Nicotine Dependence in Schizophrenia: Clinical Phenomena and Laboratory Findings
Abstract
Objective:The goal of this report is to examine the potential implications of the high prevalence of smoking in schizophrenia for our understanding of this illness.Method:A selective review of the relevant clinical and preclinical literature was conducted. The authors present a review of the clinical observations about smoking in schizophrenia, summarize the preclinical data about the complexity of the CNS nicotinic receptor family, and examine the modulatory effects of nicotine on neurotransmitter systems implicated in schizophrenia.Results:Clinical data suggest that smoking in schizophrenia may represent an attempt to self-medicate symptoms of the illness. Preclinical findings support a potential role of nicotine in medicating negative symptoms in particular. Recent preclinical and clinical data suggest that schizophrenic patients have a primary defect in the CNS nicotinergic system that leads to abnormal sensory gating. The complexity of the neuromodulatory effects of CNS nicotinic systems on other neurotransmitter systems underscores both the scope and potential importance of continued advancement of research in this area.Conclusions:Despite increasing clinical research focused on the extremely high prevalence of smoking in schizophrenia, linkages to the prodigious preclinical data about nicotine and nicotinic receptors are largely unexplored. These linkages are likely to be very important. Integrating nicotine use into our clinical and basic models of schizophrenia leads to a more complex but more realistic representation of brain dysfunction in this illness. Understanding how and why schizophrenic individuals use nicotine may lead to the development of new treatments for both schizophrenia and nicotine dependence. Am J Psychiatry 1998; 155: 1490-1501
Efforts to understand the clinical manifestations and neurobiology of schizophrenia have resulted in a greater appreciation of the complexity of the brain abnormalities that are likely to be involved in the pathophysiology of this syndrome. No single heuristic model satisfactorily explains the protean manifestations of schizophrenia. Useful models, however, must account for common clinical and behavioral manifestations associated with the disorder.
Cigarette smoking is one such behavior. Numerous studies over the past decade—across inpatient, outpatient, and community settings—have reported the prevalence of cigarette smoking among persons with schizophrenia to be 40%–100% higher than among those with other psychiatric diagnoses and as much as three times higher than the prevalence in the general population (1–5). The dramatically elevated prevalence of smoking among individuals with schizophrenia raises the intriguing possibility that the common co-occurrence of nicotine use and schizophrenia is a manifestation of shared underlying neurobiology.
There are several important reasons for understanding the relationship between smoking and schizophrenia. Smoking remains the single greatest preventable cause of death in our society (6). Smoking cessation rates are generally mediocre for heavy-smoking nonpsychiatric populations and are even lower among those with various psychiatric disorders (7, 8). At the same time, there is often the perception that smokers with schizophrenia should not be targeted for smoking cessation intervention, since smoking is one of the few subjective pleasures available to those afflicted (9). Such views are troubling and shortsighted; they represent a stigmatization of patients with schizophrenia. Indeed, a substantial proportion of smokers with schizophrenia recognize that smoking is a problem, and a subset report themselves to be interested in quitting (10, 11).
Smoking appears to engender both general and specific health risks for persons with schizophrenia. There is no doubt that cigarette smoking causes considerable morbidity and mortality in the general population (12). Unfortunately, no epidemiologic data exist that specifically describe smoking-related morbidity and mortality among smokers who have schizophrenia. However, there is considerable evidence that individuals with schizophrenia have a higher mortality rate from natural causes than control populations (13). While mortality rates from cancer for individuals with schizophrenia are not consistently elevated, the rates of cardiovascular and respiratory diseases are typically greater than—often up to twice as high as—those found in age-matched control populations (14–17). The markedly elevated prevalence of smoking among persons with schizophrenia is clearly an important potential factor in explaining this elevated mortality.
Recent data provide evidence that cigarette smoking is a risk factor for dyskinesias independent of medication exposure (18). Hence, the risk of dyskinesia due to treatment with antipsychotic medications may be increased by concurrent smoking. Since the majority of patients receiving these medications for schizophrenia are smokers, they represent a population at risk for the likely negative synergy between a health-risk behavior and an iatrogenic neurologic condition. Moreover, increased morbidity and mortality in chronic schizophrenia have been linked to tardive dyskinesia (19). To what extent that increased risk is mediated by smoking is unclear. However, these links among smoking, mortality, and dyskinesias make the development of smoking interventions important for smokers with schizophrenia.
There may be a number of reasons why individuals with schizophrenia are so much more likely to be smokers. It is possible that their illness makes them more vulnerable to the effects of nicotine. They may be more likely to be addicted and/or less likely to quit than others without the diagnosis. The effects of cigarette smoking may also modulate the symptoms of the illness or the side effects of the pharmacological agents used in the treatment of the disorder.
In this article, we review the clinical relationship between schizophrenia and smoking, describe what is known about the effects of nicotine on selected brain neurotransmitter systems, and suggest how clinical observations about smoking and schizophrenia might be explained by the known effects of nicotine on brain neurotransmitter systems thought to be involved in the pathophysiology of schizophrenia.
DESCRIPTIVE, EPIDEMIOLOGIC, AND EXPERIMENTAL STUDIES
Overview
Beginning in 1983, published reports described the rate of current smoking as dramatically elevated among patients with schizophrenia; early reports described the prevalence of smoking as up to 88% (20). Similar rates were found in other surveys around that time (1, 21). Hughes et al. (2) examined smoking rates among a relatively large (N=277), young adult, outpatient psychiatric population and compared these with rates among local and national population-based samples. That study was the first to provide data to support the hypothesis that increased smoking rates are specifically related to psychiatric diagnosis, even when other contributing factors are controlled. The rate of smoking was highest among patients with schizophrenia (88%), compared with those with mania (70%), major depressive disorder (49%), and anxiety, personality, or adjustment disorder diagnoses (45%–47%) and with the control population (30%). In the general population, smoking prevalence is greater among men and is inversely related to age, education, and socioeconomic status (22). Although they have been incompletely examined among individuals with schizophrenia, the relationships of smoking with gender, age, and socioeconomic status appear to persist (2, 3, 23). In the sample studied by Hughes et al. (2), the differences in smoking rates between subjects with a psychiatric diagnosis and control subjects were independent of effects of age, gender, socioeconomic status, marital status, and concurrent alcohol use.
In more recent studies, smoking rates among persons with schizophrenia remain high. In a sample of older, chronically hospitalized patients with schizophrenia (24), slightly more than one-half were smokers. De Leon et al. (23) examined a group of middle-aged, chronically hospitalized patients and found that 85% of those with schizophrenia were smokers, compared with 67% of those hospitalized for diagnoses other than schizophrenia. In two separate outpatient studies (3, 4), 74% and 68%, respectively, of individuals with schizophrenia were found to be smokers. Among a group of 59 patients (nearly 70% of whom were outpatients), Glynn and Sussman (25) found that 78% were current smokers. We recently reported the specificity of ever smoking and current smoking among patients with chronic schizophrenia and schizoaffective disorder compared with those who had major depression or bipolar disorder (5). The odds of ever having smoked or currently smoking were markedly higher for the schizophrenic group than for the mood disorder group. Although we did not find higher nicotine addiction scores or a greater number of cigarettes smoked per day in the schizophrenic group, other evidence suggests that smokers with schizophrenia are heavier smokers than smokers with (23) or without (26) other psychiatric disorders.
In some of these studies 3(–5, 25), a small proportion of the nonsmokers reported that they had previously smoked. Generally, sufficient details are not reported to ascertain whether there are differences in smoking history or level of addiction between current and former smokers with schizophrenia. The retrospective reports of our small group of former smokers suggested that they were less addicted smokers when they were smoking (27). There is also some suggestion that former smokers have higher levels of functioning and fewer negative symptoms than current smokers with schizophrenia (28).
