CO2-Induced Panic Attacks: A Twin Study
Abstract
Objective: The authors investigated the role of genetic factors in 35% CO2-induced panic attacks.Method: Ninety twins recruited from general population were challenged with one-vital capacity inhalations of 35% CO2–65% O2. Probandwise concordance rates were calculated and rates compared for monozygotic and for dizygotic twins.Results: A significantly higher concordance was found for 35% CO2-induced panic attacks among monozygotic than dizygotic twins (55.6% versus 12.5%).Conclusions: These results suggest a relevant role of genetic factors in 35% CO2-induced panic attacks. Am J Psychiatry 1998; 155: 1184-1188
Hypersensitivity to CO2 inhalation is one of the most widely studied laboratory markers of panic disorder, and many studies have clearly demonstrated the ability of single- or double-vital capacity inhalations of gas mixtures with high concentrations of CO2 (35%) (1-4) to induce acute anxiety/panic in patients with panic disorder. The nature of this association, however, is not yet clearly understood. Growing evidence suggests that hypersensitivity to 35% CO2 might be a trait marker for panic disorder rather than a simple state marker. Anxiety reactivity to 35% CO2 inhalations has been reported to be not significantly influenced by clinical characteristics of the disorder such as baseline anxiety, frequency of panic attacks, severity of agoraphobia, and duration of illness, as well as by age (4) and personality (5) dimensions. Recent studies have suggested that there might be a familial association between panic disorder and hypersensitivity to 35% CO2. Rates of CO2-induced panic attacks have been shown to be higher in healthy first-degree relatives of panic patients than in healthy control subjects (6) or in healthy first-degree relatives of patients with mood disorders (7), and morbidity risk for panic disorder in families of panic patients who are hypersensitive to 35% CO2 was higher than that for families of panic patients with normal sensitivity to CO2(8).
Given these data, it can be speculated that CO2 hyperreactivity might be the expression of an underlying familial vulnerability to panic directly related to its pathogenetic mechanisms. A relevant role of genetic factors in panic disorder is clearly supported by the literature. Many studies have reported the existence of a strong familial loading for panic disorder (9, 10), and twin studies have reported a higher concordance for panic disorder in monozygotic than in dizygotic twins (11-14), supporting the genetic nature of this vulnerability. Given that the etiology of panic disorder is related to genetic factors, if there is a pathogenetic link between panic disorder and CO2 hypersensitivity, it can be hypothesized that in 35% CO2-induced panic attacks, genetic factors might also play a relevant role. Twin studies may clarify the relative contributions of genetic and nongenetic factors.
In the present study, we investigated the genetic background of CO2-induced panic attacks, comparing the twin concordance rate for CO2-induced panic attacks in a sample of same-sex pairs of twins obtained from the Italian general population.
METHOD
Subjects
One hundred thirty-two same-sex pairs of twins, randomly taken from the Register-Office List of Twins covering all twin birth in Milan from 1957 to 1974, were personally contacted by telephone and were asked “to participate in a twin study dealing with the role of genetic factors in the pathogenetic mechanisms underlying anxiety disorders.” Fifty-five pairs (42%) accepted, but 10 were excluded because of contraindications related to the challenge (three for significant respiratory disorders, two for significant cardiac/circulatory disorders or hypertension, two for family history of cerebral aneurysm, one for epilepsy, and two for significant risk of pregnancy).
Exclusion criteria were significant cardiac/circulatory and respiratory disorders, personal or family history of cerebral aneurysm, significant hypertension (systolic blood pressure >180 mm Hg, diastolic >100 mm Hg), pregnancy, or epilepsy, all determined by direct physical examination and careful collection of medical histories.
At the time of the challenge, it was required that none of the subjects had taken any psychotropic medications during the last 2 weeks. They were asked to refrain from alcohol consumption for at least 36 hours, beverages containing xanthine for at least 8 hours, and food or smoking for at least 2 hours before the test.
