Apolipoprotein E ε4 Allele and Whole Brain Atrophy in Late-Onset Alzheimer's Disease
Abstract
OBJECTIVE: Diffuse brain atrophy is one of the gross pathological features of Alzheimer's disease and is a result of degenerative changes. The ε4 allele of apolipoprotein E (APOE) is a risk factor or susceptibility gene in late-onset sporadic Alzheimer's disease and may influence the pathological changes associated with the disease. The aim of this study was to examine the relationship between the APOE ε4 allele and whole brain atrophy. METHOD: Whole brain volume was quantified by using high-resolution magnetic resonance imaging and the computerized brain segmentation technique in 178 patients with late-onset sporadic Alzheimer's disease who carried no APOE ε4 alleles (N=62), one ε4 allele (N=93), or two (N=23) and had comparable clinical severity of dementia. RESULTS: An apparent positive correlation was found between normalized whole brain volume (relative to total intracranial volume) and number of APOE ε4 alleles; i.e., patients carrying two APOE ε4 alleles had the least brain atrophy. This association between the APOE ε4 allele and brain volume was similar in women and men and was independent of age, level of education, duration of illness since symptom onset, and severity of dementia. CONCLUSIONS: The results indicate that cognitive dysfunction pro~gresses before severe brain atrophy develops in patients carrying the APOE ε4 allele and suggest that an APOE ε4-allele-related mechanism that affects neuronal function before a decrement in brain matter is involved in the development of dementia.
The neuropathological changes that occur in Alzheimer's disease include neurofibrillary tangles, senile plaques, and neuronal and synaptic losses, which predominantly affect the association areas of the neocortex and regions of the hippocampus and amygdala but also diffusely affect the whole brain. Diffuse brain atrophy, which is one of the gross pathological features of Alzheimer's disease and which is a result of degenerative changes, correlates in general with global measures of dementia severity during life (1–3). Cerebral atrophy is primarily due to neurofibrillary degeneration and loss of intrinsic pyramidal cells and their processes (gray matter) and axons (white matter) (4).
Apolipoprotein E (APOE), one of the protein constituents of plasma lipoproteins and an important regulator of lipid metabolism (5), exists in three major isoforms (E2, E3, and E4) encoded by three alleles (ε2, ε3, and ε4) of the APOE gene. APOE is present in senile plaques, neurofibrillary tangles, and cerebrovascular amyloid (6). The APOE ε4 allele is a risk factor for late-onset Alzheimer's disease (7). Several postmortem studies have demonstrated an association between the APOE gene and senile plaques or neurofibrillary tangle formations. A greater accumulation of β-amyloid protein is associated with the APOE ε4 allele (8, 9). One study (10) reported a significant correlation between the APOE genotype and neurofibrillary tangle formation, whereas another study (8) found no evidence for such a correlation.
Direct measurement of brain volume with magnetic resonance imaging (MRI) has emerged as a useful method for quantifying brain atrophy. In this study we tested the hypothesis that the magnitude of diffuse brain atrophy in proportion to the severity of dementia is different in persons with the APOE ε4 allele. Using high-resolution MRI and automated volumetry software, we quantified brain atrophy by directly measuring the whole brain volume and intracranial volume of patients with probable Alzheimer's disease with mild to moderate severity of dementia. By controlling for dementia severity as well as age, disease duration, and education, which may have an influence on brain atrophy, we investigated the possible relationship between the genetic variants of APOE and the loss of brain matter in patients with Alzheimer's disease.
METHOD
Before this study began, written informed consent was obtained for all patients and from all volunteers according to the Declaration of Human Rights, Helsinki, 1975. The whole procedure strictly followed the 1993 clinical study guidelines of the Ethics Committee of the Hyogo Institute for Aging Brain and Cognitive Disorders and was approved by the internal review board.
