Cognitive Decline in Adulthood: An 11.5-Year Follow-Up of the Baltimore Epidemiologic Catchment Area Study
Abstract
OBJECTIVE: The epidemiology of cognitive decline over 11.5 years was investigated in a large community-residing population, with a special emphasis on the relationship between education and cognitive decline. METHOD: The study was an 11.5-year follow-up of a probability sample of the adult household residents of east Baltimore. From the Baltimore cohort of the Epidemiologic Catchment Area study, 1,488 participants completed the Mini-Mental State during three study waves in 1981, 1982, and 1993–1996. For each study participant, the difference in scores on the Mini-Mental State between waves 2 and 3 was calculated. RESULTS: Over a median interval of 11.5 years, the study participants’ scores on the Mini-Mental State declined a mean of 1.41 points, and the scores of 68% of the participants declined by at least 1 Mini-Mental State point. With and without adjustment for age, greater declines were associated with having 8 years or less of formal education and with being African American. CONCLUSIONS: Over a long time period, cognitive decline occurred in all age groups. Having more than 8 years of formal education was associated with less decline. However, beyond 9 years, additional education was not associated with a further reduction in cognitive decline. This suggests that a minimal amount of education during early critical periods might confer protection against cognitive decline later in life.
Cognitive capacity, a uniquely human confluence of memory, language, praxis, abstraction, and executive functioning, has multiple determinants, including genetic makeup, nutritional status, health status, formal education, and age-related developmental processes. Cognitive performance generally reaches its peak in early adulthood, and it appears to decline later in life (1). The underlying causes of this decline include disease processes, such as Alzheimer’s disease or brain injury of vascular origin, but also may include disuse and poorly characterized age-related deterioration processes (1).
Several important questions regarding the epidemiology of cognitive decline remain unanswered. For example, does decline occur at all ages or does it begin later in life? Is the rate of decline stable over an individual’s life? Who is at greatest risk for cognitive decline? Answers to these questions are important in light of the medical and public health importance of cognitive decline. Cognitive decline has been associated with impaired functioning and with increased mortality (1). In addition, cognitive decline is closely linked to dementia (2–4), a major medical and public health problem.
The most comprehensive assessments of cognitive change over the life span were conducted in the Seattle Longitudinal Study (5). That study followed a series of community-based cohorts enrolled in a health maintenance organization. The sample sizes for individual cohorts were between 500 and 997. Participants were assessed on tests of intelligence and cognitive capacity. The main findings were that individual cognitive abilities did not change much before age 60, with the exception of verbal fluency. Beyond that age there appeared to be continued decline in several areas of intellectual functioning. Owing to attrition, the Seattle Longitudinal Study did not have sufficiently large samples with which to detect small cognitive declines in younger age groups. Furthermore, few individual participants were followed for longer than 5 years.
Several studies have investigated risk factors for cognitive decline in later life (6–10). These have consistently implicated increasing age (6–9) as a risk factor for cognitive decline. In addition, a previous analysis from the Epidemiologic Catchment Area (ECA) study indicated that cognitive decline is inversely related to education (9). (That study investigated decline between ECA wave 1 [1981] and wave 2 [1982] at all five ECA sites. A subset of the data set used in those analyses was from the Baltimore site and included some of the baseline data used in the analyses reported here.) Higher social and occupational functioning also appears to be protective against later decline (8). Being female and encountering stressful life events are not associated with cognitive decline (6, 8).
Three of these risk factor studies (6–8) included relatively small samples (fewer than 350 persons), and all followed participants for less than 6 years. Jacqmin-Gadda et al. (10) recently reported findings from an epidemiologic study of 2,537 persons aged 65 and older who underwent annual cognitive assessments with the Mini-Mental State (11) for 5 years. These investigators reported small but significant mean declines on the Mini-Mental State over 5 years (between 0.02 and 0.57 Mini-Mental State point per year). Increasing age and less education were independent predictors of decline.
