Utility of Investigations, History, and Physical Examination in “Medical Clearance” of Psychiatric Patients: A Meta-Analysis
Abstract
Objective:
Few reviews and no meta-analyses have explored the utility of investigations, such as laboratory tests, among patients presenting with psychiatric symptoms, and none has explored the yield of history and physical examination. A meta-analysis of studies exploring the utility of “medical clearance” among adult psychiatric patients was conducted.
Methods:
PubMed, PsycInfo, and Web of Science were systematically searched from inception until February 15, 2021. Primary outcome was detection by investigations (e.g., bloodwork and imaging), history, or physical examination of an illness that caused or aggravated psychiatric symptoms or was comorbid and that resulted in change in the patient’s diagnosis or management (“yield”). A mixed-effects meta-analysis with inverse-variance weighting was used to pool results.
Results:
Twenty-five cross-sectional studies were included. Pooled yield of investigations was 1.1% (95% confidence interval [CI]=0.5%–2.2%), although yield was relatively higher among disoriented, agitated, or older patients. Yield was higher in the inpatient setting, compared with the emergency room, with similar results by approach (protocolized versus nonprotocolized). Compared with investigations, yield of history and physical examination was higher (15.6%, 95% CI=9.1%–25.6%, and 14.9%, 95% CI=8.1%–25.9%, respectively), with nonsignificant differences by evaluator (psychiatrist versus nonpsychiatrist) for physical examination.
Conclusions:
Investigations were of relatively low yield, especially when weighed against cost and potential harm, and they should not be routinely conducted for patients presenting with primarily psychiatric complaints, although certain subgroups may benefit. History and physical examination, by contrast, should be undertaken for all patients, ideally with participation of the consulting psychiatrist.
HIGHLIGHTS
Few reviews and no meta-analyses have explored the utility of investigations (e.g., blood work and imaging) for “medical clearance” of patients presenting with psychiatric symptoms, and none has explored yield of history and physical examination.
A meta-analysis of the results of 25 cross-sectional studies supports the undertaking of history and physical examination for all patients.
Investigations did not significantly alter diagnosis or management, and in light of their cost and potential harm, they should not be routinely conducted, although some subgroups may benefit.
Individuals presenting with psychiatric manifestations are common in most emergency rooms (ERs), with an increasing trend in some recent decades (1, 2). Indeed, psychiatrists are frequently called on to consult on patients with a variety of behavioral, affective, and cognitive symptoms and signs. Such referrals normally originate with the ER physician, who is often the first to encounter patients with such presentations and who typically initiates some or all investigations, physical examination, and history. Psychiatric symptoms may have multiple etiologies, and nonpsychiatric comorbidities, both recognized and unrecognized, may be especially high among patients with psychiatric symptoms, compared with the general population (3).
Nonetheless, the added value of initial evaluation—history, physical examination, and investigations—to the ER physician and the consultant psychiatrist is uncertain: does it contribute significantly to diagnosis or management? Given that investigations (e.g., bloodwork and imaging) are ordered for most ER visits resulting in psychiatric admission (4), such questions are of obvious importance. Indeed, initiatives such as Choosing Wisely, which seeks to evaluate the utility of commonly ordered tests or interventions by specialty, have been undertaken in recognition of the need for judicious evaluation. Current guidelines are silent on the utility of such ER evaluations (5).
The ER initial evaluation typically culminates in the designation of “medically cleared.” Broadly, this term is used to suggest the absence of nonpsychiatric comorbidity requiring further attention, typically for the purpose of psychiatric admission or referral (6). Concerns arise beyond the question of yield—that is, the useful findings from the initial evaluation. For one, evaluation may differ in scope across and even within institutions and setting (e.g., ER and inpatient). Attempts at standardization of workup (7–11) have not been widely embraced (6). What, then, is the consulting psychiatrist to assume when a patient has been “medically cleared”? Which components of the evaluation have been undertaken and for which indications? Indeed, the term has been described as “imprecise” with “greater capacity to mislead than to inform correctly” (12).