The sample included 25 pairs of dizygotic and 20 pairs of monozygotic twins. Venous blood samples, anticoagulated with EDTA, were drawn from all probands. Genomic DNA was extracted from thawed blood by salting out with saturated NaCl solution. Determination of the twins’ zygosity was made by direct administration of a questionnaire that has been reported to predict zygosity correctly in 95% of cases, when compared with results obtained by blood analysis of genetic markers (15). For each twin we also analyzed a hypervariable single locus variable number of tandem repeats marker. The alleles at the D1S80 locus (16) were amplified by using the reagents included in the AmpliFLP D1S80 PCR Amplification and Typing Kit (Perkin-Elmer Italia, Monza, Italy). Amplification protocol and interpretation of results were performed according to the manufacturer’s instructions. All 13 pairs of twins with discordant alleles at the D1S80 locus were independently judged to be dizygotic according to the questionnaire administered, thus supporting the validity of the method of determination of the twins’ zygosity applied in the study.
Twin pairs were investigated for the presence of anxiety disorders and family history of panic disorder by two experienced psychiatrists, who were blind to the results of the 35% CO2 challenge of both twins and the diagnostic assessment of the co-twin. Twins’ diagnoses were made by using the anxiety disorders section of the National Institute of Mental Health Diagnostic Interview Schedule, version III, revised (17), and data obtained were reevaluated by the same psychiatrists to generate DSM-IV diagnoses. In addition, the presence of sporadic unexpected panic attacks was assessed according to the DSM-IV definition of a panic attack. Information on history of panic disorder in first-degree relatives of twins was obtained by using a modified version of the Family History Research Diagnostic Criteria (18, 19), adapted to generate psychiatric diagnoses according to DSM-IV criteria. Consensus diagnoses for first-degree relatives of each pair of twins were obtained after a discussion of the information obtained from each twin of a pair.
Data were analyzed by Student’s t tests and chi-square analyses. To analyze concordance rates between monozygotic and dizygotic pairs of twins, the “probandwise” method was applied and rates compared calculating z scores for differences between concordance rates.
Procedure
Two gas mixtures were used: compressed air (placebo) and a mixture of 35% CO2 and 65% O2. Both gases were inhaled through the same self-administration mask connected to a respirometer used to measure the vital capacity of each subject and the volume of gas delivered in each inhalation.
The same day of the diagnostic evaluation, each twin underwent the 35% CO2 challenge in a double-blind, random, crossover design, according to the method developed by Griez et al. (1) and described in detail elsewhere (20). Twins were informed that they would be inhaling two harmless gas mixtures containing different percentages of CO2 and O2 and that they might experience some discomfort, ranging from a few neurovegetative symptoms to a definite sensation of anxiety. They were not told that one was compressed air. The possibility of a panic attack was not mentioned in order to avoid any negative cognitive bias related to expectation. The brief nature (a few seconds to 1 minute) of the panic reaction provoked by the inhalation of the 35% CO2–65% O2 gas mixture made this decision ethical. After vital capacity was measured, each subject inhaled one vital capacity of 35% CO2–65% O2 or of compressed air, in a randomly assigned order, at an interval of 25–30 minutes. At the end of each inhalation subjects were asked to hold their breaths for 4 seconds. We considered the challenge valid only if subjects had inhaled at least 80% of their previously measured vital capacities. Subjects who failed to inhale 80% or more of their vital capacities were invited to repeat the procedure after a resting interval of at least 1 day.
Immediately before and after each inhalation (air or CO2), anxiety was evaluated by a visual analogue scale for anxiety that described the degree of global subjective anxiety on a continuum from 0 (no anxiety present) to 100 (the worst anxiety ever imaginable). In addition, subjects were asked to score themselves on the Panic Symptom List III-R, a self-rating questionnaire assessing, on a 5-point scale, the 13 panic symptoms described in DSM-III-R (21) and DSM-IV. Global anxiety reactivity was evaluated as Δ% visual analogue scale for anxiety score (the percent of maximum increment or decrement possible on the visual analogue scale for anxiety; values ranged from –100 to 100) (20), calculated as follows: 1) if Δ visual analogue scale for anxiety score (post-CO2 scale values minus pre-CO2 scale values) was positive, then Δ% visual analogue scale for anxiety score=Δ scale score multiplied by 100 divided by total of 100 minus scale score before CO2); and 2) if Δ visual analogue scale for anxiety score was negative, then Δ% scale score=Δ scale score multiplied by 100 divided by scale score before CO2.
According to the scales described and DSM-IV criteria defining a panic attack, the reaction to the 35% CO2 challenge was considered to be an induced panic attack if it included a sensation of fear or panic with at least Δ% visual analogue scale for anxiety score of 26 or more, an ideal threshold previously shown by a receiver operating characteristic analysis of the 35% CO2 challenge to separate panic patients and healthy control subjects (22), and an increment of at least two points for at least four symptoms on the self-rating questionnaire.