The association between educational attainment and cognitive decline is particularly interesting. It has been well established through cross-sectional research that performance on cognitive tests is closely linked to prior educational attainment (12). There also is evidence that educational interventions increase cognitive capacity, at least in the short term (5). As already noted, low education level may be a risk factor for cognitive decline (9, 10). However, in a recent review Gilleard (12) concluded that an independent association between education and cognitive decline has not been consistently supported by the data.
Several confounders of the potential association between educational attainment and cognitive decline have not been adequately controlled in longitudinal studies. These include chronological age, birth cohort, gender, race, and prior cognitive capacity. Also, the available evidence does not speak clearly to whether the potential association between education and cognitive decline is monotonic or whether a threshold is involved (i.e., whether beyond a certain “dose” of education, additional years of education are no longer associated with less decline).
While cognitive decline and dementia are not synonymous, many older persons who exhibit cognitive decline develop dementia (2). A substantial number of epidemiologic studies of the incidence of dementia have been published in the last several years (13–26). In one of these (23), participants were followed for 15 years. The other studies had follow-up intervals of less than 5 years and focused on populations typically over 65 years of age. In these studies (13–26), increasing age, prior cognitive impairment, strokes, high blood pressure, alcohol consumption, and depression were found to be risk factors for the development of dementia. Gender has not been associated with a higher incidence of dementia (13–15), although the confounding between gender and age has not been assessed systematically. Two studies (27, 28) have suggested that lesser educational attainment is a risk factor for dementia. However, this finding has not been supported universally; some research has shown no association between educational attainment and the incidence of dementia (12, 15, 28, 29).
In this paper we report findings from the 11.5-year follow-up of 1,488 participants in the Baltimore area ECA study. The Mini-Mental State (11), a widely used quantitative measure of cognitive capacity, was administered to participants at wave 1 (1981) and at two follow-up waves in 1982 and 1993–1996. The design of the study allowed us to examine cognitive decline between waves 2 and 3 in a large epidemiologic sample containing substantial numbers of individuals of all ages. A special feature of this design was the ability to use the wave 1 Mini-Mental State score as a measure of baseline cognitive functioning. The design allowed us to address some of the unanswered questions in the epidemiology of cognitive decline and to assess more specifically the association between education and cognitive decline with adjustment for potential distortions introduced by age, sex, race-ethnicity, and baseline cognitive functioning.
METHOD
Baltimore ECA Follow-Up
The ECA program has been described in detail elsewhere (30, 31). The Baltimore arm of this five-site study first began interviewing participants in 1981, when the first wave of assessments was completed, including the baseline (wave 1) Mini-Mental State examination. A second wave of assessments (including the wave 2 Mini-Mental State) was conducted 1 year later, in 1982. The ECA target population consisted of the adult household residents of eastern Baltimore, an area with 175,211 adult inhabitants. In wave 1, 4,238 individuals were designated by probability sampling methods for interview and 3,481 (82%) completed interviews. Of these, 2,695 completed interviews at wave 2.
In 1993 all 3,481 initial participants were targeted for tracing and interviewing. It was found that 848 had died; 2,633 were presumed to be alive, of which 415 could not be successfully traced. Of the 2,218 located, 298 refused to participate and 1,920 completed interviews at follow-up. Of these, 1,488 completed the Mini-Mental State at all three study waves, approximately 11.5 years after wave 2. All study participants signed informed consent statements approved by the Institutional Review Board of the Johns Hopkins University School of Hygiene and Public Health.
The predictors of loss to follow-up and mortality included cognitive impairment, greater age, and lower education at baseline (30). These findings are important to these analyses since they imply that many of the participants lost to follow-up would have exhibited substantial cognitive declines. Thus, the findings from the “survivor” cohort in these analyses may well underestimate the degree of cognitive decline for the original cohort.
Participants
In these analyses we included only the participants who completed the Mini-Mental State at all three study waves (N=1,488). Of the original 3,481 participants at wave 1, 81 did not complete the Mini-Mental State at wave 1. Of the remaining 3,400, 2,682 completed the Mini-Mental State at wave 2 and 171 completed it at wave 3 but not at wave 2.
