Cerebral Glucose Metabolism in Women With Panic Disorder
Abstract
Objective: This study was undertaken to clarify earlier inconsistent findings in brain metabolic topography in panic disorder patients at rest.Method: Positron emission tomography (PET) with [18F]fluorodeoxyglucose was used to determine cerebral metabolic activity in six female patients with a DSM-III-R diagnosis of panic disorder and in six healthy female volunteers. All patients with panic disorder were medication free and were sensitive to lactate infusion. Results: A significant increase in glucose metabolism was found in the left hippocampus and parahippocampal area of the panic disorder subjects in comparison with that found in the healthy subjects. In addition, a significant decrease in metabolism was found in the right inferior parietal and right superior temporal brain regions of the panic disorder subjects in comparison with that of the normal subjects. There was no significant correlation between scores for the severity of panic disorder or for the severity of lactate-induced panic attack and the quantified PET abnormality. Conclusions: These data provide further support for the hypothesis of an abnormal brain metabolism in the hippocampal and parahippocampal area in individuals with panic disorder and also suggest other areas of aberrant brain metabolism in this disorder. Am J Psychiatry 1998; 155: 1178-1183
Although much remains unknown about the pathophysiology of panic disorder, in recent years there have been several interesting research developments that promise to enhance our understanding of the biological substrates of this disorder. Evidence from animal and clinical studies, for example, has suggested a neural model for panic disorder that points to certain circuits of the brain as the source of the pathophysiology (1). Both biochemical and brain imaging studies offer support for such a model, implicating a disruption in certain neurotransmitter functions involving several regions of the brain (see reference 2 for a review).
Particularly noteworthy have been studies of brain metabolism in panic disorder that used positron emission tomography (PET), which permits visualization of regional brain metabolism and neuroreceptor systems by means of a positron-labeled tracer and a model for quantitation (3). In their original work, Reiman and colleagues (4, 5) used PET with a 15O-labeled tracer to study cerebral blood flow in patients with panic disorder during the resting state. These investigators reported that patients with panic disorder who were vulnerable to lactate-induced anxiety attacks had an abnormal asymmetry of left to (higher) right parahippocampal blood flow, blood volume, and oxygen metabolism. In addition, these patients had abnormally high whole brain metabolism. However, some of the patients were receiving psychotropic medications at the time of the study. These findings suggested the hypothesis that patients with panic disorder exhibit a “trait” abnormality in the parahippocampal gyrus, which contains input and output pathways for the hippocampus. Thus, a parahippocampal abnormality, possibly triggered by inputs from the septum and amygdala, might underlie the vulnerability to panic disorder.
In a subsequent study, Nordahl and colleagues (6) examined cerebral glucose metabolism using [18F]fluorodeoxyglucose (FDG) as a tracer in PET studies of patients with panic disorder during the performance of an auditory discrimination task. This investigation confirmed the earlier findings of Reiman et al. of a hippocampal region asymmetry in blood flow but did not find global cerebral metabolic differences between patients with panic disorder and normal comparison subjects. Additional findings suggested metabolic decreases in the left inferior parietal lobule and in the anterior cingulate, as well as an increase in the metabolic rate in the medial orbital frontal cortex, in patients with panic disorder. However, results from this study may not be fully comparable to those from the work by Reiman et al., since the subjects in the study by Nordahl et al. were not identified by their lactate sensitivity.
In a study by De Cristofaro and colleagues (7), single photon emission computed tomography (SPECT) was used to study cerebral blood flow (CBF) in patients with panic disorder at rest. A group of seven patients who were sensitive to lactate and naive to psychopharmacological treatment were compared with five healthy subjects. Here, in contrast to the findings in the two previous studies, a significant decrease in the blood flow in the right and left hippocampal regions (hippocampus, parahippocampal gyrus, and amygdala) was observed. In the left occipital cortex, however, there was a significant increase in blood flow. A significant right-left asymmetry in the inferior frontal cortex was also noted in the patients with panic disorder.