The procedure followed for obtaining informed consent for the 35% CO2 challenge was approved by the Human Ethics Committee of San Raffaele Hospital, and, after the participants were given a complete description of the study, written informed consent was obtained.
RESULTS
There were no statistical differences between the two twin groups in age (monozygotic: mean=28.7 years, SD=5.2; dizygotic: mean=28.4, SD=3.7), educational level (monozygotic: mean=14.0 years, SD=3.2; dizygotic: mean=13.6 years, SD=3.1), or sex distribution (monozygotic: five male pairs and 15 female pairs; dizygotic: eight male pairs and 17 female pairs).
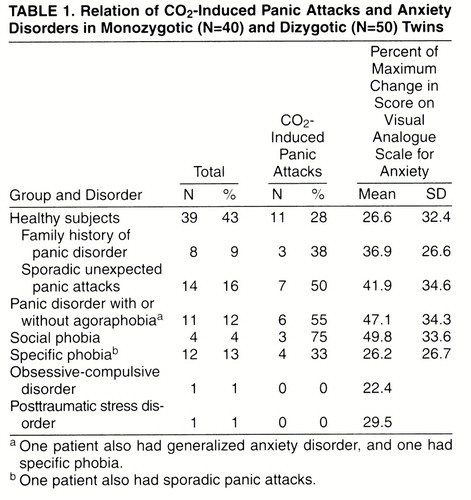
Thirty-four twins (37.7%), 18 monozygotic and 16 dizygotic, reacted with an induced panic attack to the 35% CO2 challenge. In 13 pairs of monozygotic twins and in 15 pairs of dizygotic twins, at least one of the twins reacted with an induced panic attack to the inhalation of 35% CO2–65% CO2. Table 1 reports data on diagnoses of anxiety disorders and sporadic panic attacks in our sample, family history of panic disorder in healthy twins, and the relative rates of induced panic attacks. Of the 11 twins with panic disorder, five (45%) also had agoraphobia. The rate of families with secondary cases of panic disorder in our sample of twins was 17.8% (eight of 45); no significant differences were found between rates in monozygotic (five of 20, 25%) and dizygotic (three of 25, 12%) pairs of twins. Rates of families with secondary cases of panic disorder were 100% (three of three) in pairs of twins concordant for panic disorder, 20% (one of five) in those discordant for panic disorder, and 13.5% (five of 37) in those without panic disorder.
Regarding panic disorder, among monozygotic twins three pairs were concordant and three were discordant, while among dizygotic twins none was concordant and two were discordant. Probandwise concordance rates for panic disorder were higher in monozygotic pairs (six of nine, 67%) than in dizygotic pairs (none, 0%). Regarding spontaneous panic attacks, among monozygotic twins five pairs were concordant and four were discordant, while among dizygotic twins one was concordant and nine were discordant. Probandwise concordance rates for spontaneous panic attacks were higher in monozygotic twins (10 of 14, 71%) than in dizygotic twins (two of 11, 18%).
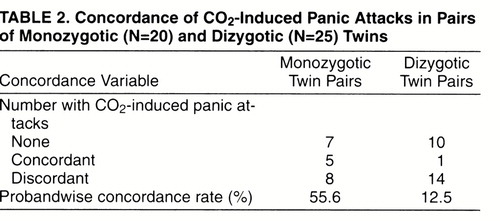
Table 2 reports data on CO2-induced panic attacks in monozygotic and dizygotic twins. Among monozygotic pairs, five were concordant and eight discordant for CO2-induced panic attacks, while among dizygotic pairs, one was concordant and 14 were discordant. Probandwise concordance rates for CO2-induced panic attacks were significantly higher in monozygotic pairs (10 of 18, 55.6%) than in dizygotic pairs (two of 16, 12.5%) (test of the difference between two proportions: z=2.3, p<0.03; 95% confidence interval for the difference=0.109–0.753), with a monozygotic/dizygotic ratio of 4.4.