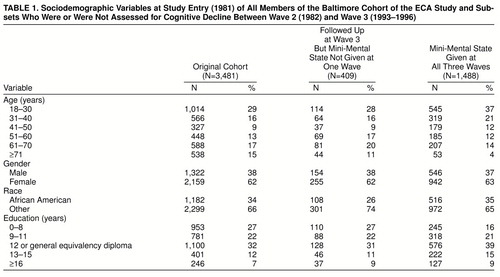
Table 1 contains data on characteristics of the original cohort of 3,481, the 409 who were interviewed at wave 3 but who did not complete the Mini-Mental State at one of the three waves, and the 1,488 participants who completed the Mini-Mental State at all three waves. The mean scores on the Mini-Mental State at wave 1 for these three groups were 27.7 (SD=3.1), 27.8 (SD=2.9), and 28.6 (SD=1.9), respectively. Compared to those who were followed-up at wave 3 but were missing a Mini-Mental State score for one of the three study waves, the subjects with Mini-Mental State scores for all three waves were younger (χ2=59.5, df=5, p<0.0001), were more likely to be black (χ2=9.6, df=1, p=0.002), were less well educated (χ2=27.0, df=4, p<0.0001), and had higher wave 1 Mini-Mental State scores (t=6.59, df=1,892, p<0.0001).
Measurement of Cognitive Decline
For each participant a difference score on the Mini-Mental State was calculated by subtracting the score at wave 3 (1993–1996) from the score at wave 2 (1982). The mean interval between the points at which these Mini-Mental State examinations were performed was 11.6 years (SD=0.39). The median interval was 11.5 years, the 25th percentile was 11.3 years, and the 75th percentile was 11.9 years. The change in score on the Mini-Mental State between waves 2 and 3 was the primary dependent variable in the analyses; it has been analyzed without recoding from this metric.
Potential Sociodemographic Predictors of Cognitive Decline
Age and sex (female/male) were recorded at study entry. The ages were grouped in years as follows: 18–30, 31–40, 41–50, 51–60, 61–70, and 71 or older. Race also was recorded at study entry and was indicated in the analyses as African American and other (mainly white, non-Hispanic). Educational attainment was recorded at wave 1 as the number of years of formal education before wave 1. Five educational subgroups were developed: 0–8 years, 9–11 years, 12 years or general equivalency diploma, 13–15 years, and 16 or more years, which conform with common educational landmarks (grade school, some high school, completed high school or equivalent, some college, completed college).
The final potential predictor of cognitive decline was Mini-Mental State score at wave 1 (1981), an independent measure of cognitive functioning obtained at the earliest point in the study timeline, well before the interval of interest. The wave 1 scores were stratified into five groups: 30, 29, 28, 25–27, and ≤24. These groups were chosen on the basis of the distribution of Mini-Mental State scores at wave 1 in this sample. In regression models, the wave 1 Mini-Mental State scores were entered without recoding. The recoding described for sociodemographic variables was specified before any of the relationships under study were estimated.
Analyses
The mean change in scores (with 95% confidence intervals) on the Mini-Mental State between waves 2 and 3 for the entire analytic cohort and for subgroups by age, gender, race, education, and wave 1 score are reported in tabular form. The relationship between the covariates and the decline in Mini-Mental State score from wave 2 to wave 3 was examined in linear regression models with Mini-Mental State change as the dependent variable and the other variables as covariates. The association of individual covariates with cognitive decline is reported in the form of a regression coefficient (and 95% confidence interval) for each covariate. Subgroups were entered into regression models individually as “dummy” variables to allow direct comparisons of regression coefficients by using one of the subgroups as reference.