One hundred seventy-eight patients with Alzheimer's disease were recruited from those who were consecutively seen in the infirmary of the Hyogo Institute, a research-oriented hospital for dementia, for examination between October 1994 and May 1996. All patients were examined by both neurologists and psychiatrists during a short-term admission and underwent routine laboratory tests, standard neuropsychological examinations including the Alzheimer's Disease Assessment Scale—Cognitive Part (11) and the Mini-Mental State examination (12), EEG, MRI of the brain, magnetic resonance angiography of the neck and head, and cerebral perfusion/metabolism studies by positron emission tomography (PET) or single photon emission computed tomog~raphy (13). The MRI and neuropsychological evaluation were performed within 1 month of each other. Among the requirements for inclusion were 1) meeting the criteria of the National Institute of Neurological Disease and Stroke/Alzheimer's Disease and Related Disorders Association for probable Alzheimer's disease (14), 2) sporadic occurrence of the disease (i.e., no family history of dementia in both first- and second-degree relatives according to an informant-based assessment), 3) onset of dementia at or after 60 years of age, 4) no complication of other neurological or mental diseases, and 5) no evidence of developmental abnormalities or significant neurological antecedents. Patients excluded from the study included 1) those who had any evidence of focal brain lesions on MRI, 2) those whose MRI was of poor quality for image analysis because of motion artifacts or for any other reason, and 3) those who carried the APOE ε2 allele, which occurs at a low frequency in patients with Alzheimer's disease.
Although 272 patients were diagnosed as having probable sporadic late-onset Alzheimer's disease in the present study period according to the inclusion criteria, 94 patients were excluded—37 because of concomitant focal brain lesions on MRI (most with lacunar infarcts), 44 with inadequate MRI scans, and 13 because they had the APOE ε2 allele. Thus, 178 patients with Alzheimer's disease met the criteria for participation in the volumetric study. The participating patients included 133 women and 45 men. Their mean age was 74.5 years (SD=6.3), the mean duration of symptoms of dementia, determined from an informant-based interview (15), was 35.0 months (SD=26.1, range=4–120), and the mean level of education was 8.6 years (SD=2.0, range=6–18). The mean Mini-Mental State score was 18.9 (SD=4.6, range=5–28), and the mean Alzheimer's Disease Assessment Scale score was 23.4 (SD=8.7, range=7–49).
APOE Genotyping
Genomic DNA was extracted from peripheral blood with a Gen~omix DNA extraction kit (Talent Corp., Trieste, Italy) according to the manufacturer's protocol. APOE genotype was determined by using polymerase chain reaction/restriction fragment length polymorphism according to the procedure described by Wenham et al. (16).
MRI Volumetry
All MRI studies were performed on a 1.5-T MRI unit (Signa Advantage 5.x, General Electric Co.) with use of a circularly polarized head coil as both transmitter and receiver. Coronal three-dimensional spoiled-gradient echo images (field of view=220 mm, matrix=256×256 pixels, 124 1.5-mm contiguous sections, TR=14 msec, TE=3 msec, flip angle=20°), perpendicular to the anterior-posterior commissure plane, that cover the whole calvarium served for the volumetric study (17). The voxel size was 1.108 mm3 ([220/256]2×1.5).
Whole brain volume and total intracranial volume were measured on coronal spoiled-gradient echo sections. The data sets of all spoiled-gradient echo images were directly transmitted to a graphic workstation (INDIGO II Extreme, Silicon Graphics, Mountain View, Calif.) from the MRI unit and were analyzed with three-dimensional MRI analyzing software developed by Yamato et al. (18). The software uses a combination of a gray scale algorithm (a three-dimensional expansion of the region-growing method), an edge-detection algorithm (a three-dimensional expansion of Sobel filtering), and some a priori knowledge (figure 1). With this software, the whole brain was segmented basically by detecting the bound~ary between the CSF and gray matter, and the calvarium was extracted by detecting the outer surface of the dura mater. The volume of each structure was calculated by multiplying the number of voxels in the extracted region by the voxel size. The caudal end of the whole brain and calvarium was manually set at the plane intersecting the occipito-atlantal junction, which is the only supervised operation required. The appropriateness of the extraction of the whole brain and calvarium was assessed by two reviewers (M.Y and H.K.) who were blind to the clinical and genetic data and who examined on-site three-dimensional reconstruction displays of elective viewpoints and two-dimensional slice images of selected sections.