Despite these concerns, few reviews have explored the role of the workup as it relates to psychiatric referral. No meta-analyses have been conducted. Only three reviews attempted any quantitative exploration of investigations (13–15). Two of these were performed more than 15 years ago (13, 15), and the third was based on only three studies (14). None has attempted quantitative review of history and physical examination. A recent position paper on “medical clearance” (16) was based largely on expert opinion. More specifically, Anfinson and Kathol (15) over a quarter-century ago and some years later Gregory et al. (13) suggested limited yield of laboratory investigations ranging from “fairly low” (13) to 0.8%–4% (15). A more recent study by Conigliaro et al. (14) noted that 0%–0.4% of abnormal results changed disposition. This more recent review was restricted to patients with protocolized (i.e., testing without clinical discretion) investigations for which clinical outcome could be evaluated; inclusion of studies with nonprotocolized tests may have offered further clinical insight. Chennapan et al. (17) also reviewed the utility of investigations and, unlike others, history and physical examination, but these authors did not provide a quantitative measure. They suggested limited benefit to investigations, compared with history and physical examination, especially among younger adults with established psychiatric history.
Questions of resource allocation and patient care demand systematic and quantitative evaluation of the utility of investigations, history, and physical examination undertaken to designate psychiatric patients as “medically cleared.” Such measures are deemed to be of utility if they result in a change in diagnosis or management, including treatment, referral, or disposition. Our meta-analysis further sought to reflect clinical practice: we contrasted approach (protocolized and nonprotocolized), evaluator (psychiatrist and nonpsychiatrist), and setting (ER and inpatient). Of note, of the reviews that assessed study quality, all have done so with tools intended for cohort studies, although no such studies exist. We incorporated a quality assessment tool (18) reflective of included study types. We hypothesized that investigations are likely to be of low utility, especially compared with history and physical examination, and that differences may be evident by site, approach, and evaluator.
Methods
Methods for study inclusion and data analysis were consistent with guidelines on the reporting of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (19) and registered at PROSPERO (ID CRD42020201250).
Data Sources and Searches
PubMed, Web of Science, and PsycInfo databases were searched for all papers published in English from inception to February 15, 2021, that included all keywords (“medical clearance” OR “clearance”) AND (“screening”) AND (“psychiatry”). Additional studies were identified by a manual search of an online registry (ClinicalTrials.gov) and of published reference lists of all studies included in this review. One reviewer (R.N.) reviewed titles and abstracts to identify potentially relevant studies. Once relevant studies were identified, we completed full-text reviews to determine eligibility for inclusion on the basis of criteria described below. Our study was exempt from institutional review board review because it examined published data.
Study Selection
Studies of any type, excluding surveys, were included if they assessed the utility of investigations, history, or physical examination among primarily adults (age ≥18) with psychiatric symptoms presenting for evaluation. In addition to studies conducted in the ER, studies that focused on “medical clearance” but were conducted in the psychiatric ER or the inpatient setting were also included if a process of “medical clearance” similar to that of a general ER was undertaken.
Data Extraction
Details on the sample, design, tests, and conclusions were extracted. To explore any differences in approach to “medical clearance,” note was made, where possible, of evaluator. Consistent with a previous study (20), illness that caused or aggravated psychiatric symptoms or was comorbid was included if it resulted in a change in diagnosis or management, including treatment, referral, or disposition. An investigation was significant only if the finding was not otherwise detected by history or physical examination. Investigations repeated and deemed to be noncontributory were not considered to be significant. When study authors outlined what they themselves considered to be significant results, these were used, provided that they were consistent with the above; in limited cases, significance was inferred from authors’ commentary. In contrast to inclusion criteria put forth by a previous review (14), not every study participant needed to have received every test. To ensure consistency, and because repeat investigations for a given patient might have been undertaken, investigations were excluded if we could not determine whether effectiveness was conveyed for number of patients rather than number of tests. Tests designated as urine culture, urinalysis, or midstream urine were treated as equivalent. For reasons of scope and heterogeneity, studies focusing on urinary drug screen (UDS), many of which involved self-harm, were excluded. We reviewed and discussed all extracted data, and discrepancies were resolved by discussion. Study authors were contacted as necessary.
Data Analysis
Yield of investigations or history and physical examination was calculated as the percentage of the total number of findings causing changes in diagnosis or management, as defined above, divided by the total sample or, in the case of individual tests, by the number of individuals receiving that test if known; if unknown, unless it was otherwise indicated, we assumed that all subjects received that test. Pooled percentages were calculated by summing the totals across studies, and 95% confidence intervals (CIs) were tabulated. Two-tailed z-scores were used in all comparisons, and significance was defined as p≤0.05.
Studies were stratified on the basis of whether investigations were undertaken at the discretion of the physician following history or physical examination (nonprotocolized) or whether investigations were undertaken independent of clinical status (protocolized). Stratification was done by setting (ER or inpatient) and by evaluator (psychiatrist or nonpsychiatrist).