RESULTS
Cognitive Decline Between Waves 2 and 3
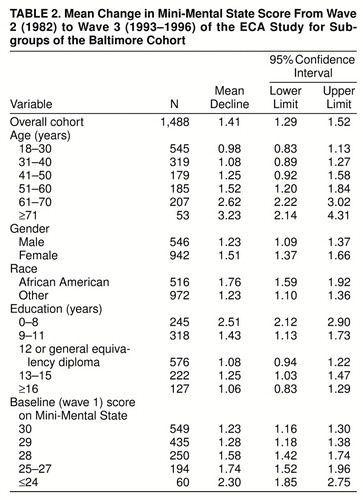
Over the 11.5 years of follow-up, there was a mean decline of 1.41 points on the Mini-Mental State (95% confidence interval=1.29–1.52). Twenty-seven (2%) of the participants showed a 3-point or greater increase (range=3–12 points), 51 (3%) showed a 2-point increase, 134 (9%) showed a 1-point increase, and 270 (18%) showed no change. The remaining 68% all showed declines: a decline of 1 point for 416 (28%), 2 points each for 257 (17%), 3 points for 140 (9%), 4 points for 72 (5%), and 5–19 points for 121 (8%).
As displayed in table 2, the magnitude of decline in Mini-Mental State score was not constant for all subgroups. With respect to our hypothesis of major emphasis, the subgroup of adults with the lowest educational attainment showed much more decline than the other education subgroups. Also, as expected, the participants who were oldest at baseline in 1981 showed large declines, relative to the other age strata (table 2).
Association Between Covariates and Cognitive Decline
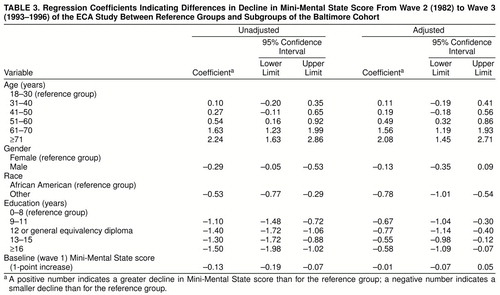
Table 3 displays the relative magnitude of declines on the Mini-Mental State for different subgroups of the cohort. The numbers presented are the regression coefficients from bivariate analyses (under “Unadjusted” heading) and multiple regression analyses (under “Adjusted” heading), as discussed previously. The multiple regression models included all the variables shown in the table. These coefficients can be interpreted as the magnitude of decline (or increase) on the Mini-Mental State after 11.5 years, relative to a reference group. For example, before adjustment the 41–50 age group exhibited a 0.27-point greater decline (positive numbers in the table) on the Mini-Mental State than the 18–30 (reference) age group, and the group with 16 or more years of education had 1.50 points less decline (negative numbers in the table) than the group with 0–8 years of education.
The evidence presented in table 3 helps us to disentangle what might be independent effects of education, age, and the other variables thought to have an influence on cognitive decline. As might be expected, many of the adults with 8 years of schooling or less also were in the older age groups, and more women than men had survived into the oldest age groups. The results from multiple regression convey the estimated relationships that link low education, age, and sex (being female) to cognitive decline, with statistical adjustments to disclose independent relationships. Low education level remained a predictor of cognitive decline, even with statistical adjustment for age and all of the other variables studied, including the wave 1 Mini-Mental State score. After adjustment for age, the wave 1 Mini-Mental State score, and the other variables, the magnitude of cognitive decline for adults with 8 years or less of education was reflected in a decline in Mini-Mental State score 0.55 to 0.77 point greater than those for participants with more education (table 3).
After adjustment for educational, male-female, and other differences, the magnitude of cognitive decline for the oldest subgroup of adults was reflected in a Mini-Mental State decline 2.08 points greater than for the youngest participants. Conversely, the adjusted estimates for the male-female variable constitute evidence that the initially observed male-female differences in magnitude of cognitive decline were not independent of the other relationships that link education, age, and other variables to cognitive decline.
Whereas our advance hypotheses led us to expect evidence of age- and education-related cognitive decline in this study, we had thought that there would be no variation in cognitive decline across race-ethnicity subgroups once we had adjusted for these other differences and for wave 1 Mini-Mental State score. Nonetheless, even with adjustments for these other variables, there remained a tangible difference between African American adults and the other (predominately white, non-Hispanic) adult household residents of eastern Baltimore: the Mini-Mental State scores of the African Americans declined by 0.78 point more than the scores of the other participants.