Changes in brain activity have also been documented when patients with panic disorder have been challenged with panic-inducing compounds such as intravenously administered lactate solution. Alterations in CBF that were found during induced panic attacks included decreased frontal cortical activity (8, 9) along with increased CBF in temporal poles, subcortical areas, and midbrain structures (10) and in the occipital cortex (8). However, it should be noted that the study of subjects’ brain activity during chemically induced panic attacks, instead of when they are “at rest,” represents a different paradigm, one that presumably identifies state-dependent characteristics rather than possible trait characteristics that are likely to be related to the vulnerability to the disorder. (The differences in the findings between these two paradigms may even have relevance for the study of patients with panic disorder who are supposedly at rest, since reported inconsistencies could conceivably reflect variations in the mental state of patients during the brain scan.)
The present investigation was undertaken to clarify these somewhat inconsistent findings by studying a maximally homogeneous group of panic disorder subjects in the resting state. We used PET with FDG to investigate possible regional differences in cerebral glucose metabolism between patients with panic disorder and healthy comparison subjects. A second phase, which explored benzodiazepine effects on lactate response and PET findings, will be reported at a later date.
METHOD
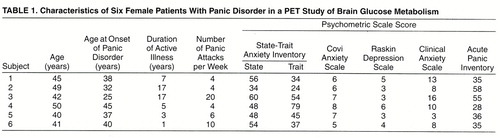
Six female patients with panic disorder and six normal female volunteers were recruited for the study through advertisements posted in local newspapers and at the North Shore University Hospital campus (Manhasset, N.Y.). (Two additional panic disorder patients had been recruited but did not panic in response to lactate infusion [see below] and were eliminated from the study.) All subjects were right-handed. The mean age of the patients with panic disorder was 44.5 years (SD=4.2, range=41–50). These subjects met the DSM-III-R criteria for panic disorder with or without agoraphobia on the basis of the Structured Clinical Interview for DSM-III-R—Patient Version (11). To ensure at least minimal severity of panic disorder, patients had to have experienced at least one panic attack per week for the 2 consecutive weeks immediately before the study. Patients with a coexisting major psychiatric disorder (including current major depression, bipolar disorder, schizophrenia, and substance abuse) were excluded from the study. The acceptable score on the Hamilton Depression Rating Scale at entry into the study was less than 15. The healthy comparison subjects had a mean age of 39.8 years (SD=9.3, range=29–50). They were screened to ensure the absence of psychiatric disorder as well as any family history of anxiety disorder (table 1).
Results of a complete medical evaluation, including history, physical examination, ECG, and laboratory tests, were within normal limits for each subject. All subjects were within 15% of the ideal weight for their height. A urine drug screen was used to confirm the absence of recent medication or substance abuse. All subjects had negative results on a serum pregnancy test before the scan.
Subjects selected for the study had taken no psychopharmacological medication for at least 1 month and no enzyme-inducing agents for at least 7 days prior to the study. A benzodiazepine and toxicology urine screen was performed again on the morning of the scan. All subjects abstained from alcohol and caffeine throughout the 24 hours before the study and fasted for at least 8 hours before the scan.
Informed written consent was obtained from each participant before the study began. The investigation had the approval of the institutional review board of the North Shore University Hospital–New York University School of Medicine.
On the morning of the PET study, following the urine screen, each subject’s symptoms were rated with psychometric instruments. All subjects were given the self-evaluation State-Trait Anxiety Inventory (12), and a psychiatric interview was used to rate symptoms on the Covi Anxiety Scale (13), the Raskin Depression Scale (14), and the Clinical Anxiety Scale (15). After completion of these scales, an intravenous line was inserted in each forearm, one for routine blood chemistries and the other for tracer and lactate infusions. The subject was then positioned in the scanner, and the PET scan began. After completion of the scan, the subject was removed from the scanner, and the lactate infusion was begun while the subject remained recumbent.