Clinical Correlates of Schizophrenia Associated With Smoking Status
In a number of cross-sectional studies (3, 4, 24), 29), current smoking in inpatient and outpatient settings has frequently been associated with younger age, earlier age at onset of schizophrenia, more hospitalizations, and higher doses of neuroleptic medication. If smoking were a form of “self-medication” for schizophrenia, one might expect to find some correlation of psychiatric symptoms with smoking status. Goff et al. (3) found that smokers had a higher total score on the Brief Psychiatric Rating Scale (BPRS) (30) and higher BPRS subscale scores for both positive and negative symptoms. Ziedonis et al. (4) also found more positive symptoms among smokers than among nonsmokers. In addition, heavy smokers (defined as those smoking more than 25 cigarettes per day) had the highest number of positive symptoms and the lowest number of negative symptoms in comparison with light smokers and nonsmokers with schizophrenia. Hall et al. (28) reported that former smokers with schizophrenia had fewer negative symptoms (BPRS subscale) than smokers. The relationships between psychiatric symptoms and smoking in cross-sectional associations are difficult to interpret. It may be that smoking is a marker of more severe psychiatric illness. Alternatively, it may be that smoking is associated with some increase in psychiatric symptoms; there are few data to support this assertion, however.
In their study, Glynn and Sussman (25) surveyed smokers with schizophrenia to assess their reasons for smoking. For the most part, subjects reported smoking for many of the same reasons that nonpsychiatrically ill smokers do, including “relaxation…, habit…, and settling nerves” (p. 1027). They also reported similar withdrawal symptoms. About 28% of the subjects said that they smoked in part because of psychiatric symptoms. Several of these patients reported increased psychiatric symptoms during withdrawal from tobacco. We have reported our own experience of three cases of exacerbation of psychiatric symptoms in smokers with schizophrenia who cut back or briefly abstained from smoking (31). Hamera et al. (32) also examined the relationship between self-reported psychiatric symptoms and nicotine use. They found that self-report of symptoms signaling an exacerbation of illness was associated with decreased nicotine use. Conclusions from these reports are limited, however, because the temporal relationship between symptom changes and smoking behavior was not prospectively studied, nor was there an objective measure of smoking (e.g., nicotine levels) beyond subjects’ self-reports about the number of cigarettes smoked.
One report addressed the specificity of the relationship between smoking and schizophrenia among chronically hospitalized psychiatric patients: de Leon et al. (23) found that schizophrenia was associated with an increased likelihood of being both a smoker and a heavy smoker (smoking more than 1.5 packs per day). However, smoking was not related to duration of hospitalization or to neuroleptic dose among patients with schizophrenia.
Correlations between smoking and movement disorders have also received special attention. Several cross-sectional reports have suggested that cigarette smoking is associated with a decrease in the likelihood of idiopathic Parkinson’s disease. It has been speculated that this may be due to the effect of nicotine on striatal dopamine systems affected in this condition (33). Similarly, there is evidence to suggest that smoking is associated with a reduced incidence of neuroleptic-induced parkinsonism. Several studies (3, 29, 34) found that measures of neuroleptic-induced parkinsonism were lower among smokers than among nonsmokers with schizophrenia who were treated with neuroleptics. This finding is not uniformly held, however (35).
Several studies suggest that tardive dyskinesia and smoking may also be associated. Yassa et al. (36) reported that tardive dyskinesia was more prevalent among smokers than among nonsmokers with schizophrenia who were treated with neuroleptics, while Goff et al. (3) reported a trend for a lower Abnormal Involuntary Movement Scale score (37) among smokers in comparison with nonsmokers. Others (35) found no difference. As noted above, Nilsson et al. (18) reported results from a large, older male population sample and found that dyskinesias were strongly and independently associated with exposure to neuroleptics and daily cigarette smoking. Indeed, the risk of dyskinesias increased with the number of cigarettes smoked per day. This raises another important public health issue, particularly for smokers who are exposed to neuroleptic medications, a group in which smokers with schizophrenia are likely to be overrepresented. Beyond long-term smoking exposure and dyskinesia, there is also some suggestion that acute exposure to nicotine may increase dyskinetic movements (38). Hence, the temporal relationship between the last cigarette smoked and evaluation for tardive dyskinesia needs to be clearly defined in order to interpret shorter-term changes in abnormal involuntary movements with smoking.
Experimental Studies of Nicotine, Smoking, and Schizophrenia: Clinical Measures
Observations linking smoking and schizophrenia have been generally cross-sectional and mostly retrospective. Clinical experimental paradigms have the potential to define these relationships further and to inform our understanding of the connections between clinical phenomena and basic neurophysiological functions central to schizophrenia and smoking. Work in this area includes studies in which nicotine exposure was the independent variable, and physiological and cognitive measures in schizophrenia were the dependent measures (39–42).
Adler et al. (39, 40) examined the effects of nicotine on sensory gating, assessed by measuring auditory evoked responses, in subjects with schizophrenia. Diminished gating of the P50 auditory evoked response to repeated stimuli is typically present in this population. The trait is shared by one-half of first-degree relatives unaffected with schizophrenia (43). Adler et al. (39) showed that nicotine delivered by nicotine gum transiently reversed the sensory gating deficit in nonsmoking relatives of individuals with schizophrenia. In addition, nicotine delivered by ad lib smoking transiently reversed the P50 auditory evoked response gating deficit in smokers with schizophrenia. These results have implications for the modulation by nicotine of abnormalities in neurophysiological function found in schizophrenia. The fact that these abnormalities occur and can be briefly reversed by nicotine administration in nonaffected relatives of probands with schizophrenia suggests a genetic basis for the deficit, possibly involving CNS nicotine receptor function. In an elegant extension of these observations (44), the same group of investigators has reported genetic linkage for this gating deficit, with the gene for one type of nicotinic receptor expressed in the human hippocampus. This finding provides genetic evidence linking nicotinic function with a potential pathophysiological trait associated with schizophrenia (also see below).
Olincy et al. (42) showed that cigarette smoking improves some of the smooth pursuit eye movement abnormalities found in schizophrenic subjects compared with nonschizophrenic subjects. This effect may be related to the higher nicotine doses self-administered by schizophrenic patients and does not appear to be a reversal of nicotine-withdrawal effects by smoking.
Levin et al. (41) followed up observations about the effects of nicotinic systems on cognitive function (45) to study the interactions of nicotine and haloperidol treatment on cognitive performance among subjects with schizophrenia. They found that nicotine (administered through a transdermal skin patch) reversed some of the haloperidol dose-related impairments in a variety of cognitive tests that assay memory performance and reaction time to a complex spatial task. In addition, nicotine administration improved attentiveness during a continuous performance task, independent of haloperidol dosage. That study suggests that smoking may have important effects in improving the cognitive side effects of treatment with typical antipsychotic agents.
Experimental Studies of Nicotine, Smoking, and Schizophrenia: Antipsychotic Medications
Smoking results in increased metabolism of neuroleptics (46, 47). This pharmacokinetic effect has been shown to result in 1) an increased average dose of antipsychotic medication to achieve similar blood levels in smokers compared with nonsmokers (46) or 2) similar average doses of antipsychotics with lower blood levels in smokers compared with nonsmokers (46, 47).
At the same time, the choice of pharmacological treatment is likely to have some influence on smoking behavior. George et al. (48) reported that outpatients with schizophrenia retrospectively reported a decrease in smoking (daily cigarette consumption) after treatment with the atypical agent clozapine compared with their smoking when treated with typical antipsychotics. This suggests that smoking behavior might be driven by the differential efficacy of clozapine and typical antipsychotics in treating positive and negative symptoms of schizophrenia or by their different side effect profiles. Dawe et al. (49) had already shown that administration of 5 mg of haloperidol to normal smokers resulted in an increase in nicotine intake compared with baseline smoking. These authors inferred that dopaminergic blockade (specifically, substantial D2 blockade) resulted in a decrease in dopaminergically mediated reward and a compensatory increase in nicotine intake to maintain levels of subjective reward.