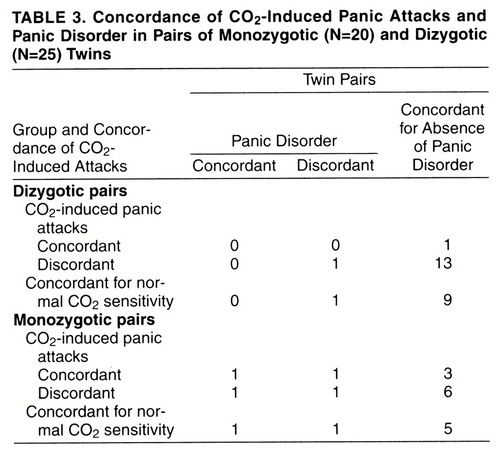
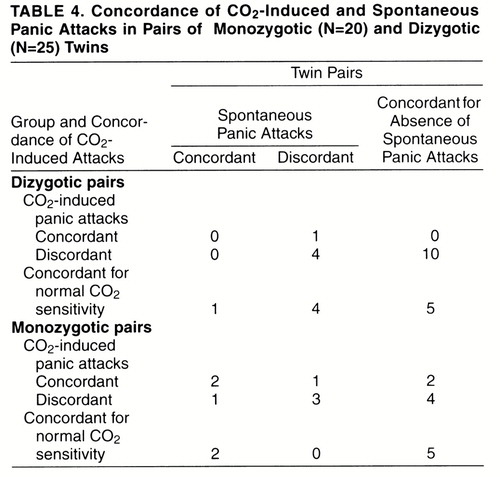
Table 3 and table 4 report monozygotic and dizygotic concordance rates for CO2-induced panic attacks and, respectively, personal history of panic disorder and spontaneous panic attacks, either sporadic or recurrent. For the pairs that were concordant for CO2-induced panic attacks, among dizygotic pairs none was concordant and none discordant for panic disorder, and none was concordant and one discordant for spontaneous panic attacks; among monozygotic pairs one was concordant and one discordant for panic disorder, and two were concordant and one was discordant for spontaneous panic attacks. Twins’ probandwise concordance rates for panic disorder and spontaneous panic attacks among those concordant for CO2-induced panic attacks were, respectively, two (67%) of three and four (80%) of five for monozygotic pairs and none and none (0%) of one for dizygotic pairs;among pairs with normal sensitivity to CO2, rates were two (67%) of three and four (100%) of four for monozygotic pairs and none (0%) of one and two (33%) of six for dizygotic pairs.
DISCUSSION
The present study addresses the role of inherited and noninherited components in 35% CO2-induced panic attacks. The results of this study might be considered preliminary, since the sample of twins examined was not very large. Some preliminary observations should be reported.
The prevalence of panic disorder in our sample (12%) is higher than the prevalence reported for general populations (9), possibly as the result of the way in which subjects were recruited, which probably made subjects who had anxiety symptoms more interested in participation in the study. The prevalence of sporadic panic attacks was similar to the prevalence reported for general populations (9, 23).
The CO2-induced panic attack rate of 28% among healthy subjects in this sample seems to be higher than values reported in previous studies (4, 6, 20) and near that for those with a family history of panic disorder (38%). It should be noted, however, that in the present study the sample examined was composed of twins, who are not independent subjects; in the other studies the groups of healthy subjects were composed of independent subjects. Thus, rates obtained in this study are not comparable to those from our previous studies. In addition, the sample of healthy subjects with a family history of panic disorder was very small (N=8), making a type II error possible.
Probandwise concordance rates for 35% CO2-induced panic attacks were significantly higher in monozygotic (55.6%) than in dizygotic (12.5%) pairs of twins, demonstrating that heritability contributes substantially to the susceptibility to react to the inhalation of hypercapnic gas mixtures with a panic-like episode. The plausibility of this idea is supported by the evidence of a relevant role of genetic factors both in panic disorder (11-14) and in chemosensitivity (24-26). It should also be recognized that although monozygotic pairs show a higher concordance rate than dizygotic pairs, half of the monozygotic pairs of twins were discordant. This finding suggests that nongenetic biological/environmental factors may also play an important role in the development of CO2-induced panic attacks.
To our knowledge, this is the first study to examine the role of genetic factors in the susceptibility to panic-provoking procedures. The observation of a relevant role of genetic factors in CO2-induced panic attacks might suggest that the recently reported association between CO2 hyperreactivity and panic disorder (6-8) might be genetic in nature.
