Partial Volume Decrease of the Thalamus in Relatives of Patients With Schizophrenia
Abstract
Objective:The authors’ goal was to compare the thalamic, total brain, and intracranial volumes of patients with schizophrenia, their healthy siblings, and normal comparison subjects.Method:Magnetic resonance imaging (MRI) brain scans were obtained for 32 same-sex siblings who were discordant for schizophrenia and 32 matched normal comparison subjects. Results:Mean total thalamic volume, corrected for total brain volume, was significantly different among affected siblings, unaffected siblings, and comparison subjects. Thalamic volume was smallest in the patients; thalamic volume in their siblings was smaller than that of comparison subjects but larger than that of the patients with schizophrenia.Conclusions:These results suggest that healthy siblings of patients with schizophrenia partially share the thalamic abnormalities of their affected relatives. Am J Psychiatry 1998; 155: 1784-1786
Schizophrenia is a chronic and prevalent psychiatric disorder that is thought to have a genetic basis. Family members of patients with schizophrenia have an elevated risk of developing the disorder; this risk increases proportionally to the degree of kinship (1). Rates of schizophrenia are higher in children of mothers with schizophrenia irrespective of whether they are raised by their biological mothers. Moreover, the concordance for schizophrenia in monozygotic twins is triple that in dizygotic twins, even in twins who are raised apart (2).
An accumulating body of evidence suggests that brain abnormalities are related to schizophrenia. The most consistent morphological finding is ventricular enlargement, which is indicative of decreased brain volume (3). There is also evidence that the thalamus, a nuclear complex with multiple connections to different parts of the brain, may be abnormal in schizophrenia. Decreased volume of the thalamus, measured with magnetic resonance imaging (MRI) (4–6), and loss of thalamic neurons (7) have been reported consistently in patients with schizophrenia. However, whether the thalamic abnormalities are related to the genotype or are secondary to environmental effects or the disease process itself is unclear. We hypothesized that the thalamic abnormalities in schizophrenia are related to the genotype. To test this hypothesis, we used MRI to measure thalamic volumes of patients with schizophrenia, their healthy same-sex siblings, and normal comparison subjects. Because healthy siblings share no more than 50% of their genes with their affected relative, we expected that thalamic abnormalities would be only partially present in the healthy siblings.
METHOD
Thirty-two same-sex siblings (24 men and eight women) who were discordant for schizophrenia and 32 normal comparison subjects, matched for age, sex, and handedness, participated in this study after written informed consent was obtained. Subjects with any major medical or neurological illness were excluded, as were patients with an IQ below 80 or a history of having received ECT. Patients’ mean age at onset of illness was 21.9 years (SD=5.0), and the mean duration of illness was 20.1 years (SD=8.0). All patients were receiving antipsychotic medication (15 were receiving atypical medication and one was receiving typical medication). Four patients were left-handed, and all healthy siblings were right-handed.
The diagnosis of DSM-IV schizophrenia was determined by using the Comprehensive Assessment of Symptoms and History and the Schedule for Affective Disorders and Schizophrenia—Lifetime Version. Personality disorders were ruled out in the healthy siblings by using the Structured Interview for DSM-IV Personality Disorders. All of the healthy siblings were at least 8 years older than the affected sibling was when he or she developed the first symptoms of schizophrenia, which implies that they were very unlikely to develop schizophrenia in the future (8).
MRIs were obtained on a Philips 1.5 T scanner. For analysis of the thalamus, total brain volume, and intracranial volume, T1-weighted scans with 1.2-mm contiguous coronal slices (TE=4.6 msec; TR=30 msec; flip angle=30º, field of view=256/80%) and T2-weighted scans with 1.6 mm thick contiguous coronal slices (TE1=14 msec; TE2=80 msec; TR=6350 msec, field of view=256/80%) of the whole head were acquired.
The MRI data were processed on a Unix 9000 series workstation. Total brain volumes and intracranial volumes were automatically measured after correction for scanner nonuniformity by using a series of operations based on intensity histogram analysis (9,10) in the T1- and T2-weighted scans. The left and right thalamus were identified and delineated in the T1-weighted scans by one rater, using ANALYZE (Mayo Clinic, Rochester, Minn.). All scans were blinded with respect to diagnosis and any identifying characteristics. The reliability of the rater, measured by intraclass correlation coefficient, was 0.84 for the total thalamus, 0.77 for the left thalamus, and 0.86 for the right thalamus.
All variables were normally distributed within groups. Differences in left and right (side) thalamic volume and differences in brain volume between groups were analyzed by using two-tailed repeated measures analysis of covariance, with a diagnosis-by-side design and with total brain volume and intracranial volume as covariants. Two-tailed t tests were used to determine between-group differences. Because it can be argued that the patients and their siblings are paired, differences in thalamic volume between patients and their siblings were analyzed by using paired t tests, whereas differences between patients and comparison subjects and differences between healthy siblings and comparison subjects were analyzed by using independent t tests.
RESULTS
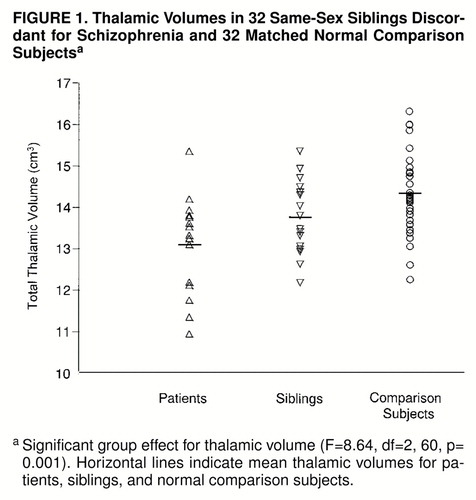
In 14 of 16 patients with schizophrenia, thalamic volumes were smaller than those of their individually matched comparison subjects (χ2=9, df=1, p<0.005), and in 12 of the 16 patients thalamic volumes were smaller than those of their healthy siblings (χ2=4, df=1, p<0.05). In 14 of the 16 healthy siblings the thalamic volumes were smaller than those of their individually matched comparison subjects (χ2=9, df=1, p<0.005).
