mitochondrial genes, including those that encode two catalytic subunits of cytochrome oxidase, may also contribute to the determination of age at onset and lifetime risk of Alzheimer’s disease (79, 80).
The results of this study suggest that alleles that confer risk of developing Alzheimer’s disease at more typical ages of symptomatic onset after age 60 may not exert their effects evenly throughout the entire age of risk. Increased platelet membrane fluidity was associated with incident cases of Alzheimer’s disease with symptomatic onsets between ages 64 and 71, following which the increase in the age-specific cumulative incidence of Alzheimer’s disease associated with this phenotype appeared to dissipate. This observation recapitulates the results of previously published cross-sectional clinical studies and family history studies of the role of platelet membrane fluidity in Alzheimer’s disease (2, 9, 10). In contrast, the APOE ε4 allele was associated with incident cases of Alzheimer’s disease across the age of risk beginning at age 64 until at least 80. The latter observation is consistent with a previous study that found that the age at onset of memory problems had no effect on the association between the ε4 alleles and Alzheimer’s disease (81). However, the results of Rebeck and co-workers (33) reflect a weakening of this association among individuals with Alzheimer’s disease whose symptomatic onset occurs after age 80. These results suggest that the dichotomous classification of Alzheimer’s disease susceptibility genes into early- and late-onset categories may warrant revision, as additional risk alleles for typical-onset cases of Alzheimer’s disease are identified and characterized.
Several limitations of our study warrant further discussion. Our risk ratio estimates, while highly significant, rely on the emergence of nine incident cases of Alzheimer’s disease. Additional incident cases will be required to produce stable estimates of risk with narrower 95% confidence intervals and to permit the cohort to serve as a resource for detecting risk factors that have more modest but still clinically relevant effect sizes. Furthermore, the Alzheimer’s disease high-risk cohort was composed entirely of first-degree relatives who were almost exclusively Caucasians. These features may affect the generalizability of our results to other populations.
Received Dec. 24, 1997; revision received April 30, 1998; accepted June 19, 1998. From the Departments of Psychiatry, Neurology, and Pathology (Neuropathology), University of Pittsburgh School of Medicine, Pittsburgh; the Department of Biological Sciences, Carnegie-Mellon University, Pittsburgh; and the Geriatric Psychiatry Branch, NIMH, Bethesda, Md. Address reprint requests to Dr. Zubenko, Western Psychiatric Institute and Clinic, Rm. E-1230, 3811 O’Hara St., Pittsburgh, PA 15213. Supported by NIMH research project grant MH-43261, National Institute on Aging Alzheimer Disease Research Center grant AG-05133, NIMH Clinical Research Center grant MH-30915, and by NIMH Independent Scientist Award MH-00540 to Dr. Zubenko. The authors thank Michelle Pfeifer, Dawna Ruhe, and Dr. John Moossy for their contributions.
 |
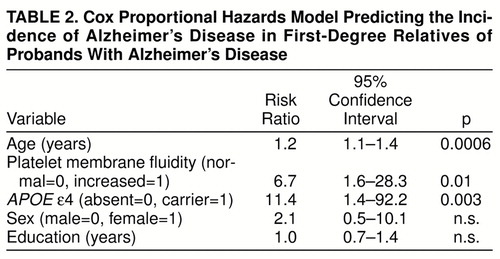 |
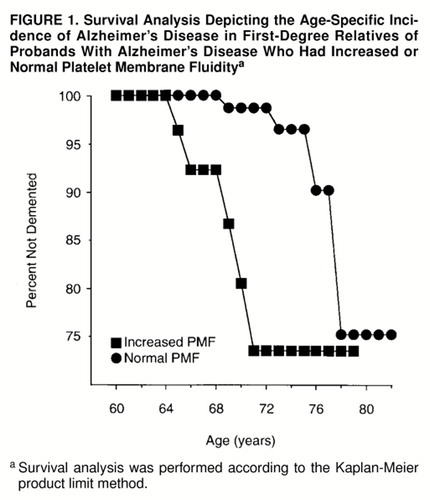
FIGURE 1. Survival Analysis Depicting the Age-Specific Incidence of Alzheimer’s Disease in First-Degree Relatives of Probands With Alzheimer’s Disease Who Had Increased or Normal Platelet Membrane Fluiditya
aSurvival analysis was performed according to the Kaplan-Meier product limit method.