Subjects were awake during PET scanning. They were studied with the GE Advance tomograph (General Electric Medical Systems, Milwaukee) at North Shore University Hospital. The performance characteristics of this instrument have been described elsewhere (16). This 18-ring bismuth germinate whole-body tomograph produces 35 slices with an axial field of view of 14.8 cm and a resolution of 4.2 mm (full width at half maximum) in all directions.
In each PET study, the subject was positioned in the scanner by means of a Laitinen stereoadapter with three-dimensional laser alignment with reference to the orbitomeatal line (17). All studies were performed with the subjects’ eyes open in a dimly lit room and with minimal auditory stimulation. The time course of fluorine-18 radioactivity was determined from blood obtained by radial artery puncture 15 minutes after the radiotracer injection (18). In all of the FDG/PET studies, we calculated global and regional cerebral metabolic rates for glucose on a pixel-by-pixel basis, using the autoradiographic method (19-21). Data analysis was performed with statistical parametric mapping, 1995 version (22, 23). The scan from each subject was transformed to a standard template in the Talairach and Tournoux atlas space (24). Images were smoothed with a Gaussian filter of 20×20×12 mm3. Images were then scaled to a global cerebral glucose metabolic rate of 50 µmol/100 ml per minute. Both decreases and increases in patients versus comparison subjects were tested pixel-by-pixel by analysis of variance with global glucose metabolism as the confounding variable. Pixel differences achieving a threshold of at least p<0.01 (uncorrected) were displayed in three orthogonal projections. The stereotactic coordinates of the peak differences were determined with reference to the Talairach and Tournoux atlas.
A standard lactate infusion challenge (25), administered immediately after the PET scan, was conducted under a single-blind condition. All six panic disorder patients and five of the six comparison subjects received the lactate infusion. The infusion sequence consisted of intravenous normal saline for 15 minutes with increased speed during the last 2 minutes to mimic the subsequent lactate infusion. An infusion of 10 cc/kg of 0.5-M sodium lactate (racemic) at body temperature was then administered over 20 minutes or until the subject experienced a panic attack, at which time the lactate infusion was discontinued, and the saline infusion was restarted for an additional 5 minutes. Subjective symptoms were assessed at the start of the saline infusion, immediately before the lactate infusion, halfway through the lactate infusion, and at the end of the lactate infusion. Symptoms were also recorded at the point at which the subject was observed to be experiencing a panic attack. The psychometric test used to assess these symptoms was the Acute Panic Inventory (26) and a 10-point acute anxiety, apprehension, and breathlessness scale. The criterion for determining that a panic attack had occurred was the abrupt onset of four or more DSM-III-R symptoms of panic plus a subjective sense of fear and impending doom. (The mere occurrence of physical symptoms alone was insufficient.) At baseline, throughout the lactate infusion,and for 3 hours afterward, ECG, heart rate, and supine blood pressurewere monitored and recorded at regular intervals.
RESULTS
Two of the eight recruited panic disorder subjects were not lactate sensitive and were not included in the study. None of the healthy comparison subjects responded to lactate.