McEvoy et al. (50, 51) studied the effect on smoking behavior of pharmacological treatment of schizophrenic patients with haloperidol and clozapine. They found that treatment with haloperidol resulted in an increase in smoking and nicotine blood levels compared with a baseline medication-free condition (50). In a similar group of smokers with schizophrenia, the number of cigarettes smoked and the amount of carbon monoxide in expired air decreased after 12 weeks of clozapine treatment compared with baseline measures during haloperidol treatment (51). It is interesting to note that clozapine treatment has also been shown to improve gating of the P50 auditory evoked response (52). This suggests that the modulation of smoking by clozapine treatment may be mediated in part by similar effects on sensory gating. It remains to be determined whether these differences are also related to changes in psychiatric symptoms (i.e., differential treatment efficacy), changes in side effects of medications, or both.
Neurochemical Correlates Relating Smoking and Schizophrenia
Current models of schizophrenia are predicated on regional differences in dopaminergic activity (53) and the interactions of glutamatergic, dopaminergic (54, 55), and serotonergic neurotransmitter systems (56). There is considerable evidence for nicotinergic modulation of midbrain dopamine neurons and cortical glutamatergic cells. The diversity of receptor subtypes for those two neurotransmitters (57, 58), the interactions between glutamatergic and dopaminergic systems, and the potential for regional specificity of dopaminergic and glutamatergic activity all make for a complex model of dopaminergic and glutamatergic interactions in brain function, with many possible sites of dysfunction in schizophrenia. The influence of nicotinergic activity in modulating these systems adds an additional layer to this complexity. However, a clearer understanding of the heterogeneity of nicotinic receptor expression and regional specificity of nicotinergic activity as a modulator of other neurotransmitter systems is necessary before one can advance the understanding of the way in which cigarette smoking and schizophrenia may be linked.
THE NICOTINIC RECEPTOR: STRUCTURE, PHARMACOLOGY, AND ROLE IN MODULATION OF NEUROTRANSMITTER SYSTEMS IMPLICATED IN SCHIZOPHRENIA
This section begins with a review of the distribution and pharmacology of the nicotinic receptors in the brain. Data describing the interactions of nicotinergic systems with dopaminergic and glutamatergic activity, respectively, are then summarized. Finally, complex interactions simultaneously involving nicotinergic, dopaminergic, and glutamatergic neurotransmission are described.
Distribution and Pharmacology of Nicotinic Receptors
The neuronal nicotinic receptor is a ligand-gated ion channel receptor similar to many glutamate and γ-aminobutyric acid (GABA) receptors. These receptors are composed of five subunits that are assembled to form an ion channel, which opens when the associated ligand binds to the proper site(s). The properties of a receptor can be modified depending on what subunits are included in the final receptor, a feature common to most families of ligand-gated ion channels. There are multiple nicotinic receptor subunits, each encoded by a separate gene. The two families of neuronal nicotinic receptor subunits are named α and β because of their homology with the muscle nicotinic receptor subunits α1 and β1. There are multiple subtypes of both the α and the β subunits (α2–α9, β2–β4). Usually, nicotinic receptors contain two α subunits and three β subunits, although there are nicotinic receptors that contain five identical α subunits (59).
Two acetylcholine molecules are required to activate neuronal nicotinergic receptors that open the ion channel, allowing calcium entry into the cell (60). These binding sites are associated with the α subunits. This activation appears to be short-lived, however, and a time-dependent decrease in activation is observed (59). This has been interpreted as suggesting that after brief stimulation, the receptor becomes insensitive to further agonist exposure, a phenomenon that is termed desensitization (59). Desensitization limits the activity of nicotinic receptors in response to ligand binding and appears to underlie some of the difficulties in understanding the possible role of nicotinic receptors in the pathophysiology of schizophrenia. In addition, the varied pharmacodynamics of the different subunit combinations create further difficulty in teasing out the function of nicotinic receptors (61).
Binding studies demonstrate at least three types of nicotinic receptors that have distinct subunit compositions (figure 1), pharmacological and electrophysiological properties, and neuroanatomical distributions (table 1). The most abundant type of receptor avidly binds [3H]nicotine. These binding sites constitute 90% of all nicotinergic sites in the brain and contain α4 and β2 subunits (59). Sites that have high affinity for the snake neurotoxin, α-bungarotoxin, are composed exclusively of α7 subunits (i.e., five α7 subunits constitute the receptor) (59). The binding sites associated with the neurotoxin neuronal bungarotoxin are, at least in part, composed of α3 subunits (62). It remains to be determined what other α and β subunits are contained in these receptors.
All of these nicotinic receptor subtypes are located in regions of the human brain that have been implicated in schizophrenia. [3H]Nicotine binding sites are abundant in the striatum and substantia nigra, have moderate levels of expression in the neocortex, and have lower levels of expression in the hippocampus (63–66). α-Bungarotoxin binding sites have also been localized in the midbrain, neocortex, and hippocampus, with lower levels in the striatum (63, 65). Neuronal bungarotoxin sites are abundant in the hippocampus and neocortex but are present in much lower levels in the midbrain (62, 63). Within the neocortex itself, the pattern of distribution of two of these binding sites is lamina-specific: [3H]nicotine sites are preferentially distributed in supragranular cortical laminae (II and III) (64), and neuronal bungarotoxin sites are preferentially localized to infragranular laminae (V and VI) (62). α-Bungarotoxin sites are less abundant in the cortex than the other two nicotinic binding sites and do not appear to display differential laminar distribution (63). The relative enrichment of the [3H]nicotine binding site in supragranular layers and the neuronal bungarotoxin binding site in infragranular layers is intriguing, given that the supragranular pyramidal neurons tend to project to other cortical regions, while infragranular pyramidal neurons typically project to subcortical structures. On the basis of these anatomical differences, there may be specific functional roles for [3H]nicotine sites in corticocortical information processing and neuronal bungarotoxin sites in cortical-subcortical communication, both of which may be disrupted in schizophrenia. It is conceivable that both the hypofrontality and the cortical-subcortical dysregulation that have been hypothesized in schizophrenia (53) may be mitigated, in part, by self-administration of nicotine that activates or desensitizes these particular receptors.
The following sections review studies examining the nicotinic modulation of dopaminergic and glutamatergic neurotransmission, with particular emphasis on studies focusing on limbic regions. Controversy surrounds the issue of which nicotine administration schedules in animal studies most accurately reflect the dosing schedule experienced by smokers. Therefore, we have summarized studies that have used one of the following dosing schedules: acute dosing (one-time injection), chronic intermittent treatment (daily injections for many days), chronic continuous infusion with minipumps, and chronic infusion followed by acute challenge.
Nicotine-Dopamine Interactions
Differences between nigrostriatal and mesocorticolimbic dopamine systems
The dopaminergic neurons in the ventral tegmental area and the substantia nigra, pars compacta, provide the majority of dopaminergic innervation to the forebrain. The ventral tegmental area projects to the nucleus accumbens, the cortex, and many limbic regions (mesocorticolimbic system), while the substantia nigra, pars compacta, projects primarily to the dorsal striatum (nigrostriatal system) (67). These two parallel systems subserve limbic and motor functions, respectively (figure 2). Nicotine treatment appears to differentially affect dopamine release, dopamine metabolism, and the electrophysiological properties of dopamine neurons in these two functional systems.