The observed evidence regarding the association between baseline Mini-Mental State score and later cognitive decline also merits scrutiny. As shown in table 3, before adjustment for other variables, a 1-point increase in wave 1 score was associated, on average, with 0.13 point less decline between waves 2 and 3. Thus, for example, participants with a wave 1 score of 27 evidenced on average 0.13 point less decline than those with a wave 1 score of 26 and 0.65 point less decline than those with a wave 1 score of 22 (calculated by multiplying 0.13 by 5, since 27–22=5). After adjustment for age, education, race, and sex, there was no longer a significant association between wave 1 Mini-Mental State score and later decline.
DISCUSSION
This paper presents estimates of the magnitude of cognitive decline after approximately 12 years in a large general population sample of adults. The study participants exhibited a mean decline of 1.41 points on the Mini-Mental State, substantially greater than a 1-point decline. Two-thirds of the sample exhibited at least a 1-point decline on the Mini-Mental State during this long time period. Virtually all demographic subgroups under study exhibited a decline. The mean declines were greatest for persons aged 71 and older, for those with 8 years or less of formal education, and for African Americans. After adjustment for other variables, the mean declines were similar for men and women and across the range of baseline Mini-Mental State scores.
The finding that older age is associated with greater cognitive decline replicates prior results (6–9, 32). These analyses extend the age-decline relationship to younger age groups and suggest that cognitive decline occurs at all ages. While this result differs from the findings of the Seattle Longitudinal Study (5), it is likely that the much larger size and longer follow-up of the ECA cohort allowed detection of sustained small declines in cognition in younger individuals.
The relationship between age and decline appeared to be monotonic across the age groups, perhaps because the Mini-Mental State has limited dispersion. Persons under age 31 declined less than 1 point per decade. This rate of decline increased with each successive decade of life. After age 50, there was an acceleration in decline by an additional 1.25 points for each successive decade of life. By age 71, the mean declines were above 3 points per decade, a value many observers would regard as clinically significant (32–34).
To illustrate the cumulative effect of these decline rate estimates for an average individual we offer the following example, using the estimates reported in this paper and under an assumption of no strong cohort or period effects. A person with a Mini-Mental State score of 29 (rounded up from an average of 28.60 for this select cohort) at age 25 would decline 0.98 point by age 37 (12 years later) to a score of 28 (rounded from 27.62), decline 1.08 points to a score of 27 (rounded from 26.54) by age 49, decline 1.25 points to 25 (rounded from 25.29) by age 61, and decline another 2.62 points to a score of 23 (rounded from 22.67) by age 73, for a total decline of almost 6 points (5.93) in a bit less than 50 years. For interpretation, it might be useful to note that many clinicians would consider a 3–4-point decline in Mini-Mental State score as clinically significant (32–34), given population Mini-Mental State norms (32) and rates of Mini-Mental State decline in patients with Alzheimer’s disease (33).
Nonetheless, the same cannot be said for a Mini-Mental State decline of 1 point over 12 years, which is quite small and at the level of the individual person might well be artifactual. For example, a person might lose 1 point on the Mini-Mental State simply by failing to know the exact location of the study test site or by forgetting a single item on a three-item recall task. One might then argue that a mean decline of about 1 point in the younger age groups should be interpreted as the consequence of such individual errors or oversights. This effect might be compounded by the fact that at wave 1 about 37% of the study participants scored a perfect 30 on the Mini-Mental State and had no room for increase at follow-up. Counterbalancing this acknowledgment of possible artifacts, there are good reasons to interpret these Mini-Mental State declines as more than measurement error.
First, small individual errors on the Mini-Mental State due to “having a bad day” ought to be random and not systematic (equally distributed among study waves). The effect on mean estimates ought to average out across the population and across waves of assessment. Second, the ceiling effect is limited to a minority of participants, those who at baseline scored 30 points. Third, there is a clear trend for the rate of decline to increase in every successive age decade. Fourth, any beneficial practice effects on the Mini-Mental State score would be unlikely to last for 11.5 years and would tend to lead to higher, not lower, Mini-Mental State scores. Fifth, this is a cohort of survivors, and in the ECA sample, survival is inversely correlated with wave 1 Mini-Mental State score (30), so that these decline figures may actually underestimate true declines.