The interoperator reliability, expressed as an intraclass correlation derived from repeated measurement of 20 randomly selected patients, was greater than 0.999 for both total intracranial volume and whole brain volume. The volume measurements of the same samples made by this technique were compared with those made by a semiautomated segmentation technique that combined density thresholding with manual tracing, whose accuracy in phantom measurements and test-retest reliability has been established (19). The mean difference from the latter method was 13.7 cm3 (0.95%; 95% confidence interval=–0.5 to 27.0 cm3) for total intracranial volume and 3.1 cm3 (0.31%; 95% confidence interval=–13.6 to 19.7 cm3) for whole brain volume. The Pearson correlation coefficients were 0.962 (df=18, p<0.0001) for total intracranial volume and 0.896 (df=18, p<0.0001) for whole brain volume.
Normalization of Whole Brain Volume
To correct the brain volume for individual differences in brain size, the observed whole brain volume (oWBV) was normalized to total intracranial volume (TIV) by the covariance method as described by Jack et al. (20). The normalized whole brain volume was then used in subsequent analyses. Each patient's normalized whole brain volume (nWBV) was calculated by the following equation:
nWBV=oWBV–B×(TIV–TIVmean),
where B is the regression coefficient of the normative intracranial volume/brain volume regression line and TIVmean is the mean value of total intracranial volume for normal subjects. A normative intracranial volume/brain volume relationship was determined in 32 normal Japanese volunteers (21 women and 11 men whose mean age was 53.9 years, SD=8.7), recruited from the community, who had no evidence of cognitive impairment (Mini-Mental State scores were 29 or 30), neurological diseases, significant medical antecedents, or abnormal findings on MRI. The mean total intracranial volume in these normal subjects was 1507 cm3 (SD=111, range=1322–1741)—1447 cm3 (SD=73) for women and 1621 cm3 (SD=74) for men. The mean whole brain volume in the normal subjects was 1161 cm3 (SD=93, range=1012–1414)—1128 cm3 (SD=77) for women and 1224 cm3 (SD=89) for men. A single regression analysis revealed a highly significant correlation between total intracranial volume and whole brain volume (r=0.466, F=8.35, df=1,30, p=0.007) and yielded a B value of 0.390. When normalized to total intracranial volume, the mean whole brain volume in the normal subjects was 1161 cm3 (SD=82).
Statistical Analysis
To analyze the relationship between the APOE ε4 allele and whole brain atrophy, the patients were divided into three groups according to their APOE genotypes: ε3/ε3, ε3/ε4, and ε4/ε4. The differences between age, disease duration, education level, and cognitive test score and total intracranial volume and observed whole brain volume across groups of patients were analyzed by one-way analysis of variance (ANOVA), and the difference in distribution of women and men across groups was assessed by a chi-square test. The association of normalized whole brain volume with age, disease duration, education level, and Mini-Mental State score was analyzed by single linear regression. To examine the effect of APOE genotype on brain atrophy, the mean normalized whole brain volume was analyzed first by one-way ANOVA and then by two-way analysis of covariance (ANCOVA) adjusted for age, disease duration, level of education, and either Mini-Mental State score or Alzheimer's Disease Assessment Scale score as potential confounding variables, with two between-subject factors (APOE genotype and gender). Since a gender difference in the Alzheimer's-disease-promoting effect of the APOE genotype has been suggested (21, 22), gender and ε4 gene “dose” were entered into the two-way ANCOVA model as independent variables. The effects of age, disease duration, education level, and severity of cognitive impairment, which may have an influence on brain volume or atrophy (23–25), were covariates in this model. Significant interactions were tested with a post hoc Scheffé test. The significance level was set at p<0.05 for all statistical analyses. Statistical analyses were performed with SAS software (Statistical Analysis Systems, Inc., Cary, N.C.).