Ventral tegmental area neurons and substantia nigra, pars compacta, neurons display basal firing rates with brief periods of very rapid firing (burst firing). Acute nicotine treatment causes an increase in both the firing rate and burst firing of substantia nigra, pars compacta, neurons but only an increase in burst firing of ventral tegmental area neurons (68, 69). Acute treatment with mecamylamine, a nicotine receptor antagonist, causes a decrease in basal firing rate in ventral tegmental area neurons but not in substantia nigra, pars compacta, neurons (68). It is interesting that chronic continuous nicotine administration has the same electrophysiological effect as the antagonist mecamylamine; this is thought to be due to desensitization of the nicotinic receptor (70).
Taken together, these studies suggest the presence of maximally driven tonic cholinergic input to the ventral tegmental area, mediated by nicotinic receptors, such that the basal firing rate of ventral tegmental area neurons is not increased by exogenous nicotine. This is in contrast to the substantia nigra, pars compacta, where there does not appear to be tonic cholinergic input. Consistent with this interpretation is the relative insensitivity to up-regulation of nicotinic receptors on ventral tegmental area neurons in comparison with substantia nigra, pars compacta, neurons (71). In addition, the differences in firing rates and burst firing result in differential dopamine release from cells originating in the ventral tegmental area and substantia nigra, pars compacta. Specifically, nicotine treatment much more efficiently causes dopamine release in the nucleus accumbens than in the dorsal striatum (72, 73) (figure 3).
Further, dopamine metabolism also appears to be differentially regulated in limbic and motor systems by nicotine treatment. Animal studies indicate that acute nicotine treatment increases dopamine synthesis and catabolism in the nucleus accumbens but not in the dorsal striatum (74, 75). On the other hand, chronic continuous nicotine treatment decreases dopamine catabolism in the dorsal striatum but not in the nucleus accumbens (76). A precedent for altered dopamine metabolism exists in humans as well. The 40% decrease in monoamine oxidase B activity in the brains of smokers compared with nonsmokers may provide an additional mechanism for enhancing the effects of nicotine-related dopamine release (77).
The net effect of nicotine on dopaminergic neuron firing and dopamine turnover is to enhance dopamine levels in the nucleus accumbens relative to the dorsal striatum. These activities are believed to be an important part of the neurobiological substrate of nicotine’s addictive properties (78). As such, these effects may not have any specific relevance to schizophrenia. On the other hand, it is possible that the anhedonic, amotivational negative symptoms of schizophrenia are a manifestation of an abnormal reward-reinforcement system (79). In that case, the observation that smokers with schizophrenia have more negative symptoms than nonsmokers with the illness suggests that smoking may be an attempt to self-medicate a disturbance in the reward circuitry in the ventral striatum. This is a speculative interpretation, and it is confounded by the ubiquitous use of antipsychotic medications, since these also affect dopaminergic systems. An alternative view is that smoking may represent an effort to overcome medication-related accumbens dopaminergic blockade (49).
In addition to differential cholinergic input, these differences in dopamine metabolism and release in the nigrostriatal and mesocorticolimbic systems may be mediated by differences in the subunit composition of nicotinic receptors in these different brain regions. Functional measures of nicotine response provide indirect evidence for heterogeneity in nicotinic receptor pharmacology, since different subunit combinations probably confer unique pharmacological properties. In particular, a potential substrate for pharmacological differences may be the difference in neuroanatomical distribution of the α3 subunit. ABT-418 and isoarecolone are nicotinic receptor agonists with a much higher affinity for receptors that contain the α4 subunit than those containing the α3 subunit (80, 81). Treatment with ABT-418 is three times less potent at activating ventral tegmental area neurons than nicotine (80), while isoarecolone is much less potent than nicotine at stimulating dopamine release in the nucleus accumbens (81). On the basis of these findings, it is conceivable that there are more α3-subunit-containing receptors in the accumbens than in the dorsal striatum, which mediates this pattern of differential dopamine release in those regions by nicotine. A potential subpopulation of receptors fitting this profile has been identified in a study by Schulz et al. (82) of the effects of aging on dopamine release. These investigators found a 2.5-fold difference in dopamine release in striatal slices from young rats in comparison with those from old rats. They suggested that diminished release in the old rats was due to an 80% reduction in a subpopulation of α3-subunit-containing receptors, determined by neuronal bungarotoxin binding. The fact that there is an appreciable effect on dopamine release when receptor composition changes provides support for the hypothesis that subunit differences may mediate regionally specific patterns of dopamine release.
The regional specificity of nicotinic receptor distribution is also supported by several studies which suggest that individual brain regions express different nicotinic receptor subunit mRNAs. The most common nicotine receptor subunits are α4 and β2, and high-affinity nicotine binding sites are associated with the concomitant presence of both (59). However, the ratio of α4 mRNA to β2 mRNA varies in different brain regions, suggesting that there are other subunits co-assembled in the final receptors in these regions (83). There is evidence to support the inclusion of other β subunits and/or α subunits in the final receptor. Nicotinic receptors composed of α4α4β2β3β4 have been recently isolated from rat striatum and shown to have high affinity for nicotine (84). In addition, midbrain dopaminergic nuclei have higher levels of α5, α6, and β3 subunits than α4 or β2 subunits (85), so there remains the potential for unique combinations of nicotinic receptor subunits and considerable complexity of nicotinic receptors. These subunit combinations may confer unique pharmacological properties.
The total nicotinic activation in a region appears to be determined by the relative proportions of different populations of nicotinic receptors with varying subunit composition. This diversity may explain some of the differences of nicotine response in limbic versus motor dopamine systems, but the confirmation of this explanation awaits further study of the specific subunit composition of nicotinic receptors in these regions. Given the evidence that at least one subtype of nicotinic receptor is differentially expressed in schizophrenia (44), it is tempting to speculate that different combinations of nicotinic receptor subunits may exist between schizophrenic and normal subjects and possibly between schizophrenic smokers and schizophrenic nonsmokers. Investigation of such potential differences would have implications for our understanding of both schizophrenia and nicotine addiction.
Differences between cortical and subcortical dopamine activity
In addition to the dissociation of nicotinic modulation of mesocorticolimbic and nigrostriatal dopaminergic systems, there appear to be significant differences in nicotinic regulation of cortical and subcortical dopamine activity. Acute nicotine treatment increases dopamine levels in the dorsal striatum and prefrontal cortex (86), while chronic nicotine treatment does not affect dopamine levels in either region (76). However, acute challenge with nicotine after chronic treatment causes an increase in dopamine levels in the prefrontal cortex but not in the nucleus accumbens (86), suggesting that chronic nicotine treatment produces an alteration in cortical nicotinic receptor activity that results in altered sensitivity to nicotine. Consistent with this idea, nicotine-mediated dopamine metabolism differs in the cortex and the striatum. Acute nicotine treatment regulates dopamine synthesis and catabolism in the nucleus accumbens but not in the prefrontal cortex (87). Conversely, both chronic intermittent and chronic continuous nicotine treatment change dopamine metabolism in the prefrontal cortex but not in the dorsal striatum (87).
Again, pharmacological data suggest that these differences in dopaminergic modulation may be due to differential subunit composition of the nicotinic receptors. Measures of agonist-induced dopamine release in cortical versus striatal synaptosomes suggest that nicotinic receptors in the cortex have a higher affinity for nicotine, but a quicker onset of desensitization after acute nicotinic agonist treatment, than subcortical nicotinic receptors (88). Hence, the different patterns of nicotine-stimulated dopamine release in cortical and subcortical structures may be due to different pharmacological properties of nicotinic receptors in these regions, which in turn are likely determined by different subunit composition.