Heterogeneity between the studies was quantified with the I2 statistic (21). An I2 value <25% was selected to represent low heterogeneity, whereas a value >75% was selected to represent high heterogeneity (21). All statistical analyses were conducted by using Comprehensive Meta-Analysis, version 3.3. A meta-analysis was performed with inverse-variance weighting. Subgroup analysis was performed by using a mixed model, i.e., assumption of random effects within subgroups and fixed effects across subgroups (22); variance was pooled (23). A funnel plot (24) was constructed, and statistical testing was performed to evaluate for potential biases, including publication bias. To explore heterogeneity over time, a meta-regression was undertaken whereby yield was regressed on publication year (25).
Quality Assessment
The methodological quality of included studies was assessed with the appraisal tool for cross-sectional studies (AXIS) (18). In contrast to quality evaluation measures used in previous reviews of this topic (18), this tool is designed and validated for use with the cross-sectional studies included in our review. The AXIS tool comprises 20 items, and each item has three rating options: yes (Y), no (N), or unknown (?). By design, a summed numerical score is not produced. We each independently assessed each study with the above tool; subsequent consensus of ratings was achieved.
Results
Search Results
Database searches and searches from other sources identified 327 citations (a PRISMA diagram is included in an online supplement to this article). A total of 129 publications were retrieved for detailed screening. We excluded articles that did not address utility or allow for inference of same (N=32) or that did not meet our research aims (N=32). Studies focused on UDS or self-harm (N=24) were excluded, as were those with pediatric (N=8) or survey-based samples (N=4). Three aimed at validation of a screening tool were not included (8, 9, 11). One study did not appear to be substantively different from earlier work (26). One author was contacted, and the study was retained. Ultimately, 25 articles were reviewed (27–51).
Study Characteristics
Table 1 provides information on all included studies. Comments were largely derived from authors’ conclusions, except in one case (33). Sample size ranged from 38 (32) to 719 (50); four samples included largely agitated or disoriented patients (32, 36, 37, 45), and two studies focused on older patients (29, 41). Eleven studies were undertaken in the general ER (27, 30, 37, 38, 40, 42–46, 49), three in the psychiatric ER (32, 33, 36), and 11 on the psychiatric inpatient ward (28, 29, 31, 34, 35, 39, 41, 47, 48, 50, 51). Nineteen studies adopted protocolized approaches (27, 29–33, 35–38, 41–43, 45, 46, 48–51), three adopted nonprotocolized approaches (34, 40, 44), and one used both (47). In two studies, the approach could not be determined (28, 39). Evaluations were conducted by a psychiatrist or psychiatric resident in nine studies (28, 29, 33, 34, 36, 39, 41, 50, 51) and a nonpsychiatrist in 13 (27, 30, 31, 35, 37, 38, 40, 42–46, 49); in three studies, the evaluator was unspecified (32, 47, 48).
| Investigationsb | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Sample | Design | Outcome | Overall | Individual | Historyb | Physical examb | Comments |
| Amin and Wang, 2009 (27) | N=375; sex and age unspecified; diagnosis, “normal mental status to florid psychosis” | Prospective, 21 months; ER; protocolized; evaluator, ER resident | Change in management among patients with a normal history and physical exam | 0% | UA, 0%; thyroid, 0%; CBC, 0% | Indet | Indet | Patients with normal history and physical exam had low likelihood of significant yield from investigations |
| Chandler and Gerndt, 1988 (28) | N=224; males, 47%; mean age, 39; diagnoses: 18% schizophrenia, 8% substance use disorder | Prospective, 4 months; inpatient; evaluator, psychiatric resident | Change in diagnosis or treatment | Indet | CBC, 0%; thyroid, 0% | 4% | 12.1% | History and physical exam played an important role in the detection and treatment of illness |