RESULTS
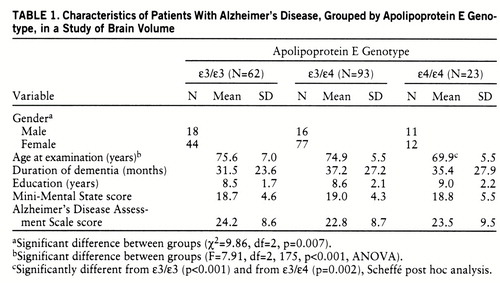
Table 1 summarizes demographic characteristics and neuropsychological test results for the groups with each APOE genotype. The proportion of women in the three genotype groups differed significantly, and there was a significant difference in the mean age at examination among the three groups; the ε4/ε4 allele group was significantly younger than the ε3/ε4 group and the ε3/ε3 group, while the ε3/ε4 group did not differ significantly in age from the ε3/ε3 group. The mean disease duration, education level, Mini-Mental State score, and Alzheimer's Disease Assessment Scale score were comparable in each of the APOE genotypes.
The mean total intracranial volume of the patients was 1460 cm3 (SD=126, range=1174–1881)—1420 cm3 (SD=100) for women and 1579 cm3 (SD=123) for men. Their mean observed whole brain volume was 983 cm3 (SD=91, range=770–1220)—970 cm3 (SD=65) for women and 1020 cm3 (SD=98) for men. The mean normalized whole brain volume of the patients was 945 cm3 (SD=65, range=796–1132), which implies a mean reduction of 18.6% as compared with the normal subjects. Simple linear regressions showed that normalized whole brain volume was correlated appropriately with the following demographic variables: age at examination (r=–0.246, F=11.35, df=1,176, p<0.001), disease duration (r=–0.184, F=6.60, df=1,176, p=0.01), and cognitive test scores (Mini-Mental State: r=0.319, F=20.00, df=1,176, p<0.001; Alzheimer's Disease Assessment Scale: r=–0.308, F=18.43, df=1,176, p<0.001). This would further rationalize covariance of these variables as confounding factors. The relationship between level of education and normalized whole brain volume was not significant.
The mean total intracranial volume, observed whole brain volume, and normalized whole brain volume in the patients classified by APOE genotype are shown in table 2. The ANOVA revealed a significant difference in the mean normalized whole brain volume among patients in the three genotype groups. In the Scheffé post hoc tests, the APOE ε4/ε4 group was significantly different from the ε3/ε3 group (p<0.001) and the ε3/ε4 group (p=0.03), but the difference between the ε3/ε3 and ε3/ε4 groups was marginal (p=0.07). In the ANCOVA, the interactions between APOE genotype and age and between APOE genotype and all covariates together with the main effects were initially tested in separate models, since the mean age was significantly different among the APOE genotype groups. Neither the interaction between APOE genotype and age nor the interaction between APOE genotype and all covariates was significant. (Figure 2 illustrates the relationship between age and normalized whole brain volume for the APOE genotypes.) Thus, these interactions were not included in the final model.
The two-way ANCOVA with age, disease duration, education level, and Mini-Mental State score as covariates revealed that the APOE genotype effect was significant (table 2), while neither the gender effect nor the interaction between APOE genotype and gender was significant. A Scheffé post hoc analysis showed pairwise differences in all pairs of genotypes: between the ε3/ε3 and ε3/ε4 groups (p=0.01), between the ε3/ε4 and ε4/ε4 groups (p=0.03), and between the ε3/ε3 and ε4/ε4 groups (p<0.001). When the Alzheimer's Disease Assessment Scale score was entered into the model as a confounder instead of the Mini-Mental State score, the results were similar (genotype effect: F=6.95, df=2,168, p=0.001; gender effect: F=2.52, df=1,168, p=0.11; interaction: F=1.53, df=2,168, p=0.22; covariates: F=6.19, df=4,168, p<0.001). In the Scheffé post hoc pairwise comparisons, for the ε3/ε3 and ε3/ε4 groups, p=0.01; for the ε3/ε4 and ε4/ε4 groups, p=0.03; and for the ε3/ε3 and ε4/ε4 groups, p<0.001. These results indicated a significant “dose”-dependent effect of the APOE ε4 alleles on normalized whole brain volume that was independent of age, gender, disease duration, level of education, and severity of cognitive impairment.