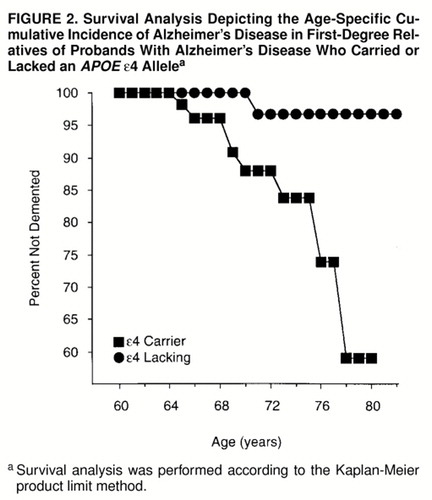
FIGURE 2. Survival Analysis Depicting the Age-Specific Cumulative Incidence of Alzheimer’s Disease in First-Degree Relatives of Probands With Alzheimer’s Disease Who Carried or Lacked an APOE ε4 Allelea
aSurvival analysis was performed according to the Kaplan-Meier product limit method.
1. Zubenko GS, Cohen BM, Growden J, Corkin S: Cell membrane abnormality in Alzheimer’s disease (letter). Lancet 1984; 2:235Crossref, Medline, Google Scholar
2. Zubenko GS, Cohen BM, Reynolds CF III, Boller F, Malinakova I, Keefe N: Platelet membrane fluidity in Alzheimer’s disease and major depression. Am J Psychiatry 1987; 144:860–868Link, Google Scholar
3. Hicks N, Brammer MJ, Hymas N, Levy R: Platelet membrane properties in Alzheimer and multi-infarct dementias. Alz Dis Assoc Disord 1987; 1(suppl 2):90–97Google Scholar
4. Eagger S, Hajimohammadreza I, Fletcher K, Levy R, Brammer M: Platelet membrane fluidity, family history, and severity and age of onset in Alzheimer’s disease. Int J Geriatr Psychiatry 1990; 5:395–400Crossref, Google Scholar
5. Hajimohammadreza I, Brammer MJ, Eagger S, Burns A, Levy R: Platelet and erythrocyte membrane changes in Alzheimer’s disease. Biochim Biophys Acta 1990; 1025:208–214Crossref, Medline, Google Scholar
6. Piletz JE, Sarasua M, Whitehouse P, Chotani M: Intracellular membranes are more fluid in platelets of Alzheimer’s disease patients. Neurobiol Aging 1991; 12:401–406Crossref, Medline, Google Scholar
7. van Rensburg SJ, Carstens ME, Potocnik FCV, Aucamp AK, Taljaard JF, Koch KR: Membrane fluidity of platelets and erythrocytes in patients with Alzheimer’s disease and the effect of small amounts of aluminum on platelet and erythrocyte membranes. Neurochem Res 1992; 17:825–829Crossref, Medline, Google Scholar
8. K�lm�n J, Dey I, Ilona SV, Matkovics B, Brown D, Janka Z, Farkas T, Jo� F: Platelet membrane fluidity and plasma malondialdehyde levels in Alzheimer’s demented patients with and without family history of dementia. Biol Psychiatry 1994; 35:190–194Crossref, Medline, Google Scholar
9. Zubenko GS: Biological correlates of clinical heterogeneity in primary dementia. Neuropsychopharmacology 1992; 6(suppl 2):77–93Google Scholar
10. Zubenko GS, Huff FJ, Beyer J, Auerbach J, Teply I: Familial risk of dementia associated with a biologic subtype of Alzheimer’s disease. Arch Gen Psychiatry 1988; 45:889–893Crossref, Medline, Google Scholar
11. Zubenko GS, Brenner RP, Teply I: Electroencepholographic correlates of increased platelet membrane fluidity in Alzheimer’s disease. Arch Neurol 1988; 45:1009–1013Crossref, Medline, Google Scholar
12. Zubenko GS, Brenner RP, Teply I: Risk factors for stroke as predictors of platelet membrane fluidity in Alzheimer’s disease. Stroke 1991; 22:997–1003Crossref, Medline, Google Scholar
13. Zubenko GS, Teply I: Longitudinal study of platelet membrane fluidity in Alzheimer’s disease. Biol Psychiatry 1988; 24:918–924Crossref, Medline, Google Scholar
14. Zubenko GS, Wusylko M, Cohen BM, Boller F, Teply I: Family study of platelet membrane fluidity in Alzheimer’s disease. Science 1987; 238:539–542Crossref, Medline, Google Scholar
15. Chakravarti A, Slaugenhaupt SA, Zubenko GS: Inheritance pattern of platelet membrane fluidity in Alzheimer’s disease. Am J Hum Genet 1989; 44:799–805Medline, Google Scholar
16. Zubenko GS, Malinakova I, Chojnacki B: Proliferation of internal membranes in platelets from patients with Alzheimer’s disease. J Neuropathol Exp Neurol 1987; 46:407–418Crossref, Medline, Google Scholar
17. Cohen BM, Zubenko GS, Babb SM: Abnormal platelet membrane composition in Alzheimer’s-type dementia. Life Sci 1987; 40:2445–2451Crossref, Medline, Google Scholar