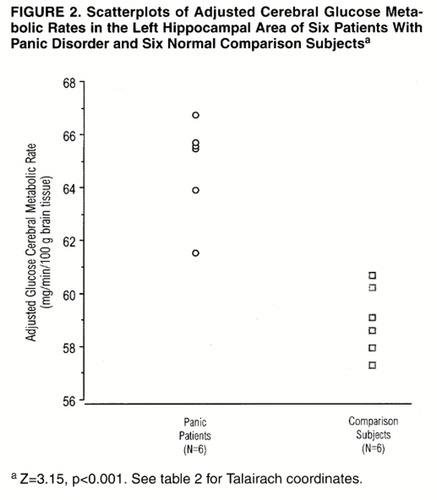
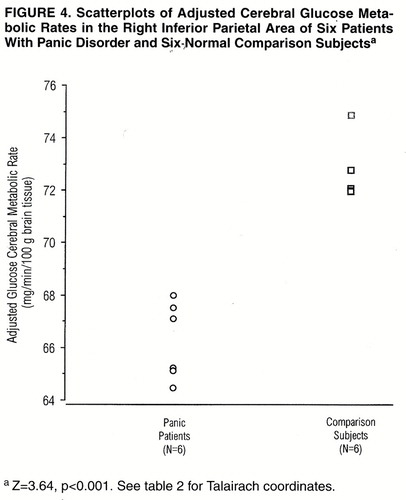
Statistical parametric mapping analyses were used to contrast the scans of the six panic disorder patients and the six normal comparison subjects. A significant difference in glucose metabolism was found, with relative increases in the left hippocampus and parahippocampal area of the panic disorder subjects compared with the values found in this region for the normal subjects (figure 1 and figure 2). Relative decreases were found in the right inferior parietal and right superior temporal brain regions of the panic disorder subjects when compared with the values found in the healthy subjects (figure 3 and figure 4). For both areas, the peak pixel differences actually achieved a level of significance of p<0.001, clearly above the threshold significance of p<0.01 (table 2). There was no overlap in the metabolic rates of the panic disorder and comparison subjects for the regions of difference. No other significant differences were found between the PET scans of the panic disorder patients and those of the comparison subjects.
We found no correlations (or even trends) between scores for the severity of panic disorder, the severity of lactate-induced panic attacks, or the scored clinical variables on the psychometric scales (Covi Anxiety Scale, State-Trait Anxiety Inventory, Raskin Depression Scale, Clinical Anxiety Scale) and the quantified PET findings, although the limitation posed by such a relatively small number of subjects on this statistical analysis must be recognized.
DISCUSSION
PET with FDG was used to compare cerebral metabolic activity in female patients with panic disorder “at rest” and healthy volunteers. The patients with panic disorder had significantly higher glucose metabolism in the left hippocampus and parahippocampal area and significantly lower glucose metabolism in the right inferior parietal and right superior temporal brain regions than did the comparison subjects. While we cannot be certain of the actual mental state of each subject during the PET scan, the scans were conducted less then 30 minutes after the State-Trait Anxiety Inventory questionnaire and the baseline Acute Panic Inventory were administered and indicated a state of relative calmness. Moreover, all subjects appeared to be at rest during the scan, including during the arterial sampling (which was done with local anesthesia). Repeated administration of the State-Trait Anxiety Inventory might have yielded relevant information about mental state during the scan but itself could have been a source of stimulation.
Despite inconsistencies in the observed side of abnormality, all studies now seem to point to the hippocampal and parahippocampal area as a site of aberrant metabolism in individuals with panic disorder who are “at rest.” Whether this abnormality reflects altered blood flow, a change in local neuronal activity, or unusual input into or output from the area is not discernible at present. However, this region, by virtue of its neural inputs from all sensory modalities and outputs to the amygdala, hypothalamus, and brain stem, clearly plays a critical role in mediating affective states. A trait abnormality in this region could produce a vulnerability to misprocessing anxiety-producing stimuli. It is also noteworthy that the locus ceruleus, which contains a rich supply of noradrenergic neurons whose activity is typically blunted by medications effective against panic attacks, has ample projections to the hippocampal formation that are presumably relevant to the anxiety response (27). The hippocampal formation, with its connection to the amygdala, may also play a role in contextual conditioning, that is, conditioning to a place or situation in which environmental stimuli become associated with fearful behavior (28). This is the form of conditioning postulated to be pertinent to the characteristic responses of persons who develop panic disorder and agoraphobia (29).