DISCUSSION
Our volumetry results confirm substantial brain atrophy in patients with Alzheimer's disease and confirm correlations of age, disease duration, and cognitive impairment with brain volume. These findings further indicate the appropriateness of the samples and methods used in the study. Our results show a significant positive correlation of brain volume with an increasing number of APOE ε4 alleles in patients with comparable clinical severity of dementia. In other words, among patients with Alzheimer's disease, brain atrophy was the least in those carrying two APOE ε4 alleles. This association between the APOE ε4 allele and brain volume was similar in both genders and was not induced by effects of age, level of education, duration of dementia from the onset of symptoms, or severity of dementia as measured by two cognitive tests. The mean age of the APOE ε4 homozygous group was 5 or 6 years younger at the time brain volume was determined than that of the APOE ε3 homozygous and APOE ε3/ε4 groups. However, this difference in age reportedly accounts for only about 10 cm3 of the reduction in brain volume (24, 25). Thus, our results suggest that cognitive decline was disproportionately severe compared with the magnitude of brain atrophy, implying that cognitive dysfunction preceded brain atrophy in patients with the APOE ε4 allele.
Since the APOE ε4 allele is a powerful risk factor for Alzheimer's disease (7), the data developed in this study would be the opposite of what one might expect. However, our finding implies that the mechanism by which APOE influences risk of Alzheimer's disease occurs before the onset of brain atrophy. This finding is consistent with other studies showing 1) that PET changes occur in ε4 carriers before the predicted average onset of symptoms (26, 27), 2) that memory and cognitive functions decline in association with the APOE ε4 allele in nondemented elderly subjects (28–30), and 3) that the APOE genotype modulates the accumulation of β-amyloid protein in the cerebral cortex of elderly subjects without dementia, with the most extensive deposition occurring in persons with the ε4 allele in general autopsy series (31). Our data are in contrast to the recent studies of Lehtovirta et al. (32), who found a pronounced volume loss in the hippocampus and the amygdala with an increase in the number of ε4 alleles of patients with Alzheimer's disease. Their processing of volumetric data differed from the method used in the present study in that they normalized the volumes of the hippocampus, the amygdala, and the frontal lobe by dividing them by the brain areas at a determined level. The volume of the frontal lobe of their patients tended to increase with the number of ε4 alleles, although they provided no data on whole brain volume.
Postmortem studies on brains of persons with Alzheimer's disease have shown increased accumulation of βA4-amyloid (6, 8, 33) and decreased choline acetyltransferase activity in acetylcholine-containing neurons (34) in association with two APOE ε4 alleles. Neurofibrillary tangle formation is a proportional marker of disease progression (35) and is responsible for brain atrophy (4). A recent study (8) showed that neurofibrillary tangles correlated closely with clinical measures of the duration and severity of dementia, and APOE ε4 was associated with earlier onset and longer duration, indicating that an indirect interaction could account for the correlation between APOE ε4 and neurofibrillary tangles. Terry et al. (2) studied the correlations between a number of neuropathological and neurochemical variables and cognitive functions and concluded that synapse loss was the major correlate of cognitive impairment. They also found that mean brain weight was not significantly different between patients with Alzheimer's disease and normal subjects. They postulated that synapse loss may occur before the disappearance of the neuronal perikaryon. Katzman et al. (36) reported that a subgroup of patients whose cognitive performance was more preserved than that of age-matched control subjects without brain pathology showed pathological features of mild Alzheimer's disease, with many neocortical plaques, higher brain weights, and a greater number of neurons. Notably, the magnitude of brain atrophy in patients with Alzheimer's disease was quite variable, although neither the study of Terry et al. nor that of Katzman et al. provided information on APOE genotypes. A recent study (37) using in situ hybridization found that in an affected cortical region in Alzheimer's disease, not only neurons bearing neurofibrillary tangles but also tangle-free neurons showed a decrease in cytochrome oxidase subunit III messenger RNA, which is a marker of mitochondrial energy metabolism. The investigators postulated that impaired synaptic function may precede tangle formation. Therefore, it is conceivable that before the occurrence of neuronal cell loss, neurofibrillary tangle formation, and brain atrophy, neurochemical changes and other unknown factors accompanied by the APOE ε4 allele lead to a derangement of neural function, thereby resulting in cognitive dysfunction. Further neurochemical and neuropathological studies are necessary to elucidate this mechanism.