| Colgan and Philpot, 1985 (29) | N=167; females, 71%; mean age: 79 for females, 75 for males; diagnosis, 41% dementia | Retrospective, 18-month chart review; inpatient; protocolized; evaluator, admitting psychiatrist | Change in management among older inpatients | 49% | CBC, 8.1%; glucose, 2.8%; folate, 3.8%; B12, 5.3%; thyroid, .7%; syphilis, .8%; UA, 17.5%; CT head, 3.5%; CXR, 7.7%; ECG, 14.3% | Indet | Indet | CBC, serum folate, urea, electrolytes, and UA recommended |
| Crede et al., 2011 (30) | N=604; males, 67%; median age, 29; diagnosis, “acute psychosis” | Retrospective, 6-month review; ER; protocolized; evaluator, ER physician | Change in diagnosis or management | .3% | LP, 0%; thyroid, 0% | Indet | Indet | Investigations provided little additional information to that obtained by history and physical exam |
| Dolan and Mushlin, 1985 (31) | N=250; females, 60%; mean age, 42; diagnosis, 58% major affective disorder | Retrospective, 11 months; inpatient; protocolized; evaluator, internist | Change in diagnosis or management | 4.4% | Na, .4%; thyroid, 3%; CR, 0%; syphilis, 1.1%; CBC, 3.8%; WBC, 3% | 20.8% | Indet | Routine investigations unnecessary; select tests in patients with high pretest probability of disease may be of benefit |
| Dubin et al., 1983 (32) | N=38; males, 53%; mean age, 58; diagnosis, organic brain syndrome (89% disoriented) | Prospective, 1 year; psychiatric ER; protocolized; evaluator unspecified | Utility of physical exam and investigations | 16% | Glucose, 7.9%; BUN, 7.9% | Indet | Indet | Investigations largely not helpful in determination of etiology of organic brain syndrome |
| Eastwood et al., 1970 (33) | N=100; males, 53%; mean age, 34; diagnoses: 60% affective disorder, 8% schizophrenia | Prospective, study period unspecified; psychiatric ER; protocolized; evaluator, psychiatrist | Frequency of unrecognized nonpsychiatric illness | 1% | CBC, 1% | Indet | 11% | Excluding anemia, all patients had symptoms or signs apparent on history or physical exam |
| Ferguson and Dudleston, 1986 (34) | N=650; females, 55%; 26–35 age group, 21%; diagnosis, “affective psychosis” | Retrospective, 2-year chart review; inpatient; nonprotocolized; evaluator, psychiatrist | Incidence of tests resulting in change in treatment | .8% | Syphilis, 0%; thyroid, 0%; UA, .8% | Indet | Indet | Routine investigations of little value |
| Hall et al., 1980 (35) | N=100; females, 55%; mean age, 28; diagnosis, 38% schizophrenia; no “significant medical illness”; majority under mental health warrant | Retrospective, study period unspecified; inpatient; protocolized; evaluator, general practitioner | Illnesses that caused or exacerbated psychiatric symptoms | Unspecified | UA, 6%; ECG, 9%; CBC, 15% | 28% | 40% | Detailed history and physical exam and select investigations (e.g., ECG, CBC, UA) recommended |
| Hatta et al., 1998 (36) | N=259; males, 100%; age unspecified; diagnosis, “severely disturbed involuntary patients” | Prospective, 18 months; psychiatric intensive care unit; protocolized; evaluator, psychiatrist | Contributions of physical exam, history, and investigations toward new diagnosis | 21.6% | K+, 2.3%; CK, 13.9%; WBC, 3.1% | Indet | 2.7% | Investigations more important than history, VS, or physical exam in detecting illness among uncooperative patients |
| Henneman et al., 1994 (37) | N=100; males, 63%; mean age, 38; diagnoses: 13% schizophrenia, 63% “organic,” 66% agitated, 60% disoriented; “no medical complaints or physical findings” | Prospective, 9 months; ER; protocolized; evaluator, ER physician or internist | Admission or diagnosis of etiology of psychiatric symptoms | Unspecified | SMA 7, 10%; CBC, 5%; LP, 8%; CT head, 8% | 27% | 6% | Results support investigations for patients with new psychiatric symptoms |
| Janiak and Atteberry, 2012 (38) | N=502; sex, age and diagnosis unspecified | Retrospective, 8-month chart review; inpatient; protocolized; evaluator, ER physician | Influence of inpatient investigations on ER management | .2% | Indet | Indet | Indet | Routine investigations neither cost-effective nor necessary |