Differences in neural plasticity may also distinguish cortical and subcortical nicotinic receptors. Several studies suggest that cortical nicotinic receptor expression is regulated by chronic nicotine treatment (89, 90). Further, this change in expression appears to result in electrophysiological activity of cortical neurons (91) and enhanced cortical dopamine release in response to nicotine challenge (86). These preclinical data suggest that chronic nicotine treatment affects cortical sensitivity to nicotine challenges to a greater extent than treatment does for subcortical nicotinic receptors. This is consistent with the hypothesis that schizophrenia is associated with a dissociation of cortical-subcortical dopamine activity (53). Perhaps schizophrenic individuals smoke to stimulate cortical activity without altering subcortical activity.
Nicotine-Glutamate Interactions
The interaction of nicotine and glutamate is much less well characterized than nicotine-dopamine interactions, but evidence supports a facilitating role for nicotine in glutamatergic neurotransmission (92, 93). Nicotine appears to enhance fast glutamatergic synaptic transmission in the cortex, and this phenomenon is likely mediated by a direct effect on nicotinic receptors (92). Recent evidence suggests that this may be more specifically regulated by α7-subunit-containing nicotinic receptors, since the nicotine-induced increase in glutamate levels in the hippocampus is inhibited by α-bungarotoxin (93). This effect on hippocampal glutamate release may underlie the important set of findings concerning sensory gating abnormalities in schizophrenia. As mentioned earlier, persons with schizophrenia have abnormalities in prepulse inhibition (94). In normal subjects, the second P50 wave associated with this paradigm is diminished, but in subjects with schizophrenia it is not (95). Recently, this gating abnormality has been linked to the gene encoding the α7 subunit of the nicotinic receptor (44). Further, it has been hypothesized that this sensory gating phenomenon is mediated, at least in part, by the hippocampus and associated anatomical structures (96). Since α7 nicotinic receptors are relatively abundant in the hippocampus, Freedman et al. (96) examined α-bungarotoxin binding in the postmortem hippocampus of schizophrenic and matched control subjects and found a decrease in binding in the subjects with schizophrenia.
To explore this phenomenon further, a rodent model has been developed that uses evoked potential recordings to measure the N40 wave that appears to correspond to the P50 wave in humans (97). This wave appears to be localized to CA3 and CA4 pyramidal neurons of the hippocampus. The ability to gate auditory sensory information appears to rely on increased inhibitory input to these neurons resulting from the first tone (97). It is interesting to note that this particular subfield receives extensive cholinergic innervation. In fact, lesions of the cholinergic fibers projecting to the hippocampus diminish the gating response, and nicotine treatment can, in turn, normalize gating in lesioned animals (98). Further, either α-bungarotoxin treatment or α7 subunit antisense oligonucleotide treatment can disrupt sensory gating (97). Both treatments, in effect, serve to decrease α7-subunit-containing receptor activity, strongly suggesting a role for α7-subunit-containing receptors in this electrophysiological phenomenon. One model posits that the first sound activates α7-subunit-containing nicotinic receptors, and this in turn facilitates glutamate release (97). Subsequently activated glutamate receptors located on GABA-ergic neurons then cause GABA release, which then inhibits pyramidal neurons in the CA3-CA4 subfields of the hippocampus and dampens response to the second stimulus (97). The role of glutamate in this model suggests that nicotine-glutamate interactions in the hippocampus may be critical to the process of sensory gating and could be a site of neurochemical dysregulation in schizophrenia. It may well be that smoking is an attempt to self-medicate this physiological deficit in schizophrenia.
DISCUSSION
Nicotine treatment modulates both dopaminergic and glutamatergic neurotransmission, and these effects are specific both to brain region and functional system. These differences may be due to the unique aspects of the circuitry of the nigrostriatal and mesocorticolimbic dopamine systems. It has been proposed that the cortico-striato-pallido-thalamic circuit links glutamatergic and dopaminergic activity in limbic areas (54, 55) (figure 2). Such a circuit model may help to clarify the modulatory effects mediated by nicotinic receptors. Glutamatergic corticostriatal neurons presynaptically innervate dopaminergic projections to the striatum. Neurons in the striatum that receive this innervation project to the globus pallidus, which projects back to both the midbrain (by way of the subthalamic nucleus) and the thalamus. The thalamus projects back to the cortex, creating several integrated circuits (54, 55). Any disruption in glutamatergic or dopaminergic activity may potentially disinhibit thalamic outflow, which would then alter cortical activity.
The dissociation of cortical-subcortical dopaminergic activity that is proposed to be related to psychotic symptoms in schizophrenia may be viewed in the context of this model (53) and provides a role for nicotinic receptors to modulate, and potentially normalize, this disturbance. The negative symptoms of schizophrenia seem to be associated with cortical hypoactivity, while the positive symptoms appear to be associated with subcortical dopaminergic hyperactivity (53). Animal models of cortical hypoactivity demonstrate concomitant alterations in subcortical activity, which can be normalized by nicotine treatment (99). This suggests that nicotine, through increased glutamatergic transmission in the cortex, can affect striatal dopamine levels and provides direct evidence for a complex interaction of nicotine with both dopaminergic and glutamatergic systems (100) (figure 3).
Dopamine is the neurotransmitter most often associated with the pathophysiology of schizophrenia, but converging lines of evidence also implicate glutamatergic dysfunction in the disorder. Nicotine appears to modulate the function of both of these neurotransmitter systems, suggesting that this modulation may underlie some of the clinical findings of a high prevalence of nicotine use among individuals with schizophrenia. In general, increases in dopamine levels in cortical regions appear to be more sensitive to chronic nicotine treatment than those in subcortical regions, which may be associated with a potential correction of the cortical-subcortical dissociation of dopamine activity putatively associated with schizophrenia. In addition, the mesocorticolimbic dopaminergic system, which is most often associated with psychotic symptoms, appears to be much more sensitive to the effects of nicotine than the extrapyramidal motor system, lending support to a hypothesis of self-medication of psychotic symptoms for nicotine use in schizophrenia. The less well characterized interaction between nicotinergic and glutamatergic systems suggests that nicotine treatment is associated with increased glutamatergic activity in limbic regions implicated in schizophrenia, particularly the frontal cortex and hippocampus. The hypofrontality associated with schizophrenia, as well as the auditory gating lesions in schizophrenic subjects, may be partially normalized by nicotine treatment through a glutamatergic mechanism.
The clinical and epidemiologic observations of a high rate of smoking among persons with schizophrenia has led to greater understanding of the possible interactions between nicotine’s effects and signs and symptoms of schizophrenic illness. Considerable clinical evidence supports the contention that nicotine exposure, through smoking, and schizophrenia may have a pathophysiological link. The neuronal nicotinic receptors present considerable pharmacodynamic complexity and regional specificity, with implications for dopaminergic and glutamatergic cross-regulation. Our understanding of these relationships, however, is far from complete. We suggest that preclinical and clinical investigations of schizophrenia and nicotine addiction can be more profitably linked across disorders to extend our understanding of these conditions as both individual and commonly comorbid phenomena. In addition, understanding how and why schizophrenic persons use nicotine to self-medicate symptoms may also lead to the development of new treatments for both schizophrenia and nicotine dependence.