According to table 1, in the present sample, CO2 hypersensitivity seems to be not specifically related to the diagnosis of panic disorder but also a characteristic of other anxiety disorders (social and specific phobia) and of a relevant sample of nonclinical subjects. Thus, hypersensitivity to CO2 might be considered not a simple marker of panic disorder but the expression of a vulnerability to a spectrum of disorders, whose main clinical expression might be represented by panic disorder.
Similar to CO2-induced panic, concordance rates for panic disorder were higher in monozygotic than in dizygotic pairs of twins, confirming data previously reported in the literature (13, 14). It should be observed, however, that there was only a partial overlap between CO2-induced panic and panic disorder among monozygotic pairs of twins, and thus, at least some of the genetic factors involved in these two phenotypes might be different. In addition, the higher concordance rates for panic disorder and spontaneous panic attacks in monozygotic than in dizygotic twin pairs were present both in pairs concordant for CO2-induced panic attacks and in those concordant for normal sensitivity to CO2, confirming that there might not be a complete match between the genetic factors of “clinical panic attacks” and those of “CO2-induced panic attacks” and that other inherited factors, not related to CO2 sensitivity, might play a role in the pathogenesis of panic disorder.
CO2-induced panic attacks might be considered a phenotypic expression of a genetic vulnerability to panic disorder even before the clinical onset of panic disorder. Formal and molecular genetic studies of panic disorder, in particular studies of families (i.e., segregation analysis), might consider healthy relatives of panic patients hypersensitive to panic as affected members.
CO2 hypersensitivity might be the expression of an impairment, under genetic control, at some level of the respiratory control system. This impairment might be anatomically related to specific medullary H+-sensitive neurons (27, 28), as well as to an imbalance of neurotransmitters regulating respiratory control mechanisms. Recent studies (29, 30) have reported that treatment with antipanic agents (selective serotonin reuptake inhibitors, tricyclic antidepressants) significantly modulates CO2 hypersensitivity in panic patients. The main neurotransmitters modulated by these antipanic medications have been reported to influence respiration (31-33). Serotonergic system activation and α-adrenoceptor or cholinergic receptor blockade reduce CO2 sensitivity (33), and it has been claimed that the modulation of these neurotransmitters plays a role in the pathogenetic mechanisms of panic disorder (34-37). Finally, it is noteworthy that Bernard et al. (38) have found “chemosensitive sites in the raphe, the locus ceruleus, the nucleus tractus solitarius, and near the surface of ventro-lateral medulla,” all CNS areas reported to play a significant role in the pathogenesis of panic disorder (39). Abnormalities at these levels of the respiratory control mechanisms might explain why analogous stimulations of the respiratory centers trigger abnormal responses in panic patients but not in healthy subjects. These abnormalities might be explained by a specific alarm system developed to control for possible risks of suffocation (40-42).
Some limitations of the present study must be recognized. The relatively small size of our sample makes a type I error possible. Since we used a cutoff measure of 80% of vital capacity to consider valid the result of the 35% CO2 challenge, below this threshold the amount of CO2–O2 gas mixtures inhaled by each twin of a pair might differ and data obtained might not be completely comparable. Finally, given the method used to determine zygosity of pairs of twins, the possibility cannot be excluded that some pairs might be misdiagnosed as monozygotic or dizygotic.
In conclusion, our findings suggest a relevant role of genetic factors in CO2-induced panic attacks. If these data are confirmed by other studies, CO2 hypersensitivity might become a valid tool in etiologic and genetic investigations of panic disorder.
Received Aug. 11, 1997; revision received Jan. 5, 1998; accepted Feb. 9, 1998. From the Anxiety Disorders Clinical and Research Unit, Department of Neuropsychiatric Sciences, Istituto Scientifico H.S. Raffaele.. Address reprint requests to Prof. Bellodi, Department of Neuropsychiatric Sciences, Istituto Scientifico H.S. Raffaele, 29 via Prinetti 20127, Milan, Italy.