18. Zubenko GS: Endoplasmic reticulum abnormality in Alzheimer’s disease: selective alteration in platelet NADH-cytochrome C reductase activity. J Geriatr Psychiatry Neurol 1989; 2:3–10Crossref, Medline, Google Scholar
19. Noguchi S, Murakami K, Yamada N: Apolipoprotein E genotype and Alzheimer’s disease (letter). Lancet 1993; 342:737Crossref, Medline, Google Scholar
20. Saunders AM, Strittmatter WJ, Schmechel D, St George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-McLachlan DR, Alberts MJ, Hulette C, Crain B, Goldgaber D, Roses AD: Association of apolipoprotein E allele ε4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 1993; 43:1467–1472Crossref, Medline, Google Scholar
21. Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD: Apolipoprotein E: high-avidity binding to β-amyloid and increased frequency of type 4 allele in late onset familial Alzheimer disease. Proc Natl Acad Sci USA 1993; 90:1977–1981Crossref, Medline, Google Scholar
22. Ueki A, Kawano M, Namba Y, Kawakami M, Ikeda K: A high frequency of apolipoprotein E4 isoprotein in Japanese patients with late-onset nonfamilial Alzheimer’s disease. Neurosci Lett 1993; 163:166–168Crossref, Medline, Google Scholar
23. Chartier-Harlin M-C, Parfitt M, Legrain S, Perez-Tur J, Brousseau T, Evans A, Berr C, Vidal O, Roques P, Gourle V, Fruchart J-C, Delacourte A, Rossor M, Amouyel P: Apolipoprotein E, ε4 allele as a major risk factor for sporadic early and late-onset forms of Alzheimer’s disease: analysis of the 19q13.2 chromosomal region. Hum Mol Genet 1994; 3:569–574Crossref, Medline, Google Scholar
24. Dai XY, Nanko S, Hattori M, Fukuda R, Nagata K, Isse K, Ueki A, Kazamatsuri H: Association of apolipoprotein E4 with sporadic Alzheimer’s disease is more pronounced in early onset type. Neurosci Lett 1994; 175:74–76Crossref, Medline, Google Scholar
25. Yoshizawa T, Yamakawkobayashi K, Komatsuzaki Y, Arinami T, Oguni E, Mizusawa H, Shoji S, Hamaguchi H: Dose-dependent association of apolipoprotein E allele epsilon 4 with late-onset, sporadic Alzheimer’s disease. Ann Neurol 1994; 36:656–659Crossref, Medline, Google Scholar
26. Zubenko GS, Stiffler S, Stabler S, Kopp U, Hughes HB, Cohen BM, Moossy J: Association of the apolipoprotein E ε4 allele with clinical subtypes of autopsy-confirmed Alzheimer’s disease. Am J Med Genet Neuropsychiatr Genet 1994; 54:199–205Crossref, Medline, Google Scholar
27. Hendrie HC, Hall KS, Hui S, Unverzagt FW, Yu CE, Lahiri DK, Sahota A, Farlow M, Msick B, Class CA: Apolipoprotein E genotypes and Alzheimer’s disease in a community study of elderly African Americans. Ann Neurol 1995; 37:118–120Crossref, Medline, Google Scholar
28. Maestre G, Ottman R, Stern Y, Gurland B, Chun M, Tang MX, Shelanski M, Tycko B, Mayeux JR: Apolipoprotein E and Alzheimer’s disease: ethnic variation in genotypic risks. Ann Neurol 1995; 37:254–259Crossref, Medline, Google Scholar
29. Myklebost O, Rogne S: A physical map of the apolipoprotein gene cluster on human chromosome 19. Hum Genet 1988; 78:244–247Crossref, Medline, Google Scholar
30. Zubenko GS, Teply I, Winwood E, Huff FJ, Moossy J, Sunderland T, Martinez AJ: Prospective study of increased platelet membrane fluidity as a risk factor for Alzheimer’s disease: results at five years. Am J Psychiatry 1996; 153:420–423Link, Google Scholar
31. Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA: Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993; 261:921–923Crossref, Medline, Google Scholar
32. Payami H, Montee KR, Kaye JA, Bird TD, Yu C-E, Wijsman EM, Schellenberg GD: Alzheimer’s disease, apolipoprotein ε4, and gender. JAMA 1994; 271:1316–1317Crossref, Medline, Google Scholar
33. Rebeck GW, Reiter JS, Strickland DK, Hyman BT: Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron 1993; 11:575–580Crossref, Medline, Google Scholar