Received July 25, 1997; revision received April 15, 1998; accepted May 5, 1998. From the Ann Arbor VA Medical Center; and the Department of Psychiatry and the Mental Health Research Institute, University of Michigan, Ann Arbor. Address reprint requests to Dr. Dalack, Ann Arbor VAMC, 2215 Fuller Road, 116C, Ann Arbor, MI 48105; [email protected] (e-mail). Supported by the Psychiatry Research Committee, University of Michigan; the Ann Arbor VA Medical Center; NIMH grant MH-15794 to Dr. Healy; and an APA/Lilly Psychiatry Fellowship Award to Dr. Healy.
 |
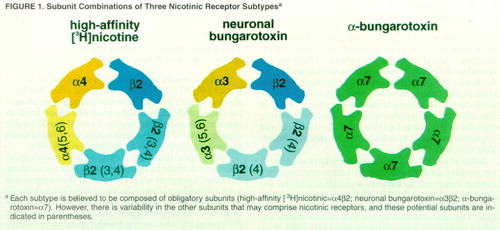
FIGURE 1. Subunit Combinations of Three Nicotinic Receptor Subtypes A
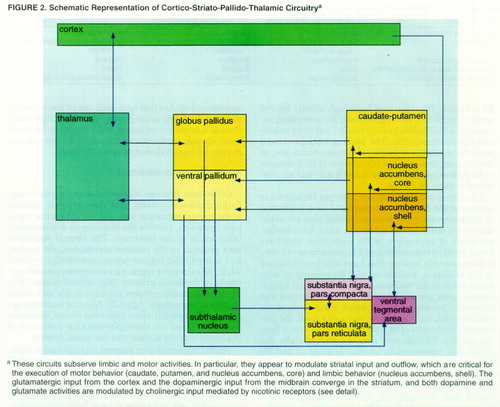
FIGURE 2. Schematic Representation of Cortico-Striato-Pallido-Thalamic Circuitrya
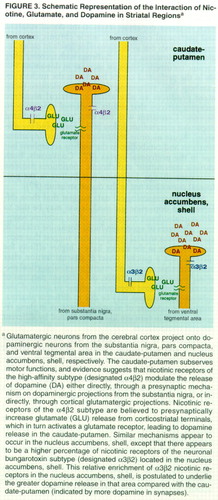
FIGURE 3. Schematic Representation of the Interaction of Nicotine, Glutamate, and Dopamine in Striatal RegionsA
1. Masterson E, O’Shea B: Smoking and malignancy in schizophrenia. Br J Psychiatry 1984; 145:429–432Crossref, Medline, Google Scholar
2. Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA: Prevalence of smoking among psychiatric outpatients. Am J Psychiatry 1986; 143:993–997Link, Google Scholar
3. Goff DC, Henderson DC, Amico E: Cigarette smoking in schizophrenia: relationship to psychopathology and medication side effects. Am J Psychiatry 1992; 149:1189–1194Link, Google Scholar
4. Ziedonis DM, Kosten TR, Glazer WM, Frances RJ: Nicotine dependence and schizophrenia. Hosp Community Psychiatry 1994; 45:204–206Abstract, Google Scholar
5. Diwan A, Castine M, Pomerleau CS, Meador-Woodruff JH, Dalack GW: Differential prevalence of cigarette smoking in patients with schizophrenic vs mood disorders. Schizophr Res 1998; 33:113–118Crossref, Medline, Google Scholar
6. Reducing the Health Consequences of Smoking: 25 Years of Progress—a Report of the Surgeon General: DHHS publication CDC-89-8411. Rockville, Md, US Department of Health and Human Services, Public Health Service, Centers for Disease Control, 1989Google Scholar
7. Glassman AH, Covey LS, Dalack GW, Stetner F, Rivelli SK, Fleiss J, Cooper TB: Smoking cessation, clonidine, and vulnerability to nicotine among dependent smokers. Clin Pharmacol Ther 1993; 54:670–679Crossref, Medline, Google Scholar
8. Covey L, Hughes DC, Glassman AH, Blazer DG, George LK: Ever-smoking, quitting, and psychiatric disorders: evidence from the Durham, North Carolina, Epidemiologic Catchment Area. Tobacco Control 1994; 3:222–227Crossref, Google Scholar
9. Eastwood R: Weeding out (letter). Lancet 1993; 341:1316Crossref, Medline, Google Scholar
10. Addington J, el-Guebaly N, Addington D, Hodgins D: Readiness to stop smoking in schizophrenia. Can J Psychiatry 1997; 42:49–52Crossref, Medline, Google Scholar
11. Ziedonis DM, George TP: Schizophrenia and nicotine use: report of a pilot smoking cessation program and review of neurobiological and clinical issues. Schizophr Bull 1997; 23:247–254Crossref, Medline, Google Scholar
12. Cigarette smoking-attributable mortality and years of potential life lost—United States, 1990. MMWR Morb Mortal Wkly Rep 1993; 42:645–649Medline, Google Scholar
13. Tsuang MT, Woolson RF, Fleming JA: Premature deaths in schizophrenia and affective disorders: an analysis of survival curves and variables affecting the shortened survival. Arch Gen Psychiatry 1980; 37:979–983Crossref, Medline, Google Scholar
14. Allebeck P, Wistedt B: Mortality in schizophrenia. Arch Gen Psychiatry 1986; 43:650–653Crossref, Medline, Google Scholar
15. Buda M, Tsuang MT, Fleming JA: Causes of death in DSM-III schizophrenics and other psychotics (atypical group). Arch Gen Psychiatry 1988; 45:283–285Crossref, Medline, Google Scholar
16. Mortensen PB, Juel K: Mortality and causes of death in schizophrenic patients in Denmark. Acta Psychiatr Scand 1990; 81:372–377Crossref, Medline, Google Scholar
17. Tabbane K, Joober R, Spadone C, Poirier MF, Olié JP: Mortalité et causes de décès dans la schizophrénie: revue de la littérature. L’Encéphale 1993; 19:23–28Medline, Google Scholar
18. Nilsson A, Waller L, Rosengren A, Adlerberth A, Wilhelmsen L: Cigarette smoking is associated with abnormal involuntary movements in the general male population—a study of men born in 1933. Biol Psychiatry 1997; 41:717–723Crossref, Medline, Google Scholar
19. Youssef HA, Waddington JL: Morbidity and mortality in tardive dyskinesia: associations in chronic schizophrenia. Acta Psychiatr Scand 1987; 75:74–77Crossref, Medline, Google Scholar
20. O’Farrell TJ, Connors GJ, Upper D: Addictive behaviors among hospitalized psychiatric patients. Addict Behav 1983; 18:329–333Crossref, Google Scholar
21. Gopalaswamy AK, Morgan R: Smoking in chronic schizophrenia (letter). Br J Psychiatry 1986; 149:523Medline, Google Scholar
22. Cigarette smoking among adults—United States, 1991. MMWR Morb Mortal Wkly Rep 1993; 42:230–233Medline, Google Scholar
23. de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM: Schizophrenia and smoking: an epidemiological survey in a state hospital. Am J Psychiatry 1995; 152:453–455Link, Google Scholar
24. Sandyk R, Kay SR: Tobacco addiction as a marker of age of onset of schizophrenia. Int J Neurosci 1991; 57:259–262Crossref, Medline, Google Scholar
25. Glynn SM, Sussman S: Why patients smoke (letter). Hosp Community Psychiatry 1990; 41:1027–1028Abstract, Google Scholar
26. Olincy A, Young DA, Freedman R: Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry 1997; 42:1–5Crossref, Medline, Google Scholar
27. Dalack GW, Becks L, Pomerleau C, Meador-Woodruff JH: Cigarette smoking and psychiatric illness: an outpatient survey in a VA mental health clinic (abstract). Addiction 1997; 92:624Google Scholar
28. Hall RG, Duhamel M, McClanahan R, Miles G, Nason C, Rosen S, Schiller P, Tao-Yonenaga L, Hall SM: Level of functioning, severity of illness, and smoking status among chronic psychiatric patients. J Nerv Ment Dis 1995; 183:468–471Crossref, Medline, Google Scholar
29. Sandyk R: Cigarette smoking: effects on cognitive functions and drug-induced parkinsonism in chronic schizophrenia. Int J Neurosci 1993; 70:193–197Crossref, Medline, Google Scholar
30. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
31. Dalack GW, Meador-Woodruff JH: Smoking, smoking withdrawal and schizophrenia: case reports and a review of the literature. Schizophr Res 1996; 22:133–141Crossref, Medline, Google Scholar
32. Hamera E, Schneider JK, Deviney S: Alcohol, cannabis, nicotine and caffeine use and symptom distress in schizophrenia. J Nerv Ment Dis 1995; 183:559–565Crossref, Medline, Google Scholar