 |
 |
 |
 |
1. Griez EJL, Lousberg H, van den Hout MA, van der Molen GM: CO2 vulnerability in panic disorder. Psychiatry Res 1987; 20:87–95Crossref, Medline, Google Scholar
2. Fyer MR, Uy J, Martinez J, Goetz R, Klein DF, Fyer A, Liebowitz MR, Gorman J: CO2 challenge of patients with panic disorder. Am J Psychiatry 1987; 144:1080–1082Link, Google Scholar
3. Gorman JM, Papp LA, Martinez J, Goetz RR, Hollander E, Liebowitz MR, Jordan F: High dose carbon dioxide challenge test in anxiety disorder patients. Biol Psychiatry 1990; 28:743–757Crossref, Medline, Google Scholar
4. Perna G, Battaglia M, Garberi A, Arancio C, Bertani A, Bellodi L: Carbon dioxide/oxygen challenge test in panic disorder. Psychiatry Res 1994; 52:159–171Crossref, Medline, Google Scholar
5. Perna G, Arancio C, Bertani A, Gabriele A, Bellodi L: Personality dimensions and reactivity to 35% CO2 in panic patients and healthy controls. Eur Psychiatry 1994; 9:236–240Google Scholar
6. Perna G, Cocchi S, Bertani A, Arancio C, Bellodi L: Sensitivity to 35% CO2 in healthy first-degree relatives of patients with panic disorder. Am J Psychiatry 1995; 152:623–625Link, Google Scholar
7. Coryell WH: Hypersensitivity to carbon dioxide as a disease specific trait marker. Biol Psychiatry 1997; 41:259–263Crossref, Medline, Google Scholar
8. Perna G, Bertani A, Caldirola D, Bellodi L: Family history of panic disorder and hypersensitivity to CO2 in patients with panic disorder. Am J Psychiatry 1996; 153:1060–1064Link, Google Scholar
9. Weissman MM: Family genetic studies of panic disorder. J Psychiatr Res 1993; 27(suppl 1):69–78Google Scholar
10. Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lepine JP, Newman SC, Oakley-Browne MA, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen HU, Yeh EK: The cross-national epidemiology of panic disorder. Arch Gen Psychiatry 1997; 54:305–309Crossref, Medline, Google Scholar
11. Skre I, Onstad S, Torgersen S, Lygren S, Kringlen E: A twin study of DSM III-R anxiety disorders. Acta Psychiatr Scand 1993; 88:85–92Crossref, Medline, Google Scholar
12. Kendler KS, Neale MC, Kessler RC, Heat AC, Eaves LJ: Panic disorder in women: a population-based twin study. Psychol Med 1993; 23:397–406Crossref, Medline, Google Scholar
13 Torgersen S: Genetic factors in anxiety disorders. Arch Gen Psychiatry 1983; 40:1085–1089Crossref, Medline, Google Scholar
14. Perna G, Caldirola D, Arancio C, Bellodi L: Panic attacks: a twin study. Psychiatry Res 1997; 66:6–71Crossref, Google Scholar
15. Torgersen S: The determination of twin zygosity by means of a mailed questionnaire. Acta Genet Med Gemellol (Roma) 1979; 28:225–236Crossref, Medline, Google Scholar
16. Budowle B, Chakraborty R, Giusti AM, Eisenberg AJ, Robert CA: Analysis of the VNTR Locus D1S80 by the PCR followed by high-resolution PAGE. Am J Hum Genet 1991; 48:137–144Medline, Google Scholar
17. Robins LN, Helzer JE, Cottler L, Golding E: National Institute of Mental Health Diagnostic Interview Schedule, version III, revised. St Louis, Washington University, Department of Psychiatry, 1989Google Scholar
18. Andreasen NC, Endicott J, Spitzer RL, Winokur G: The family history method using diagnostic criteria: reliability and validity. Arch Gen Psychiatry 1977; 34:1229–1235Crossref, Medline, Google Scholar
19. Andreasen NC, Rice J, Endicott J, Reich T, Coryell W: The family history approach to diagnosis: how useful is it? Arch Gen Psychiatry 1986; 43:421–429Google Scholar
20. Perna G, Bertani A, Arancio C, Ronchi P, Bellodi L: Laboratory response of patients with panic and obsessive-compulsive disorders to 35% CO2 challenges. Am J Psychiatry 1995; 152:85–89Link, Google Scholar
21. Pols H, Zandbergen J, de Loof C, Griez E: Attenuation of carbon dioxide-induced panic after clonazepam treatment. Acta Psychiatr Scand 1991; 84:585–586Crossref, Medline, Google Scholar