A significant between-group effect for thalamic volume was found with total brain volume as covariant (figure 1). Thalamic volumes in the affected siblings were smaller than those in the comparison group (t=4.1, df=46, p<0.001, independent t test). Moreover, thalamic volumes of the healthy siblings were smaller than those in the comparison group (t=2.1, df=46, p<0.05, independent t test) but larger than those in their affected siblings (t=2.6, df=15, p=0.02, paired t test). The left thalamus was smaller than the right (F=63.8, df=1, 61, p<0.001), but no significant interaction effect between group and side was found. There were no effects of sex on the thalamic volume measurements.
A significant difference in total brain volume was found by using intracranial volume as covariant (F=4.8, df=2, 60, p=0.01): the patients had significantly smaller volumes than their healthy siblings (t=2.3, df=60, p=0.03) and the comparison subjects (t=3.0, df=60, p=0.01). No difference in brain volume was found between healthy siblings and the normal comparison subjects (t=0.4, df=60, p=0.70).
DISCUSSION
This study reports an abnormal decrease in thalamic volume in schizophrenic patients, which was also found to a lesser extent in their healthy siblings. The smallest thalamic volumes were found in the siblings affected with schizophrenia. The thalamic volumes of their healthy siblings, who are at greater risk for developing schizophrenia, were of intermediate size, whereas the largest thalamic volumes were found in the normal comparison group. This suggests that thalamic volume may be related to a genetic liability to develop schizophrenia. After correction for intracranial volume, differences in total brain volumes were also found. However, in contrast to the differences in thalamic volumes, brain volumes of the healthy siblings were similar to those of normal comparison subjects; only the affected siblings showed smaller brain volumes. Perhaps the decreased brain volume in our patients may be secondary to the pathophysiology of schizophrenia or the result of additional environmental effects rather than related to genotype.
Several explanations for the partial decrease in thalamic volume in the healthy siblings of schizophrenic patients may be contemplated. If an interaction of several genes is necessary to develop schizophrenia (2), then an incomplete number of such genes might result in partial thalamic volume decrease. Alternatively, environmental factors (e.g., obstetric complications or viral infections) combined with a genetic constitution for developing schizophrenia may be necessary to result in a complete thalamic volume loss that would not have been present without these risk factors.
The strength of this study is that it relates thalamic volume decrease to kinship with schizophrenic patients in healthy siblings. However, a larger number of subjects is required to draw more definitive conclusions. Future family studies with larger numbers of subjects may provide the necessary statistical power to confirm our findings.
Received Feb. 9, 1998; revision received June 25, 1998; accepted July 6, 1998. From the Department of Psychiatry, University Hospital Utrecht. Address reprint requests to Dr. Staal, Department of Psychiatry, University Hospital Utrecht, Heidelberglaan 100 3584 CX, Utrecht, The Netherlands. Supported by Solvay Duphar B.V.
1. Gottesman II: Schizophrenia Genesis: The Origins of Madness. New York, WH Freeman, 1991Google Scholar
2. McGuffin P, Owen MJ, Farmer AE: Genetic basis of schizophrenia. Lancet 1995; 346:678–682Crossref, Medline, Google Scholar
3. Woodruff PWR, Lewis SW: Structural brain imaging in schizophrenia, in Brain Imaging in Psychiatry. Edited by Lewis S, Higgins N: Oxford, UK, Blackwell Science, 1996, pp 188–214Google Scholar
4. Andreasen NC, Arndt S, Swayze V II, Cizadlo T, Flaum M, O’Leary D, Ehrhardt JC, Yuh WTC: Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science 1994; 266:294–298Crossref, Medline, Google Scholar
5. Flaum M, Swayze VW II, O’Leary DS, Yuh WTC, Ehrhardt JC, Arndt SV, Andreasen NC: Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am J Psychiatry 1995; 152:704–714Link, Google Scholar
6. Buchsbaum MS, Someya T, Teng CY, Abel L, Chin S, Najafi A, Haier RJ, Wu J, Bunney WE Jr: PET and MRI of the thalamus in never-medicated patients with schizophrenia. Am J Psychiatry 1996; 153:191–199Link, Google Scholar
7. Pakkenberg B: The volume of the mediodorsal thalamic nucleus in treated and untreated schizophrenics. Schizophr Res 1992; 7:95–100Crossref, Medline, Google Scholar
8. Belmaker R, Pollin W, Wyatt RJ, Cohen S: A follow-up of monozygotic twins discordant for schizophrenia. Arch Gen Psychiatry 1974; 30:219–222Crossref, Medline, Google Scholar
9. Sled JG, Zijdenbos AP, Evans AC: A comparison of retrospective intensity non-uniformity correction methods for MRI, in Anonymous Information Processing in Medical Imaging. New York, Springer, 1997, pp 459–464Google Scholar
10. Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P: Multi-modality image registration by maximizing of mutual information. IEEE Trans Med Imaging 1997; 16:187–198Crossref, Medline, Google Scholar