34. Zubenko GS: Molecular neurobiology of Alzheimer’s disease (syndrome?). Harvard Rev Psychiatry 1997; 5:177–213Crossref, Medline, Google Scholar
35. Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD: Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA 1993; 90:9649–9653Crossref, Medline, Google Scholar
36. Petersen RC, Smith GE, Ivnik RJ, Tangalos EG, Schaid DJ, Thibodeau SN, Kokmen E, Waing SC, Kurland L: Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA 1995; 273:1274–1278Crossref, Medline, Google Scholar
37. Myers RH, Schaefer EJ, Wilson PWF, D’Agostino R, Ordovas JM, Espino A, Au R, White RF, Knoefel JE, Cobb JL, McNulty KA, Beiser A, Wolf PA: Apolipoprotein E ε4 association with dementia in a population-based study: the Framingham study. Neurology 1996; 46:673–677Crossref, Medline, Google Scholar
38. Heston LL, Mastri AR, Anderson VE, White J: Dementia of the Alzheimer type: clinical genetics, natural history, and associated conditions. Arch Gen Psychiatry 1981; 38:1085–1090Crossref, Medline, Google Scholar
39. Heyman A, Wilkinson WE, Hurwitz BJ, Schmechel D, Sigmon AH, Weinberg T, Helms MJ, Swift M: Alzheimer’s disease: genetic aspects and associated clinical disorders. Ann Neurol 1983; 14:507–515Crossref, Medline, Google Scholar
40. Breitner JCS, Murphy EA, Folstein MF: Familial aggregation in Alzheimer dementia, II: clinical genetic implications of age-dependent onset. J Psychiatr Res 1986; 20:45–55Crossref, Medline, Google Scholar
41. Mohs RC, Breitner JCS, Silverman JM, Davis KL: Alzheimer’s disease: morbid risk among first-degree relatives approximates 50% by 90 years of age. Arch Gen Psychiatry 1987; 44:405–408Crossref, Medline, Google Scholar
42. Huff FJ, Auerbach J, Chakravarti A, Boller F: Risk of dementia in relatives of patients with Alzheimer’s disease. Neurology 1988; 38:786–790Crossref, Medline, Google Scholar
43. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer"s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer"s Disease. Neurology 1984; 34:939–944Crossref, Medline, Google Scholar
44. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
45. Mattis S: Mattis Organic Mental Syndrome Screening Examination (MOMSSE), in Geriatric Psychiatry: A Handbook for Psychiatrists and Primary Care Physicians. Edited by Bellak L, Karasu TB. New York, Grune & Stratton, 1976, pp 102–177Google Scholar
46. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL: A new clinical scale for the staging of dementia. Br J Psychiatry 1982; 140:566–572Crossref, Medline, Google Scholar
47. Tomlinson BE, Corsellis JAN: Aging and the dementias, in Greenfield’s Neuropathology, 4th ed. Edited by Adams HJ, Corsellis JAN, Duchen LW. New York, John Wiley & Sons, 1984, pp 951–1025Google Scholar
48. Khachaturian ZS: Diagnosis of Alzheimer’s disease. Arch Neurol 1985; 42:1097–1105Crossref, Medline, Google Scholar
49. Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L: The Consortium to Establish a Registry for Alzheimer"s Disease (CERAD), part II: standardization of the neuropathologic assessment of Alzheimer"s disease. Neurology 1991; 41:479–486Crossref, Medline, Google Scholar
50. Miller SA, Dykes DD, Polesky HF: A simple salting out procedure for extracting DNA from human nucleated cells. Nucl Acids Res 1988; 16:1215Crossref, Medline, Google Scholar
51. Zubenko GS, Stiffler S, Farr J, Kopp U, Hughes H, Kaplan BB, Moossy J: Lack of variation in the nucleotide sequence corresponding to the transmembrane domain of the β-amyloid precursor protein in Alzheimer’s disease. Am J Med Genet Neuropsychiatr Genet 1993; 48:131–136Crossref, Medline, Google Scholar