22. Battaglia M, Perna G: The 35% CO2 challenge in panic disorder: optimization by receiver operating characteristic (ROC) analysis. J Psychiatr Res 1995; 29:111–119Crossref, Medline, Google Scholar
23. Norton G, Harrison B, Hauch J, Rhodes L: Characteristics of people with infrequent PAs. J Abnorm Psychol 1985; 94:216–221Crossref, Medline, Google Scholar
24. Arkinstall WW, Nirmel K, Klissouras V, Milic-Emili J: Genetic differences in the ventilatory response to inhaled CO2. J Appl Physiol 1974; 36:6–11Crossref, Medline, Google Scholar
25. Beral V, Read DJC: Insensitivity of respiratory centre to carbon dioxide in the Enga people of New Guinea. Lancet 1971; 2:1290–1294Crossref, Medline, Google Scholar
26. Saunders NA, Leeder SR, Rebuch AS: Ventilatory response to carbon dioxide in young athletes: a family study. Am Rev Resp Dis 1976; 113:497–502Medline, Google Scholar
27. Arita H, Ichikawa K, Kuwana S, Kogo N: Possible locations of pH-dependent central chemoreceptors: intramendullary regions with acidic shift of extracellular fluid pH during hypercapnia. Brain Res 1989; 485:285–293Crossref, Medline, Google Scholar
28. Kogo N, Arita H: In vivo study on medullary H+-sensitive neurons. J Appl Physiol 1990; 69:1408–1412Crossref, Medline, Google Scholar
29. Bertani A, Perna G, Caldirola D, Arancio C, Bellodi L: Pharmacologic effect of paroxetine, sertraline and imipramine on reactivity to the 35% CO2 challenge: a double blind, random, placebo controlled study. J Clin Psychopharmacol 1997; 17:97–101Crossref, Medline, Google Scholar
30. Perna G, Bertani A, Gabriele A, Politi E, Bellodi L: Modification of 35% CO2 carbon dioxide hypersensitivity across one week of treatment with clomipramine and fluvoxamine: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol 1997; 17:173–178Crossref, Medline, Google Scholar
31. Bonham AC: Neurotransmitters in the CNS control of breathing. Respiration Physiol 1995; 101:219–230Crossref, Medline, Google Scholar
32. Eldridge FL, Millhorn DE: Central regulation of respiration by endogenous neurotransmitters and neuromodulators. Ann Rev Physiol 1981; 43:121–135Crossref, Medline, Google Scholar
33. Mueller AR, Lundberg DBA, Breese GR, Hedner J, Hedner T, Jonason Y: The neuropharmacology of respiration control. Pharmacol Rev 1982; 34:255–279Medline, Google Scholar
34. Brambilla F, Perna G, Garberi A, Nobile P, Bellodi L: α2-Adrenergic receptor sensitivity in panic disorder, I: GH response to GH-RH and clonidine stimulation in panic disorder. Psychoneuroendocrinology 1995; 20:1–9Crossref, Medline, Google Scholar
35. Brambilla F, Bellodi L, Arancio C, Nobile P, Perna G: α2-Adrenergic receptor sensitivity in panic disorder, II: cortisol response to clonidine stimulation in panic disorder. Psychoneuroendocrinology 1995; 20:11–19Crossref, Medline, Google Scholar
36. Cooney JM, Lucey JV, Dinan TG: Enhanced growth hormone responses to pyridostigmine challenge in patients with panic disorder. Br J Psychiatry 1997; 170:159–161Crossref, Medline, Google Scholar
37. Coplan JD, Gorman JM, Klein DF: Serotonin related functions in panic-anxiety: a critical overview. Neuropsychopharmacology 1992; 6:189–200Medline, Google Scholar
38. Bernard DG, Li A, Nattie EE: Evidence of central chemoreception in the midline raphe. J Appl Physiol 1995; 80:108–115Google Scholar
39. Gorman JM, Liebowitz MR, Fyer AJ, Stein J: A neuroanatomical hypothesis for panic disorder. Am J Psychiatry 1989; 146:148–161Link, Google Scholar
40. Klein DF: False suffocation alarms, spontaneous panics, and related conditions: an integrative hypothesis. Arch Gen Psychiatry 1993; 50:306–317Crossref, Medline, Google Scholar
41. Klein DF: Testing the false alarm theory of panic disorder. Anxiety 1994; 1:1–7Crossref, Medline, Google Scholar
42. Klein DF: Panic disorder and agoraphobia: hypothesis hothouse. J Clin Psychiatry 1996; 57:21–27Medline, Google Scholar