The diminished glucose metabolism observed in the right inferior parietal and superior temporal regions was not anticipated. Nordahl et al. (6) also reported a decrease in the inferior parietal lobule and in the anterior cingulate, but it was on the left side and during an auditory discrimination task. The temporal lobe does receive input from the limbic system, suggesting a mediating role between affective states and behavioral and physiological responses. Both cortical areas are also functionally related to the premotor frontal areas. There is evidence from a [123I]iomazenil SPECT study (30) of patients with panic disorder of abnormal, elevated benzodiazepine receptor uptake of the labeled benzodiazepine antagonist in the right prefrontal cortex. This may indicate elevated activity of the γ-aminobutyric acid receptor system in the prefrontal cortex, thereby producing a decrease of metabolism “downstream” in areas that are functionally connected, such as the temporal cortex and parietal cortex (24).
The consistent presence of the abnormalities in our patients’ left hippocampal and parahippocampal area and right inferior parietal and superior temporal regions, in contrast to abnormalities reported in the opposite hemisphere by other investigators (4-6), is puzzling. (It is interesting that the coordinates of the maximal change in the parahippocampal region in our subjects are almost identical to those reported by Reiman et al. [5], though in the opposite hemisphere.) We would raise several potentially relevant considerations in attempting to account for the differences. First, there is the homogeneity of our subjects, as all were right-handed women of normal weight, and all were medication free. In addition, all of the patients had untreated panic disorder without a comorbid major psychiatric disorder, and all were proven lactate responders. These criteria did not apply to all of the Reiman et al. (5) or the Nordahl et al. (6) subjects. (Of course, we do not know how applicable our findings are to men with panic disorder.) The importance of a homogeneous experimental group in studies of brain glucose metabolism has been emphasized by Wiesel (31). Furthermore, we measured glucose metabolism, rather than blood flow and oxygen metabolism, which were the basis for the work of Reiman et al. (4, 5), although Nordahl et al. did use FDG. Finally, the Reiman et al. studies were performed on a lower-resolution PET tomograph than ours and used manually placed regions of interest analysis with no reference to a standardized anatomical space, unlike our method.
In our study we had made no a priori decision regarding where any between-group differences would be and therefore adopted a hypothesis-generating approach by choosing statistical parametric mapping as an appropriate exploratory statistic for this form of analysis. While false positives could occur at our chosen level of significance (p<0.01), and while relative image distortion and loss of spatial resolution are introduced by morphing images to a single template, this technique is useful in identifying changes in the distribution of pixels that may be related to different conditions. To ensure the validity of our findings, confirmation with a larger study group is required. In addition, a method of analysis based on a specific region of interest, with magnetic resonance imaging coregistration, may be valuable as a hypothesis-testing approach.
Although the results of our study should be considered preliminary, they offer further support for the concept of a functional brain abnormality in individuals—at least women—who have panic disorder and are lactate sensitive. We assume that this abnormality, presumably located in the hippocampus and the parahippocampal area and possibly in the superior temporal region and the inferior parietal region, plays a role in the pathophysiology of panic disorder. However, a number of questions remain. Do men demonstrate the same findings? Are these genuinely enduring traits that show test-retest reliability? Are the abnormal brain findings reversible with pharmacological or behavioral intervention? Does true asymmetry exist in the regional brain findings, and might this contribute to the pathophysiology of panic disorder? How do the abnormalities actually operate to facilitate the vulnerability to panic disorder? And finally, but perhaps most critically, how or why does abnormal brain function come to be present in individuals who experience panic disorder? Answers to these important questions await further research.