More education was associated with less cognitive decline in this study, which lends support to speculations about the value of education in relation to cognitive decline (9, 26, 27). This association was reduced but remained significant after adjustment for age, sex, race, and baseline cognitive functioning. The presence of an association between education and cognitive decline after adjustment for wave 1 Mini-Mental State score is of particular note since it suggests that the impact of education on rate of cognitive decline is not wholly mediated by cognitive reserve, in so far as reserve can be indicated by Mini-Mental State score at baseline. However, this result also might be interpreted as suggesting that receiving more education is independently protective against later cognitive decline and that the association between education and slower decline is not simply a reflection of education’s putative influence on cognitive reserve.
The latter argument is further strengthened by the fact that in these analyses the association between education and later cognitive decline was not monotonic. More education beyond the 9-year level was not associated with additional reductions in decline. This suggestion of a threshold for a benefit of education is in line with the idea that for education to be protective against later cognitive decline it has to be of a certain minimal duration and to occur during a critical period in life. Beyond such a critical period, additional education might not confer additional protection against cognitive decline later in life. Given evolving knowledge in neuroscience about critical periods in brain development, this might suggest a mechanism for how education exerts a protective effect. Education might prevent loss of neuronal connections and/or strengthen neuronal connections in the brain thorough regular stimulation of higher cognitive functions, in ways analogous to the manner in which exposure to light prevents blindness in human eyes.
There are other potential explanations of the relationship between lesser educational attainment and later cognitive decline. Persons of lower education might be less able to earn an income sufficient for maintaining good nutrition and health over the years. Similarly, education is confounded with socioeconomic class, so that this association may reflect a relationship between socioeconomic class and cognitive decline.
We can do no more than speculate about reasons for the race/ethnicity variations in cognitive decline. Perhaps this finding reflects the limitations of the Mini-Mental State as a measure of cognitive functioning in African Americans. But it seems equally plausible that the variation reflects chronic effects of hypertension, cerebrovascular disease, and other insults to the brain, more common among African Americans, traceable to denial of access to health care, good nutrition, and other benefits of material wealth and equity, among other things.
Another potential explanation for race/ethnicity variations is the nature of the truncated analytic sample, because of cross-sectional sampling across survivors in all age groups and also because of out-migrations between wave 1 and the follow-up assessments. To some extent, the out-migrations and losses to follow-up stemmed from age-associated impairments that prompted entry into nursing homes and other facilities distant from Baltimore, to which we could not send assessors for the in-person Mini-Mental State testing. To the extent that African Americans with cognitive impairments are more likely to remain in community households or move to nearby congregate housing facilities or nursing homes, they would have been more likely to be seen in person and tested. To the extent that white, non-Hispanic participants with cognitive impairment are more likely to move to more distant facilities (e.g., in Florida), they would not have been in the segment of the follow-up sample that was tested with the Mini-Mental-State. (We conducted telephone interviews with participants who moved more than about 150 miles away but could not administer the Mini-Mental State over the telephone.)
Lower Mini-Mental State scores on study entry were associated with greater declines before adjustment. However, after adjustment for other variables, this association was no longer statistically significant. The finding that wave 1 Mini-Mental State score was not associated with decline after adjustment for age, education, race, and gender was unexpected since it suggests that cognitive reserve in adulthood is a less important predictor of decline than previously believed. Nonetheless, we acknowledge that the Mini-Mental State is not a superb measure of cognitive reserve.
A notable limitation of this study is the fact that it was a sampling of survivors from a baseline cohort. Other limitations are loss to follow-up and mortality and limitations in the measurement of cognitive decline. Cognitive functioning at baseline was a predictor of both mortality and loss to follow-up in the ECA study (30). Given that lower cognitive functioning was associated with greater cognitive decline, these estimates of decline may be underestimates, particularly for older age groups, in which the losses to follow-up and mortality were greatest. Finally, as mentioned previously, the Mini-Mental State is not a sensitive measure of cognitive functioning, cognitive change, or cognitive reserve. However, given its ease of use and widespread application, it was the most practical brief assessment of cognitive functioning at the time the multisite ECA study was planned, in the late 1970s.