In conclusion, we found an apparent negative correlation between the magnitude of brain atrophy and the number of APOE ε4 alleles in patients with Alzheimer's disease with mild to moderate severity of dementia. The implication of this finding in a clinical setting is evident: in the evaluation of MRI or computed tomography scans of patients with Alzheimer's disease, it should be taken into account that brain atrophy may be less marked and may not be matched with the severity of dementia in patients carrying the APOE ε4 allele.
 |
 |
Received June 16, 1997; revision received Oct. 20, 1997; accepted Oct. 29, 1997. From the Division of Neurosciences, the Neuropharmacology Section, the Neurology Service, the Psychiatry Service, and the Neuropsychology Unit, and the Division of Neuroimaging Research and Radiology Service, Hyogo Institute for Aging Brain and Cognitive Disorders; and the Department of Psychiatry and Neurology, Kobe University School of Medicine, Kobe, Japan. Address reprint requests to Dr. Yasuda, Hyogo Institute for Aging Brain and Cognitive Disorders, 520 Saisho-ko, Himeji 670-0981, Japan; [email protected] (e-mail). Supported in part by grants from the Japan Pharmacopsychiatry Research Foundation and the Kobe Shinryokukai Association to Dr. Yasuda. The authors thank Shoji Kobashi, M.S., Yutaka Hata, Ph.D., and Kazuharu Yamato, Ph.D., for technical assistance and the physicians and staff of Hyogo Institute for Aging Brain and Cognitive Disorders for performing the clinical evaluations.
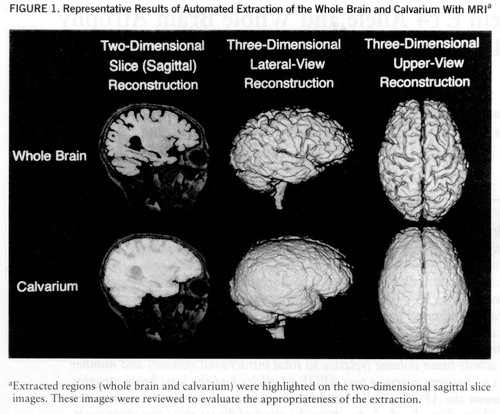
FIGURE 1. Representative Results of Automated Extraction of the Whole Brain and Calvarium With MRIa
aExtracted regions (whole brain and calvarium) were highlighted on the two-dimensional sagittal slice images. These images were reviewed to evaluate the appropriateness of the extraction.