Presented in part at the annual meeting of the American College of Neuropsychopharmacology, San Juan, Puerto Rico, Dec. 9–13, 1996.Received Aug. 15, 1997; ; revision received Dec. 30, 1997; accepted Jan. 6, 1998. From the Department of Psychiatry and the Department of Neurology, North Shore University Hospital–New York University School of Medicine; the Department of Psychiatry, College of Physicians and Surgeons, Columbia University, New York; and Pharmacia & Upjohn Co., Kalamazoo, Mich.. Address reprint requests to Dr. Katz, North Shore University Hospital, 400 Community Dr., Manhasset, NY 11030. Supported by research grant M/2002/0038 from Pharmacia & Upjohn Co. and by NIMH Research Scientist Award MH-00416 to Dr. Gorman.The authors thank Kathy Friend, M.D., Mensud Kurjacovic, M.D., Vijay Dhawan, Ph.D., Laszlo Papp, M.D., Laura K. Pearson, Carol Himelein, and the staff of the PET program at North Shore University Hospital for their assistance with this study.
 |
 |


1. Gorman JM, Liebowitz MR, Fyer AJ, Stein J: A neuroanatomical hypothesis for panic disorder. Am J Psychiatry 1989; 146:148–161Link, Google Scholar
2. Johnson MR, Lydiard RB: The neurobiology of anxiety disorders. Psychiatr Clin North Am 1995; 18:681–725Crossref, Medline, Google Scholar
3. Wiesel FA: Positron emission tomography in psychiatry. Psychiatr Developments 1989; 7:19–47Google Scholar
4. Reiman EM, Raichle ME, Butler FK, Herscovitch P, Robins E: Focal brain abnormality in panic disorder, a severe form of anxiety. Nature 1984; 310:683–685Crossref, Medline, Google Scholar
5. Reiman EM, Raichle ME, Robins E, Butler FK, Herscovitch P, Fox P, Perlmutter J: The application of positron emission tomography to the study of panic disorder. Am J Psychiatry 1986; 143:469–477Link, Google Scholar
6. Nordahl TE, Semple WE, Gross M, Mellman TA, Stein MB, Goyer P, King AC, Uhde TW, Cohen RM: Cerebral glucose metabolic differences in patients with panic disorder. Neuropsychopharmacology 1990; 3:261–272Medline, Google Scholar
7. De Cristofaro MT, Sessarego A, Pupi A, Biondi F, Faravelli C: Brain perfusion abnormalities in drug-naive, lactate-sensitive panic patients: a SPECT study. Biol Psychiatry 1993; 33:505–512Crossref, Medline, Google Scholar
8. Stewart RS, Devous MD Sr, Rush AJ, Lane L, Bonte FJ: Cerebral blood flow changes during sodium-lactate-induced panic attacks. Am J Psychiatry 1988; 145:442–449Link, Google Scholar
9. Woods SW, Koster K, Krystal JK, Smith EO, Zubal IG, Hoffer PB, Charney DS: Yohimbine alters regional cerebral blood flow in panic disorder (letter). Lancet 1988; 2:678Crossref, Medline, Google Scholar
10. Reiman EM, Raichle ME, Robins E, Mintun MA, Fusselman MJ, Fox PT, Price JL, Hackman KA: Neuroanatomical correlates of a lactate-induced anxiety attack. Arch Gen Psychiatry 1989; 46:493–500Crossref, Medline, Google Scholar
11. Spitzer RL, Williams JBW: Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P). New York, New York State Psychiatric Institute, Biometrics Research, 1986Google Scholar
12. Spielberger CD, Gorsuch RL, Lushene RD: Manual for the State-Trait Anxiety Inventory. Palo Alto, Calif, Consulting Psychologists Press, 1997Google Scholar
13. Lipman RS, Covi L: Outpatient treatment of neurotic depression: medication and group psychotherapy, in Evaluation and Psychological Therapies. Edited by Spitzer R, Klein D. Baltimore, Johns Hopkins University Press, 1976, pp 178–218Google Scholar