33. Morens DM, Grandinetti A, Reed D, White LR, Ross GW: Cigarette smoking and protection from Parkinson’s disease: false association or etiologic clue? Neurology 1995; 45:1041–1045Google Scholar
34. Decina P, Caracci G, Sandik R, Berman W, Mukherjee S, Scapicchio P: Cigarette smoking and neuroleptic-induced parkinsonism. Biol Psychiatry 1990; 28:502–508Crossref, Medline, Google Scholar
35. Menza MA, Grossman N, Van Horn M, Cody R, Forman N: Smoking and movement disorders in psychiatric patients. Biol Psychiatry 1991; 30:109–115Crossref, Medline, Google Scholar
36. Yassa R, Lal S, Korpassy A, Ally J: Nicotine exposure and tardive dyskinesia. Biol Psychiatry 1987; 22:67–72Crossref, Medline, Google Scholar
37. Munetz MR, Benjamin S: How to examine patients using the Abnormal Involuntary Movement Scale. Hosp Community Psychiatry 1988; 39:1172–1177Abstract, Google Scholar
38. Wirshing WC, Engle J, Levin E, Cummings JL, Rose J: The acute effects of smoking on tardive dyskinesia, in 1989 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 1989, p 89Google Scholar
39. Adler LE, Hoffer LJ, Griffith J, Waldo MC, Freedman R: Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry 1992; 32:607–616Crossref, Medline, Google Scholar
40. Adler LE, Hoffer LD, Wiser A, Freedman R: Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry 1993; 150:1856–1861Link, Google Scholar
41. Levin ED, Wilson W, Rose JE, McEvoy J: Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology 1996; 15:429–436Crossref, Medline, Google Scholar
42. Olincy A, Ross RG, Young DA, Roath M, Freedman R: Improvement in smooth pursuit eye movements after cigarette smoking in schizophrenic patients. Neuropsychopharmacology 1998; 18:175–185Crossref, Medline, Google Scholar
43. Waldo MC, Carey G, Myles-Worsley M, Cawthra E, Adler LE, Nagamoto HT, Wender P, Byerley W, Plaetke R, Freedman R: Codistribution of a sensory gating deficit and schizophrenia in multi-affected families. Psychiatry Res 1991; 39:257–268Crossref, Medline, Google Scholar
44. Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W: Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA 1997; 94:587–592Crossref, Medline, Google Scholar
45. Levin ED: Nicotinic systems and cognitive function. Psychopharmacology (Berl) 1992; 108:417–431Crossref, Medline, Google Scholar
46. Ereshefsky L, Jann MW, Saklad SR, Davis CM, Richards AL, Burch NR: Effects of smoking on fluphenazine clearance in psychiatric inpatients. Biol Psychiatry 1985; 20:329–332Crossref, Medline, Google Scholar
47. Jann MW, Saklad SR, Ereshefsky L, Richards AL, Harrington CA, Davis CM: Effects of smoking on haloperidol and reduced haloperidol plasma concentrations and haloperidol clearance. Psychopharmacology (Berl) 1986; 90:468–470Crossref, Medline, Google Scholar
48. George TP, Sernyak MJ, Ziedonis DM, Woods SW: Effects of clozapine on smoking in chronic schizophrenic outpatients. J Clin Psychiatry 1995; 56:344–346Medline, Google Scholar
49. Dawe S, Gerada C, Russell MAH, Gray JA: Nicotine intake in smokers increases following a single dose of haloperidol. Psychopharmacology (Berl) 1995; 117:110–115Crossref, Medline, Google Scholar
50. McEvoy JP, Freudenreich O, Levin ED, Rose JE: Haloperidol increases smoking in patients with schizophrenia. Psychopharmacology (Berl) 1995; 119:124–126Crossref, Medline, Google Scholar
51. McEvoy J, Freudenreich O, McGee M, VanderZwaag C, Levin E, Rose J: Clozapine decreases smoking in patients with chronic schizophrenia. Biol Psychiatry 1995; 37:550–552Crossref, Medline, Google Scholar
52. Nagamoto HT, Adler LE, Hea RA, Griffith JM, McRae KA, Freedman R: Gating of auditory P50 in schizophrenics: unique effects of clozapine. Biol Psychiatry 1996; 40:181–188Crossref, Medline, Google Scholar
53. Davis KL, Kahn RS, Ko G, Davidson M: Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 1991; 148:1474–1486Link, Google Scholar
54. Carlsson M, Carlsson A: Interactions between glutamatergic and monoaminergic systems within the basal ganglia—implications for schizophrenia and Parkinson’s disease. Trends Neurosci 1990; 13:272–276Crossref, Medline, Google Scholar
55. Carlsson M, Carlsson A: Schizophrenia: a subcortical neurotransmitter imbalance syndrome? Schizophr Bull 1990; 16:425–432Google Scholar
56. Meltzer HY: Clinical studies on the mechanism of action of clozapine: the dopamine-serotonin hypothesis of schizophrenia. Psychopharmacology (Berl) 1989; 99:S18–S27Google Scholar
57. Joyce JN, Meador-Woodruff JH: Linking the family of D2 receptors to neuronal circuits in human brain: insights into schizophrenia. Neuropsychopharmacology 1997; 16:375–384Crossref, Medline, Google Scholar
58. Hollmann M, Heinemann S: Cloned glutamate receptors. Annu Rev Neurosci 1994; 17:31–108Crossref, Medline, Google Scholar
59. McGehee DS, Role LW: Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol 1995; 57:521–546Crossref, Medline, Google Scholar
60. Jackson MB: Dependence of acetylcholine receptor channel kinetics on agonist concentration in cultured mouse muscle fibers. J Physiol 1988; 397:555–583Crossref, Medline, Google Scholar
61. Alkondon M, Reinhardt S, Lobron C, Hermsen B, Maelicke A, Albuquerque EX: Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons, II: rundown and inward rectification of agonist-elicited whole-cell currents and identification of receptor subunits by in situ hybridization. J Pharmacol Exp Ther 1994; 271:494–506Medline, Google Scholar
62. Schulz DW, Loring RH, Aizenman E, Zigmond RE: Autoradiographic localization of putative nicotinic receptors in rat brain using 125I-neuronal bungarotoxin. J Neurosci 1991; 11:287–297Crossref, Medline, Google Scholar
63. Sugaya K, Giacobini E, Chiappinelli VA: Nicotinic acetylcholine receptor subtypes in human frontal cortex: changes in Alzheimer’s disease. J Neurosci Res 1990; 27:349–359Crossref, Medline, Google Scholar
64. Perry EK, Court JA, Johnson M, Piggott MA, Perry RH: Autoradiographic distribution of [3H]nicotine binding in human cortex: relative abundance in subicular complex. J Chem Neuroanat 1992; 5:399–405Crossref, Medline, Google Scholar
65. Rubboli F, Court JA, Sala C, Morris C, Chini B, Perry E, Clementi F: Distribution of nicotinic receptors in the human hippocampus and thalamus. Eur J Neurosci 1994; 6:1596–1604Crossref, Medline, Google Scholar
66. Adem A, Nordberg A, Jossan SS, Sara V, Gillberg P: Quantitative autoradiography of nicotinic receptors in large cryosections of human brain hemispheres. Neurosci Lett 1989; 101:247–252Crossref, Medline, Google Scholar
67. Cooper JR, Bloom FE, Roth RH: The Biochemical Basis of Neuropharmacology, 7th ed. New York, Oxford University Press, 1996, pp 293–351Google Scholar