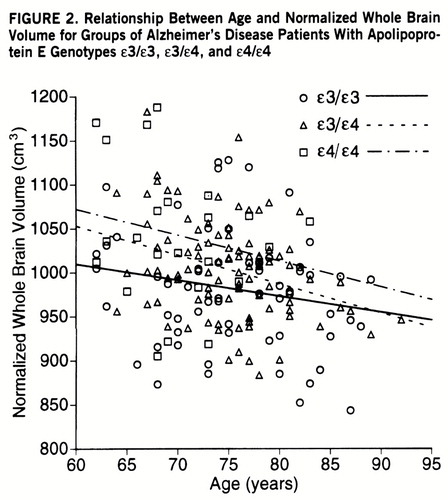
FIGURE 2. Relationship Between Age and Normalized Whole Brain Volume for Groups of Alzheimer's Disease Patients With Apolipoprotein E Genotypes ε3/ε3, ε3/ε4, and ε4/ε4
1 Neary D, Snowden JS, Mann DMA, Bowen DM, Sims NR, Northen B, Yates PO, Davison AN: Alzheimer's disease: a correlative study. J Neurol Neurosurg Psychiatry 1986; 49:229–237Crossref, Medline, Google Scholar
2 Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R: Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 1991; 30:572–580Crossref, Medline, Google Scholar
3 Bierer LM, Hof PR, Purohit DP, Carlin L, Schmeidler J, Davis KL, Perl DP: Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer's disease. Arch Neurol 1995; 52:81–88Crossref, Medline, Google Scholar
4 Mann DM: The topographic distribution of brain atrophy in Alzheimer's disease. Acta Neuropathol 1991; 83:81–86Crossref, Medline, Google Scholar
5 Mahley RW: Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 1988; 240:622–630Crossref, Medline, Google Scholar
6 Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K: Apo~lipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res 1991; 541:163–166Crossref, Medline, Google Scholar
7 Saunders AM, Strittmatter WJ, Schmechel D, St George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, Hulette C, Crain B, Goldgaber D, Roses AD: Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology 1993; 43:1467–1472Crossref, Medline, Google Scholar
8 Gomez-Isla T, West HL, Rebeck GW, Harr SD, Growdon JH, Locascio JJ, Perls TT, Lipsitz LA, Hyman BT: Clinical and pathological correlates of apolipoprotein E epsilon 4 in Alzheimer's disease. Ann Neurol 1996; 39:62–70Crossref, Medline, Google Scholar
9 Frisoni GB, Govoni S, Geroldi C, Bianchetti A, Calabresi L, Fran~ceschini G, Trabucchi M: Gene dose of the epsilon 4 allele of apolipoprotein E and disease progression in sporadic late-onset Alzheimer's disease. Ann Neurol 1995; 37:596–604Crossref, Medline, Google Scholar
10 Ohm TG, Kirca M, Bohl J, Scharnagl H, Gross W, Marz W: Apolipoprotein E polymorphism influences not only cerebral senile plaque load but also Alzheimer-type neurofibrillary tangle formation. Neuroscience 1995; 66:583–587Crossref, Medline, Google Scholar
11 Mohs RC, Rosen WG, Davis KL: The Alzheimer's Disease Assessment Scale: an instrument for assessing treatment efficacy. Psychopharmacol Bull 1983; 19:448–450Medline, Google Scholar
12 Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
13 Ishii K, Kitagaki H, Kono M, Mori E: Decreased medial temporal oxygen metabolism in Alzheimer's disease shown by PET. J Nucl Med 1996; 37:1159–1165Medline, Google Scholar
14 McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34:939–944Crossref, Medline, Google Scholar
15 Sano M, Devanand DP, Richards M, Miller LW, Marder K, Bell K, Dooneief G, Bylsma FW, Lafleche G, Albert M, Folstein M, Stern Y: A standardized technique for establishing onset and duration of symptoms of Alzheimer's disease. Arch Neurol 1995; 52:961–966Crossref, Medline, Google Scholar
16 Wenham PR, Price WH, Blandell G: Apolipoprotein E genotyping by one-stage PCR. Lancet 1991; 337:1158–1159Crossref, Medline, Google Scholar
17 Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McGarley RW: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604–612Crossref, Medline, Google Scholar
18 Yamato K, Hata Y, Kamiura N, Kobashi S, Mori E: Automatic extraction of cerebral and intracranial regions from MRI images for functional cerebral diagnosis. Visual Computing 1995; 6:96–97Google Scholar
19 Mori E, Hirono N, Yamashita H, Imamura T, Ikejiri Y, Ikeda M, Kitagaki H, Shimomura T, Yoneda Y: Premorbid brain size as a determinant of reserve capacity against intellectual decline in Alzheimer's disease. Am J Psychiatry 1997; 154:18–24Link, Google Scholar