14. Raskin A, Schulterbrandt J, Reatig N, McKeon JJ: Replication of factors of psychopathology in interview, ward behavior and self-report ratings of hospitalized depressives. J Nerv Ment Dis 1969; 148:87–98Crossref, Medline, Google Scholar
15. Snaith RT, Baught FJ, Clayden AD, Husain A, Sipple MA: The Clinical Anxiety Scale: an instrument derived from the Hamilton Anxiety Scale. Br J Psychiatry 1982; 141:518–523Crossref, Medline, Google Scholar
16. Robeson W, Dhawan V, Takikawa S, Babchyck B, Zanzi I, Margouleff D, Eidelberg D: SuperPETT 3000 time-of-flight PET tomograph: optimization of factors affecting quantification. IEEE Transactions on Nuclear Science 1993; 40:135–142Crossref, Google Scholar
17. Hariz MI, Erikson AT: Reproducibility of repeated mounting of a noninvasive CT/MRI stereoadapter. Applied Neurophysiology 1986; 49:336–347Medline, Google Scholar
18. Takikawa S, Dhawan V, Spetsieris P, Robeson W, Chaly T, Dahl JR, Margouleff D, Eidelberg D: Noninvasive quantitative fluorodeoxyglucose PET studies with an estimated input function derived from a population-based arterial blood curve. Radiology 1993; 188:131–136Crossref, Medline, Google Scholar
19. Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE: Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol 1979; 6:371–388Crossref, Medline, Google Scholar
20. Eidelberg D, Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Chaly T, Robeson W, Dahl JR, Margouleff D: Assessment of disease severity in parkinsonism with fluorine-18-fluorodeoxyglucose and PET. J Nucl Med 1995; 36:378–383Medline, Google Scholar
21. Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, Chaly T, Robeson W, Margouleff D, Przedborski S: The metabolic topography of parkinsonism. J Cereb Blood Flow Metab 1994; 14:783–801Crossref, Medline, Google Scholar
22. Friston KJ, Ashburner J, Poline J, Frith C, Heather J, Frackowiak RSJ: Spatial realignment and normalization of images. Human Brain Mapping 1995; 2:165–189Crossref, Google Scholar
23. Friston KJ, Holmes A, Worsley K, Poline J, Frith C, Heather J, Frackowiak RSJ: Statistical parametric maps in functional imaging: a general approach. Human Brain Mapping 1995; 2:189–210Crossref, Google Scholar
24. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical, 1988Google Scholar
25. Liebowitz MR, Fyer AJ, Gorman JM, Dillon D, Appelby IL, Levy G, Anderson S, Levitt M, Palij M, Davies SO, Klein DF: Lactate provocation of panic attacks, I: clinical and behavioral findings. Arch Gen Psychiatry 1984; 41:764–770Crossref, Medline, Google Scholar
26. Dillon DJ, Gorman JM, Liebowitz MR, Fyer AJ, Klein DF: Measurement of lactate-induced panic and anxiety. Psychiatry Res 1987; 20:97–105Crossref, Medline, Google Scholar
27. Gray JA: The Neuropsychology of Anxiety: An Enquiry Into the Functions of the Septo-Hippocampal System. Oxford, England, Oxford University Press, 1982Google Scholar
28. Phillips RG, Le Doux JP: Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 1992; 106:274–285Crossref, Medline, Google Scholar
29. Goddard AW, Charney DS: Toward an integrated neurobiology of panic disorder. J Clin Psychiatry 1997; 58:4–11Medline, Google Scholar
30. Kuikka JT, Pitkanen A, Lepola U, Partanen K, Vainio P, Bergstrom KA, Wieler HJ, Kaiser KP, Mittelbach L, Koponen H, Leinonen E, Riekkinen PJ: Abnormal regional benzodiazepine receptor uptake in the prefrontal cortex in patients with panic disorder. Nucl Med Commun 1995; 16:273–280Crossref, Medline, Google Scholar
31. Wiesel FA: Glucose metabolism in psychiatric disorders: how can we facilitate comparisons among studies? J Neural Transm 1992; 37:1–18Google Scholar