We conclude that cognitive decline occurs across all age groups and is greatest in older persons and among those with less formal education. Increasing age and less than 9 years of education early in life are associated with cognitive decline over the life span. Additionally, cognitive decline appears to be more rapid in African Americans. An understanding of the precise nature and mechanisms of the relationship between these variables (and their relationship to other important variables) invites further investigation, including systematic replication.
Received Dec. 2, 1997; revision received March 6, 1998; accepted June 15, 1998. From the Neuropsychiatry and Memory Group, Department of Psychiatry and Behavioral Sciences, School of Medicine, The Johns Hopkins University. Address reprint requests to Dr. Lyketsos, Osler 320, The Johns Hopkins Hospital, 600 North Wolfe St., Baltimore, MD 21287; [email protected] (e-mail). Supported by grant MH-47447 for the Baltimore Epidemiological Catchment Area follow-up. The authors thank Donald Hoover, Ph.D., for statistical consultation.
 |
 |
 |
1. Zec RF: The neuropsychology of aging. Exp Gerontol 1995; 30:431–442Crossref, Medline, Google Scholar
2. Bickel H, Cooper B: Incidence and relative risk of dementia in an urban elderly population: findings of a prospective field study. Psychol Med 1994; 24:179–192Crossref, Medline, Google Scholar
3. O’Brien JT, Beats B: Benign senescent forgetfulness and age associated memory impairment, in Dementia. Edited by Burns A, Levy R. New York, Chapman & Hall Medical, 1994, pp 295–308Google Scholar
4. Huppert FA, Brayne C: What is the relationship between dementia and normal aging? in Dementia and Normal Aging. Edited by Huppert FA, Brayne C, O’Connor DW. Cambridge, UK, Cambridge University Press, 1994, pp 3–11Google Scholar
5. Schaie KW: The course of adult intellectual development. Am Psychologist 1994; 49:304–313Crossref, Medline, Google Scholar
6. Grimby A, Berg S: Stressful life events and cognitive functioning in late life. Aging (Milano) 1995; 7:35–39Medline, Google Scholar
7. Hultsch DF, Hertzog C, Small BJ, McDonald-Miszczak L, Dixon RA: Short-term longitudinal change in cognitive performance in later life. Psychol Aging 1992; 7:571–584Crossref, Medline, Google Scholar
8. Johansson B, Zarit SH, Berg S: Changes in cognitive functioning of the oldest old. J Gerontol 1992; 47:75–80Crossref, Medline, Google Scholar
9. Farmer ME, Kittner SJ, Rae DS, Bartko JJ, Regier DA: Education and change in cognitive function: the Epidemiologic Catchment Area study. Ann Epidemiol 1995; 5:1–7Crossref, Medline, Google Scholar
10. Jacqmin-Gadda H, Fabrigoule C, Commenges D, Dartigues JF: A 5-year longitudinal study of the Mini-Mental State Examination in normal aging. Am J Epidemiol 1997; 145:498–506Crossref, Medline, Google Scholar
11. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
12. Gilleard CJ: Education and Alzheimer’s disease: a review of recent international epidemiological studies. Aging Ment Health 1997; 1:33–46Crossref, Google Scholar
13. Minami Y, Tsuji I, Fukao EA: Physical status and dementia risk: a three year prospective study in urban Japan. Int J Soc Psychiatry 1995; 41:47–54Crossref, Medline, Google Scholar
14. Yoshitake T, Kiyohara Y, Kato I: Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population. Neurology 1995; 45:1161–1168Crossref, Medline, Google Scholar
15. Letenneur L, Commenges D, Dartigues JF, Barberger-Gateau P: Incidence of dementia and Alzheimer’s disease in elderly community residents of south-western France. Int J Epidemiol 1994; 23:1256–1261Crossref, Medline, Google Scholar
