Functional Hypofrontality and Working Memory Dysfunction in Schizophrenia
Abstract
Objective: Hypofrontality is a common but not invariable finding in schizophrenia. Inconsistencies in the literature may reflect, in part, the fact that abnormal physiological responses in the prefrontal cortex are best identified under conditions that place well-specified functional demands on this region. Method: The authors studied eight patients with schizophrenia and eight matched comparison subjects using [15O]H2O positron emission tomography and the “N-back” task, which activates the prefrontal cortex as a function of working memory load in normal subjects. Results: Under low-working-memory-load conditions, the accuracy of both groups in the N-back task was equal, but when the memory load increased, the patients’ performance deteriorated more than did that of the comparison subjects. The regional cerebral blood flow response to increased working memory load was significantly reduced in the patients’ right dorsolateral prefrontal cortex.Conclusions: These results confirm the importance of using tasks that tap specific cognitive functions, linked to specific neural systems, in studies of brain-behavior relationships in schizophrenia. Hypofrontality is reliably demonstrated in schizophrenia during tasks that engage working memory functions of the prefrontal cortex. Am J Psychiatry 1998; 155: 1281-1284
Studies of humans and nonhuman primates with brain injuries have shown that the prefrontal cortex plays a critical role in initiating and organizing complex behaviors (1). It is not surprising that disturbances of the prefrontal cortex are proposed to underlie negative symptoms and disorganization in schizophrenia. In a computationally explicit formulation of this hypothesis, Servan-Schreiber et al. (2) proposed that failure of dopamine-related prefrontal cortex mechanisms responsible for representing and maintaining context contributes to cognitive dysfunction in schizophrenia.
In functional neuroimaging studies, hypofrontality is frequently reported in schizophrenia. Two reviews (3, 4) suggested that despite widely varying methods, patient status, and efforts to control mental state, a majority of studies (11 of 17 and 25 of 45) reported reduced prefrontal cortex metabolism or blood flow in schizophrenia.
Notwithstanding this convergence in the literature and the logic that prefrontal cortex dysfunction underlies apathetic and dysexecutive features of schizophrenia, the reliability of hypofrontality has been questioned. In a more recent review emphasizing resting studies, Gur and Gur (5) strongly questioned the replicability and significance of this finding. These authors suggested that functional hypofrontality, more narrowly defined as a failure to activate frontal systems during prefrontal-cortex-related cognitive activity (3), “may still merit further investigation.” It is our view that relating neurophysiological mechanisms to cognitive processes is essential to understanding the pathophysiology of schizophrenia. We tested the reliability of hypofrontality in schizophrenia by examining cortical activation associated with increasing working memory demands.
Deficits in working memory are seen in medicated and unmedicated patients with schizophrenia (2, 6, 7), and a number of neuroimaging studies have shown reduced prefrontal cortex activation during neuropsychological tasks proposed to involve this function (3). However, such tasks are complex, engaging a variety of cognitive processes, not all of which reflect working memory. The “N-back” task was designed to manipulate task factors engaging working memory. This task reliably activates the dorsolateral prefrontal cortex (8). Activation is sustained during mnemonic activity and varies monotonically with memory load (8). We hypothesized that patients with schizophrenia would show reduced dorsolateral prefrontal cortex activation associated with impaired performance under conditions of working memory load during the N-back task.
METHOD
Eight patients with schizophrenia and eight healthy comparison subjects, matched individually for age, sex, and parental education, participated in the study. The subjects with schizophrenia were clinically stable, medicated outpatients. Diagnoses were confirmed by using the Structured Clinical Interview for DSM-III-R. Comparison subjects were excluded for any lifetime axis I disorder or first-degree family history of psychotic disorder. Subjects with substance abuse within 6 months, neurological illness, previous head trauma, or mental retardation were excluded. All participants provided written informed consent and were paid for their participation.
Subjects were scanned under high and low working memory conditions of the N-back task. Subjects observed random sequences of single letters at the center of a display. They pressed a button whenever a target appeared. In the low-memory-load condition, a target was the letter X. In the high-memory-load condition, a target was any letter that was the same as the letter two back in the sequence. Both conditions require that subjects encode and evaluate each stimulus and respond to targets. The high-memory-load condition has the added requirements that subjects maintain the identity and order of two previous letters and continuously update this representation. Stimuli were presented for 500 msec, with a 2500-msec interstimulus interval. Frequency of targets (20%) and repeated letters were identical across conditions.
Subjects performed two blocks of each condition, in A-B-B-A order (low-high-high-low). During each block, a 1-minute [15O]H2O positron emission tomography (PET) scan was acquired. Subjects first practiced to ensure that they understood and could perform both tasks. Details of PET procedures and analysis have been reported previously (9). Because our hypothesis focused on the dorsolateral prefrontal cortex, the threshold for significance of these comparisons was set at z=2.33 (p<0.01, one-tailed). A second, exploratory analysis of whole-brain group data was also undertaken; the threshold for significance in this analysis was set at z=3.09 (p<0.001, one-tailed).
RESULTS
Patients responded more slowly than comparison subjects (p<0.04, analysis of variance), and both groups responded more slowly under the high-memory-load condition than the low-memory-load condition (p<0.001). There was no group-by-load interaction (p<0.28). All subjects were more accurate under the low-memory-load condition than the high-memory-load condition (p<0.0001), comparison subjects were more accurate than patients (p<0.01), and the groups differed as a function of load (group-by-task interaction: p<0.003). Patients were less accurate under the high-memory-load condition (p<0.0001) but not the low-memory-load condition (p>0.88). Within each group and for both together, accuracy and response time were either negatively or not significantly positively correlated (Pearson product-moment correlation r values <0.02), indicating an absence of speed and accuracy tradeoffs. Since the two conditions were not psychometrically matched, we cannot rule out a generalized performance deficit, although other studies suggested a specific working memory deficit in schizophrenia (2, 6). However, our results are consistent with the predicted working memory deficit.