52. Hixson JE, Vernier DT: Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res 1990; 31:545–548Medline, Google Scholar
53. Amaducci L, Lippi A: Risk factors for dementia, in Dementia. Edited by Burns A, Levy R. London, Chapman & Hall, 1994, pp 129–141Google Scholar
54. Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R: Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 1994; 271:1004–1010Crossref, Medline, Google Scholar
55. Canadian Study of Health and Aging: Risk factors for Alzheimer’s disease in Canada. Neurology 1994; 44:2073–2080Crossref, Medline, Google Scholar
56. Fratiglioni L, Grut M, Forsell Y, Vitanen M, Grafstrom M, Holm�m K, Ericsson K, Backman L, Ahlbom A, Winblad B: Prevalence of Alzheimer’s disease and other dementias in an elderly urban population: relationship with age, sex, and education. Neurology 1991; 41:1886–1892Crossref, Medline, Google Scholar
57. Beard CM, Kokmen E, Offord KP, Kurland LT: Lack of association between Alzheimer’s disease and education, occupation, marital status, or living arrangement. Neurology 1992; 42:2063–2068Crossref, Medline, Google Scholar
58. Chartier-Harlin M-C, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, Goate A, Rossor M, Roques P, Hardy J, Mullan M: Early-onset Alzheimer’s disease caused by mutations at codon 717 of the β-amyloid precursor protein gene. Nature 1991; 353:844–846Crossref, Medline, Google Scholar
59. Goate AM, Chartier-Harlin CM, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, Mant R, Newton P, Rooke K, Roques P, Talbot C, Pericak-Vance M, Roses A, Williamson R, Rossor M, Owen M, Hardy J: Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 1991; 349:704–706Crossref, Medline, Google Scholar
60. Murrell J, Farlow M, Ghetti B, Benson MD: A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science 1991; 254:97–99Crossref, Medline, Google Scholar
61. Mullan M, Crawford F, Axelman K, Houlden H, Lulius L, Winblad B, Lannfelt L: A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of β-amyloid. Nature Genet 1992; 1:345–347Crossref, Medline, Google Scholar
62. Schellenberg GD, Bird TD, Wijsman EM, Orr HT, Anderson L, Nemens E, White JA, Bonnycastle L, Weber JL, Alonso ME, Potter H, Heston LL, Martin GM: Genetic linkage evidence for a familial Alzheimer’s disease locus on chromosome 14. Science 1992; 58:668–671Crossref, Google Scholar
63. St George-Hyslop P, Haines J, Rogaev E, Mortilla M, Vaula G, Pericak-Vance M, Foncin J-F, Montesi M, Bruni A, Sorbi S: Genetic evidence for a novel familial Alzheimer’s disease locus on chromosome 14. Nat Genet 1992; 2:330–334Crossref, Medline, Google Scholar
64. van Broeckhoven C, Backhovens H, Cruts M, de Winter G, Bruyland M, Cras P, Martin JJ: Mapping of a gene predisposing to early-onset Alzheimer’s disease to chromosome 14q24.3. Nat Genet 1992; 2:335–339Crossref, Medline, Google Scholar
65. Nechiporuk A, Fain P, Kort E, Nee LE, Frommelt E, Polinsky RJ, Korenberg JR, Pulst SM: Linkage of familial Alzheimer disease to chromosome 14 in 2 large early-onset pedigrees: effects of marker allele frequencies on LOD scores. Am J Med Genet 1993; 48:63–66Crossref, Medline, Google Scholar
66. Alzheimer’s Disease Collaborative Group: The structure of the presenilin 1 (S182) gene and identification of six novel mutations in early onset AD families. Nat Genet 1995; 11:219–222Crossref, Medline, Google Scholar
67. Campion D, Flaman J-M, Brice A, Hennequin D, Dubois B, Martin C, Moreau V, Charbonnier F, Didierjean O, Tardieu S, Penet C, Puel M, Pasquier F, LeDoze F, Bellis G, Calenda A, Heilig R, Martinez M, Mallet J, Bellis M, Clerget-Darpoux F, Agid Y, Frebourg T: Mutations of the presenilin 1 gene in families with early-onset Alzheimer’s disease. Hum Mol Genet 1995; 4:2373–2377Crossref, Medline, Google Scholar