16. Hebert LE, Scherr PA, Beckett LA: Age-specific incidence of Alzheimer’s disease in a community population. JAMA 1995; 273:1354–1359Crossref, Medline, Google Scholar
17. Payami H, Montee K, Kaye J: Evidence for familial factors that protect against dementia and outweigh the effect of increasing age. Am J Hum Genet 1994; 54:650–657Medline, Google Scholar
18. Boothby H, Blizard R, Livingston G, Mann AH: The Gospel Oak Study stage III: the incidence of dementia. Psychol Med 1994; 24:89–95Crossref, Medline, Google Scholar
19. Shen YC, Li G, Li YT: Epidemiology of age related dementia in China. Chin Med J 1994; 107:60–64Medline, Google Scholar
20. Paykel ES, Brayne C, Huppert FA: Incidence of dementia in a population older than 75 years in the United Kingdom. Arch Gen Psychiatry 1994; 51:325–332Crossref, Medline, Google Scholar
21. Morgan K, Lilley JM, Arie T: Incidence of dementia in a representative British sample. Br J Psychiatry 1993; 163:467–470Crossref, Medline, Google Scholar
22. Bachman DL, Wolf PA, Linn RT: Incidence of dementia and probable Alzheimer’s disease in a general population: the Framingham study. Neurology 1993; 43:515–519Crossref, Medline, Google Scholar
23. Hagnell O, Franck A, Grasbeck A: Vascular dementia in the Lunby study. Neuropsychobiology 1992; 26:43–49Crossref, Medline, Google Scholar
24. Copeland JR, Davidson IA, Dewey ME: Alzheimer’s disease, other dementias, depression, and pseudodementia: prevalence, incidence, and three year outcome in Liverpool. Br J Psychiatry 1992; 161:230–239Crossref, Medline, Google Scholar
25. Mann AH, Livingston G, Boothby H, Blizard R: The Gospel Oak Study: the prevalence and incidence of dementia in an inner city area of London. Neuroepidemiology 1992; 11(suppl 1):76–79Google Scholar
26. Li G, Shen YC, Chen CH: A three year follow-up study of age related dementia in an urban area of Beijing. Acta Psychiatr Scand 1991; 83:99–104Crossref, Medline, Google Scholar
27. Stern Y, Gurland B, Tatemichi TK: Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 1994; 271:1004–1010Crossref, Medline, Google Scholar
28. Ott A, Breteler MMB, van Harskamp F, Claus JJ, van der Cammen TJ, Grobbee DE, Hofman A: Prevalence of Alzheimer’s disease and vascular dementia: association with education: the Rotterdam study. Br Med J 1995; 310:970–973Crossref, Medline, Google Scholar
29. Beard CM, Kokmen E, Offord KP, Kurland LT: Lack of association between Alzheimer’s disease and education, occupation, marital status, or living arrangement. Neurology 1992; 42:2063–2068Crossref, Medline, Google Scholar
30. Eaton WW, Anthony JC, Gallo J, Cai G, Tien A, Romanoski A, Lyketsos CG, Chen LS: Natural history of Diagnostic Interview Schedule DSM-IV major depression. Arch Gen Psychiatry 1997; 54:993–999Crossref, Medline, Google Scholar
31. Eaton WW, Kessler LG (eds): Epidemiologic Field Methods in Psychiatry: The NIMH Epidemiologic Catchment Area Program. Orlando, Fla, Academic Press, 1985Google Scholar
32. Crum RM, Anthony JC, Basset SS, Folstein MF: Population based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993; 269:2386–2391Crossref, Medline, Google Scholar
33. Rebok G, Brandt J, Folstein M: Longitudinal cognitive decline in Alzheimer’s disease. J Geriatr Psychiatry Neurol 1990; 3:91–97Crossref, Medline, Google Scholar
34. Schmand B, Lindeboom J, Launer L, Dinkgerve M, Hooijer C, Jonker C: What is a significant score change on the Mini-Mental State examination? Int J Geriatr Psychiatry 1995; 10:411–414Google Scholar