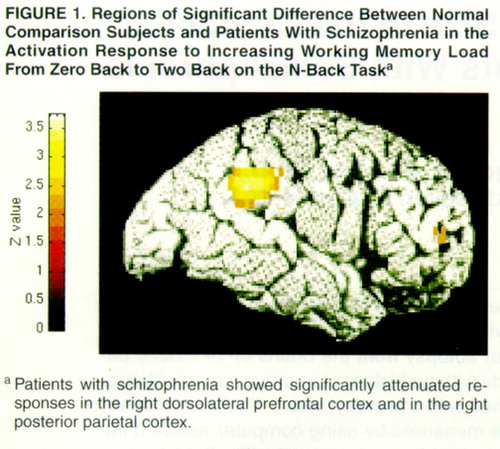
The increase in right dorsolateral prefrontal cortex activation with increased memory load was significantly less in the patients with schizophrenia than in the comparison subjects. Exploratory analysis also revealed decreased activation in the posterior parietal cortex (BA40) of the patients with schizophrenia. These two regions are shown in figure 1. Inspection of individual normalized regional cerebral blood flow (CBF) values of the maxima in both regions showed virtually no overlap between patients and comparison subjects in the high-memory-load condition. We compared the two groups’ dorsolateral prefrontal cortex and posterior parietal cortex activity under the low-memory-load condition by obtaining normalized regional CBF values for the maximum pixel location identified in the group contrast of the activation response to working memory load. Patients showed lower activity in the dorsolateral prefrontal cortex but not in the posterior parietal cortex under the low-memory-load condition. Patients were not more variable than comparison subjects in either region (Bartlett’s test of homogeneity of variance was conducted separately for each condition.
DISCUSSION
As hypothesized, patients with schizophrenia showed attenuated dorsolateral prefrontal cortex activation under conditions of increased working memory load. This suggests that hypofrontality is a reliable finding in schizophrenia when the behavioral demands of the imaging protocol specifically engage cognitive functions that rely on the integrity of this region. A decreased regional CBF response during the N-back task was also observed in the right posterior parietal cortex, a region previously shown to coactivate with the dorsolateral prefrontal cortex during working memory performance (8).
Since the N-back task taps several component processes of working memory, we cannot conclude that hypofrontality is specifically related to impaired maintenance processes. Further studies are needed using tasks that isolate component processes to fully define the nature of working memory dysfunction in schizophrenia.
The fact that the dorsolateral prefrontal cortex regional CBF was reduced in patients under the low-memory-load condition but that they performed as well as comparison subjects suggests that failure to activate this region with increased memory load was not due to poor performance per se. Notably, regional CBF in the posterior parietal cortex was not reduced in patients under the low-memory-load condition, suggesting that within the neural system subserving working memory, the dorsolateral prefrontal cortex of patients with schizophrenia is uniquely sensitive to the low level of working memory load in this condition.
These results, although preliminary because of the small number and medication status of the patients, confirm that under conditions known to place demands on the dorsolateral prefrontal cortex, patients with schizophrenia show a physiological deficit in this region. They are also consistent with mechanistic accounts of prefrontally based cognitive dysfunction in schizophrenia (2). Further studies applying advances in functional brain imaging that address this hypothesis with greater specificity are underway.
Received Sept. 19, 1997; revision received March 23, 1998; accepted April 8, 1998. From the Department of Psychiatry, University of Pittsburgh, Western Psychiatric Institute and Clinic.. Address reprint requests to Dr. Carter, Department of Psychiatry, University of Pittsburgh, Western Psychiatric Institute and Clinic, 3811 O’Hara St., Pittsburgh, PA 15213; [email protected] (e-mail).
1. Goldman-Rakic PS: Circuitry of primate prefrontal cortex and regulation of behavior by representational memory, in Handbook of Physiology: The Nervous System, vol 5. Edited by Plum F. Bethesda, Md, American Physiological Society, 1987, pp 373–417Google Scholar
2. Servan-Schreiber DSS, Cohen JD, Steingard S: Schizophrenic deficits in the processing of context: an empirical test of a theoretical model. Arch Gen Psychiatry 1996; 53:1105–1112Crossref, Medline, Google Scholar
3. Andreasen NC, Rezai K, Alliger R, Swayze VW II, Flaum M, Kirchner P, Cohen G, O’Leary DS: Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia: assessment with Xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry 1992; 49:943–958 Crossref, Medline, Google Scholar
4. Buchsbaum M: The frontal lobes, basal ganglia and temporal lobes as sites for schizophrenia. Schizophr Bull 1990; 16:379–389Crossref, Medline, Google Scholar
5. Gur RC, Gur RE: Hypofrontality in schizophrenia: RIP. Lancet 1995; 345:1383–1384Crossref, Medline, Google Scholar
6. Park S, Holzman PS: Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry 1992; 49:975–982Crossref, Medline, Google Scholar
7. Carter CS, Robertson LC, Nordahl TE, Kraft L, Chaderjian M, Oshora-Celaya L: Spatial working memory deficits and their relationship to negative symptoms in unmedicated schizophrenia patients. Biol Psychiatry 1996; 40:930–932Crossref, Medline, Google Scholar
8. Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE: Temporal dynamics of brain activation during a working memory task. Nature 1997; 386:604–608Crossref, Medline, Google Scholar
9. Carter CS, Mintun M, Nichols T, Cohen JD: Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry 1997; 154:1670–1675Link, Google Scholar