68. Grenhoff J, Aston-Jones G, Svensson TH: Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand 1986; 128:351–358Crossref, Medline, Google Scholar
69. Mereu G, Yoon KP, Boi V, Gessa GL, Naes, Westfall TC: Preferential stimulation of ventral tegmental area dopaminergic neurons by nicotine. Eur J Pharmacol 1987; 141:395–399Crossref, Medline, Google Scholar
70. Rasmussen K, Czachura JF: Nicotine withdrawal leads to increased firing rates of midbrain dopamine neurons. Neuroreport 1995; 7:329–332Crossref, Medline, Google Scholar
71. Pauly JR, Marks MJ, Gross SD, Collins AC: An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J Pharmacol Exp Ther 1991; 258:1127–1136Medline, Google Scholar
72. Benwell MEM, Balfour DJK: Regional variation in the effects of nicotine on catecholamine overflow in rat brain. Eur J Pharmacol 1997; 325:13–20Crossref, Medline, Google Scholar
73. Imperato A, Mulas A, Di Chiara G: Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol 1986; 132:337–338Crossref, Medline, Google Scholar
74. Andersson K, Fuxe K, Agnati LF: Effects of single injections of nicotine on the ascending dopamine pathways in the rat. Acta Physiol Scand 1981; 112:345–347Crossref, Medline, Google Scholar
75. Clarke PBS, Fu DS, Jakubovic A, Fibiger HC: Evidence that mesolimbic dopaminergic activation underlies the locomotor stimulant action of nicotine in rats. J Pharmacol Exp Ther 1988; 246:701–708Medline, Google Scholar
76. Kirch DG, Gerhardt GA, Shelton RC, Freedman R, Wyatt RJ: Effect of chronic nicotine administration on monoamine and monoamine metabolite concentrations in rat brain. Clin Neuropharmacol 1987; 10:376–383Crossref, Medline, Google Scholar
77. Fowler JS, Volkow ND, Wang G-J, Pappas N, Logan J, MacGregor R, Alexoff D, Shea C, Schyler D, Wolf AP, Warner D, Zezulkova I, Cilento R: Inhibition of monoamine oxidase B in the brains of smokers. Nature 1996; 379:733–736Crossref, Medline, Google Scholar
78. Pontieri FE, Tanda G, Orzi F, Di Chiara G: Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 1996; 382:255–257Crossref, Medline, Google Scholar
79. Glassman AH: Cigarette smoking: implications for psychiatric illness. Am J Psychiatry 1993; 150:546–553Link, Google Scholar
80. Brioni JD, Kim DJB, Brodie MS, Decker MW, Arneric SP: ABT-418: discriminative stimulus properties and effect on ventral tegmental cell activity. Psychopharmacology (Berl) 1995; 119:368–375Crossref, Medline, Google Scholar
81. Mirza NR, Pei Q, Stolerman IP, Zetterstrom TSC: The nicotinic receptor agonist (-)-nicotine and isoarecolone differ in their effects on dopamine release in the nucleus accumbens. Eur J Pharmacol 1996; 295:207–210Crossref, Medline, Google Scholar
82. Schulz DW, Kuchel GA, Zigmond RE: Decline in response to nicotine in aged rat striatum: correlation with a decrease in a subpopulation of nicotinic receptors. J Neurochem 1993; 61:2225–2232Crossref, Medline, Google Scholar
83. Liu C, Nordberg A, Zhang X: Differential coexpression of nicotinic acetylcholine receptor α4 and β2 subunit genes in various regions of rat brain. Neuroreport 1996; 7:1645–1649Crossref, Medline, Google Scholar
84. Forsayeth JR, Kobrin E: Formation of oligomers containing the β3 and β4 subunits of the rat nicotinic receptor. J Neurosci 1997; 17:1531–1538Crossref, Medline, Google Scholar
85. Le Novere N, Zoli M, Changeaux J-P: Neuronal nicotinic receptor α6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur J Neurosci 1996; 8:2428–2439Crossref, Medline, Google Scholar
86. Nisell M, Nomikos GG, Hertel P, Panagis G, Svensson TH: Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse 1996; 22:369–381Crossref, Medline, Google Scholar
87. Vezina P, Blanc G, Glowinski J, Tassin J: Nicotine and morphine differentially activate brain dopamine in prefrontocortical and subcortical terminal fields: effects of acute and repeated injections. J Pharmacol Exp Ther 1992; 261:484–490Medline, Google Scholar
88. Whiteaker P, Garcha HS, Wonnacott S, Stoleraman IP: Locomotor activation and dopamine release produced by nicotine and isoarecolone in rats. Br J Pharmacol 1995; 116:2097–2105Crossref, Medline, Google Scholar
89. Sanderson EM, Drasdo AL, McCrea K, Wonnacott S: Upregulation of nicotinic receptors following continuous infusion of nicotine is brain-region-specific. Brain Res 1993; 617:349–352Crossref, Medline, Google Scholar
90. Collins AC, Romm E, Wehner JM: Dissociation of the relationship between nicotine tolerance and upregulation of nicotinic receptors. Brain Res Bull 1990; 25:373–379Crossref, Medline, Google Scholar
91. Abdulla FA, Calaminici M, Wonnacott S, Gray JA, Sinden JD, Stephenson JD: Sensitivity of rat frontal cortical neurones to nicotine is increased by chronic administration of nicotine and by lesions of the nucleus basalis magnocellularis: comparison with numbers of [3H]nicotine binding sites. Synapse 1995; 21:281–288Crossref, Medline, Google Scholar
92. Vidal C, Changeaux J-P: Nicotinic and muscarinic modulations of excitatory synaptic transmission in the rat prefrontal cortex in vitro. Neuroscience 1993; 56:22–32Crossref, Google Scholar
93. Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA: Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature 1996; 383:713–716Crossref, Medline, Google Scholar
94. Braff DL, Saccuzzo DP: The time course of information-processing deficits in schizophrenia. Am J Psychiatry 1985; 142:170–174Link, Google Scholar
95. Freedman R, Adler LE, Baker N, Waldo M, Mizner G: Candidate for inherited neurobiological dysfunction in schizophrenia. Somat Cell Mol Genet 1987; 13:479–484Crossref, Medline, Google Scholar
96. Freedman R, Hall M, Adler LE, Leonard S: Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry 1995; 38:22–33Crossref, Medline, Google Scholar
97. Leonard S, Adams C, Breese CR, Adler LE, Bickford P, Byerley W, Coon H, Griffith JM, Miller C, Myles-Worsley M, Nagamoto HT, Rollins Y, Stevens KE, Waldo M, Freedman R: Nicotinic receptor function in schizophrenia. Schizophr Bull 1996; 22:431–445Crossref, Medline, Google Scholar
98. Bickford PC, Wear K: Fimbria-fornix lesions disrupt auditory sensory gating in the rat hippocampus. Brain Res 1995; 705:235–240Crossref, Medline, Google Scholar
99. Svensson TH, Grenhoff J, Engberg G: Effect of nicotine on dynamic function of brain catecholaminergic neurons, in The Biology of Nicotine Dependence. Edited by Bock G. New York, NY, Plenum, 1990, pp 169–185 Google Scholar
100. Garcia-Munoz M, Patino P, Young SJ, Groves PM: Effects of nicotine on dopaminergic nigrostriatal axons requires stimulation of presynaptic glutamatergic receptors. J Pharmacol Ther 1996; 277:1685–1693Medline, Google Scholar