20 Jack CR, Twomey CK, Zinsmeister AR, Scarborough FW, Petersen R, Cascino GD: Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology 1989; 172:549–554Crossref, Medline, Google Scholar
21 Yasuda M, Maeda K, Shimada K, Kakigi T, Mori E, Nakai M, Nishio H, Tanaka C: Apolipoprotein E ε4 allele and gender difference in risk of Alzheimer's disease. Alzheimer Res 1995; 1:77–81Google Scholar
22 Payami H, Zareparsi S, Montee KR, Sexton GJ, Kaye JA, Bird TD, Yu CE, Wijsman EM, Heston LL, Litt M, Schellenberg GD: Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet 1996; 58:803–811Medline, Google Scholar
23 Andreasen NC, Ehrhardt JC, Swayze VW II, Alliger RJ, Yuh WT, Cohen G, Ziebell S: Magnetic resonance imaging of the brain in schizophrenia: the pathophysiologic significance of structural abnormalities. Arch Gen Psychiatry 1990; 47:35–44Crossref, Medline, Google Scholar
24 Ho K, Roessmann U, Straumfjord JV, Monroe G: Analysis of brain weight: adult brain weight in relation to sex, race and age. Arch Pathol Lab Med 1980; 104:140–145Google Scholar
25 Dekaban AS, Sadowsky D: Changes in brain weight during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol 1978; 4:345–356Crossref, Medline, Google Scholar
26 Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D: Preclinical evidence of Alzheimer's disease in persons homozygous for the ε4 allele for apolipoprotein E. N Engl J Med 1996; 334:752–758Crossref, Medline, Google Scholar
27 Small GW, Mazziotta JC, Collins MT, Baxter LR, Phelps ME, Mandelkern MA, Kaplan A, La Rue A, Adamson CF, Chang L, Guze BH, Corder EH, Saunders AM, Haines JL, Pericak-Vance MA, Roses AD: Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA 1995; 273:942–947Crossref, Medline, Google Scholar
28 Bondi MW, Salmon DP, Monsch AU, Galasko D, Butters N, Klauber MR, Thal LJ, Saitoh T: Episodic memory changes are associated with the APOE-ε4 allele in nondemented older adults. Neurology 1995; 45:2203–2206Crossref, Medline, Google Scholar
29 Reed T, Carmelli D, Swan GE, Breitner JC, Welsh KA, Jarvik GP, Deeb S, Auwerx J: Lower cognitive performance in normal older adult male twins carrying the apolipoprotein E epsilon 4 allele. Arch Neurol 1994; 51:1189–1192Crossref, Medline, Google Scholar
30 Helkala EL, Koivisto K, Hanninen T, Vanhanen M, Kervinen K, Kuusisto J, Mykkanen L, Kesaniemi YA, Laakso M, Riekkinen P Sr: The association of apolipoprotein E polymorphism with memory: a population-based study. Neurosci Lett 1995; 191:141–144Crossref, Medline, Google Scholar
31 Polvikoski T, Sulkava R, Haltia M, Kainulainen K, Vuorio A, Verkkoniemi A, Niinisto L, Halonen P, Kontula K: Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N Engl J Med 1995; 333:1242–1247Crossref, Medline, Google Scholar
32 Lehtovirta M, Soininen H, Laakso MP, Partanen K, Helisalmi S, Mannermaa A, Ryynanen M, Kuikka J, Hartikainen P, Riekkinen PJ Sr: SPECT and MRI analysis in Alzheimer's disease: relation to apolipoprotein E ε4 allele. J Neurol Neurosurg Psychiatry 1996; 60:644–649Crossref, Medline, Google Scholar
33 Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD: Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA 1993; 90:9649–9653Crossref, Medline, Google Scholar
34 Poirier J: Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends Neurosci 1994; 17:525–530Crossref, Medline, Google Scholar
35 Arriagada PV, Marzloff K, Hyman BT: Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology 1992; 42:1681–1688Crossref, Medline, Google Scholar
36 Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A: Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol 1988; 23:138–144Crossref, Medline, Google Scholar
37 Hatanpaa K, Brady DR, Stoll J, Rapoport SI, Chandrasekaran K: Neuronal activity and early neurofibrillary tangles in Alzheimer's disease. Ann Neurol 1996; 40:411–420Crossref, Medline, Google Scholar