68. Cruts M, Backhovens H, Wang S-Y, Gassen GV, Theuns J, DeJonghe C, Wehnert A, DeVoecht J, DeWinter G, Cras P, Bruyland M, Datson N, Weissenbach J, den Dunnen JT, Martin J-J, Hendriks L, van Broeckhoven C: Molecular genetic analysis of familial early-onset Alzheimer’s disease linked to chromosome 14q24.3. Hum Mol Genet 1995; 4:2363–2371Crossref, Medline, Google Scholar
69. Sherrington R, Govaev EI, Liang Y, Rogaeva EA, Levesque G, Ikdea M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin J-F, Bruni AC, Montesi MP, Sorbi S, Rainero I, Piness L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, DaSilva HAR, Haines JL, Pericak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH: Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 1995; 375:754–760Crossref, Medline, Google Scholar
70. Sahara N, Yahagi Y-I, Takagi H, Kondo T, Okochi M, Susami M, Shirasawa T, Mari H: Identification and characterization of presenilin I-467, I-463, and I-374. FEBS Lett 1996; 381:7–11Crossref, Medline, Google Scholar
71. Levy-Lahad E, Wijsman EM, Nemens E, Anderson L, Goddard KAB, Weber JLJ, Bird TD, Schellenberg GD: A familial Alzheimer’s disease locus on chromosome 1. Science 1995; 269:970–972Crossref, Medline, Google Scholar
72. Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu C-E, Jondro PD, Schmidt SD, Wang K, Crowley AC, Fu Y-H, Guenette SY, Galas D, Nemens E, Wijsman EM, Bird TD, Schellenberg GD, Tanzi RE: Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 1995; 269:973–977Crossref, Medline, Google Scholar
73. Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holamn K, Tsuda T, Mar L, Sorbi S, Nacmias B, Piacentini S, Amaducci L, Chumaker I, Cohen D, Lannfelt L, Fraser PE, Rommens JM, St George-Hyslop PH: Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature 1995; 376:775–778Crossref, Medline, Google Scholar
74. Holland AJ: Down’s syndrome and dementia of the Alzheimer type, in Dementia. Edited by Burns A, Levy R. London, Chapman & Hall, 1994, pp 695–708Google Scholar
75. Pericak-Vance MA, Bebout JL, Gaskell PC, Yamaoka LH, Hung WY, Alberts MJ, Walker AP, Bartlett RJ, Haynes CA, Welsh KA, Earl NL, Heyman A, Clark CM, Roses AD: Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. Am J Hum Genet 1991; 8:1034–1050Google Scholar
76. Pericak-Vance MA, Bass MP, Yamaoka LH, Gaskell PC, Scott WK, Terwedow HA, Menold MM, Conneally PM, Small GW, Vance JM, Saunders AM, Roses AD, Haines JL: Complete genomic screen in late-onset familial Alzheimer disease. JAMA 1997; 278:1237–1241Crossref, Medline, Google Scholar
77. Zubenko GS, Stiffler JS, Hughes HB, Hurtt MR, Kaplan BB: Initial results of a genome survey for novel Alzheimer’s disease risk genes: association with a locus on the X chromosome. Am J Med Genet Neuropsychiatr Genet 1998; 81:98–107; correction, 81:196–205Crossref, Medline, Google Scholar
78. Zubenko S, Hughes HB, Stiffler JS, Hurtt MR, Kaplan BB: A genome survey for novel Alzheimer’s disease risk loci: results at 10cM resolution. Genomics 1998; 50:121–128Crossref, Medline, Google Scholar
79. Hutchin TP, Heath PR, Pearson RCA, Sinclair AJ: Mitochondrial DNA mutations in Alzheimer’s disease. Biochem Biophys Res Comm 1997; 241:221–225Crossref, Medline, Google Scholar
80. Davis RE, Miller S, Herrnstadt C, Ghosh SS, Fahy E, Shinobu LA, Galasko D, Thal LJ, Beal MF, Howell N, Parker WD: Mutations in mitochondrial cytochrome c oxidase genes segregate with late-onset Alzheimer disease. Proc Natl Acad Sci USA 1997; 94:4526–4531Crossref, Medline, Google Scholar
81. Mayeux R, Stern Y, Ottman R, Tatemichi TK, Tang MX, Maestre G, Ngai C, Tycko B, Ginsberg H: The apolipoprotein ε4 allele in patients with Alzheimer’s disease. Ann Neurol 1993; 34:752–754Crossref, Medline, Google Scholar