| Johnson, 1968 (39) | N=250; sex, age, and diagnosis unspecified; | Prospective, study period unspecified; inpatient; evaluator, psychiatrist | Utility of physical exam | Indet | Indet | Indet | 12% | Physical exam is an important tool in the exploration of psychiatric presentation |
| Kagel et al., 2017 (40) | N=682; males, 78%; 18–41 age group, 94%; diagnosis, “chief psychiatric complaints” (e.g., depression, anxiety) | Retrospective, 2-year chart review; ER; nonprotocolized; evaluator, ER physician | Percentage of dispositions that changed from admission to psychiatric service to medical service | .1% | UA, .1%; BAL, .1%; thyroid, 0%; ASA, 0%; APAP, 0%; CBC, 0%; pregnancy, 0% | Indet | Indet | Mandatory investigations rarely altered disposition |
| Kolman, 1985 (41) | N=68; females, 75%; mean age, 78; diagnosis, 51% dementia | Prospective, 7 months; psychogeriatric unit; protocolized; evaluator, psychiatric resident | Changes in diagnosis or management | Indet | UA, 13%; CXR, 8.5%; B12, 1.5%; ECG, 1.5%; glucose, 1.5%; urea, 1.5% | Indet | Indet | Author suggested UA, CXR, B12, ECG, and urea |
| Korn et al., 2000 (42) | N=80; sex unspecified; age range, 17–83; diagnosis, 20% “bizarre behavior,” 11% depression | Retrospective, 5-month chart review; ER; protocolized; evaluator, ER physician | Changes in management | 0% | CBC, 0%; Cr, 0%; BUN, 0%; pregnancy, 0%; CXR, 0% | Indet | Indet | No benefit to investigations |
| Olshaker et al., 1997 (43) | N=345; males, 64%; mean age, 35; diagnosis, “psychiatric complaints” (e.g., depression, psychosis) | Retrospective, 2-month review; ER; protocolized; evaluator, ER physician | Identification of “acute medical conditions” | .9% | CBC, .9%; glucose, .3%; K+, .6% | 17.7% | 9.6% | No benefit to investigations |
| Parmar et al., 2012 (44) | N=191; sex, age, and diagnosis unspecified | Prospective, two periods of 7 and 13 months; ER; nonprotocolized evaluator, ER residents and physicians | Change in disposition from admission to psychiatric ward | .5% | CBC, 0%; thyroid, 0%; ECG, 0%; UA, 0%; liver, 0% | Indet | Indet | Mandatory investigations did not change disposition of patients after initial history and physical exam |
| Saloojee, 2009 (45) | N=339; males, 69%; mean age, 26; diagnosis, aggressive patients requiring sedation | Retrospective, 11-month review; ER; protocolized; evaluator, ER physician | Utility of investigations, history, and physical exam in exclusion of illness contributing to aggression | 28.9% | CBC, 13.9%; glucose, 5.3% | Indet | Indet | Results support use of investigations for aggressive patients in the ER |
| Schauer and Goolsby, 2015 (46) | N=204; males, 70%; mean age, 24; diagnoses: 52% “suicidal ideation,” 16% “depression” | Retrospective, 11-month chart review; ER; protocolized; evaluator, ER physician | Admission to medical or surgical unit | 0% | ETOH, 0%; CBC, 0%, APAP, 0%; ASA, 0%; pregnancy, 0%, UA, 0% | Indet | Indet | Investigations infrequently resulted in disposition changes |
| Sheline and Kehr, 1990 (47) | N=252; males, 59%; mean age, 33; diagnoses: 57% schizophrenia, 21%, bipolar disorder | Retrospective, 3-month chart review; inpatient; protocolized and nonprotocolized; evaluator unspecified | Change in management or diagnosis | 6.6% | Protocolized: CBC, 3.3%; UA, .6%; nonprotocolized: CBC, 6%; UA, 3.7% | Indet | Indet | Investigations were of low utility; nonprotocolized testing had higher yield than protocolized |
| Thomas, 1979 (48) | N=613; sex, age, and diagnosis unspecified | Retrospective, 12-month review; inpatient; protocolized; evaluator unspecified | Influence of investigations on management | 10% | Syphilis, 0%; thyroid, 2.1%; B12, 8%; CBC, 2.2%; liver, 1%; CXR, 3.1% | Indet | Indet | Investigations of high utility |
| Tobin et al., 2020 (49) | N=441; males, 57%; 18–33 age group, 69%; diagnoses: 52% suicidal ideation, 21% anxiety | Retrospective, 8-month chart review; ER; protocolized; evaluator, ER physician | Changes in disposition | .9% | Indet | Indet | Indet | Protocolized investigations rarely altered patient disposition |
| White and Barraclough, 1989 (50) | N=719; males, 71%; 25–64 age group, 62%; diagnosis unspecified | Retrospective, 15-month chart review; inpatient; protocolized; evaluator, psychiatrist | Change in management or diagnosis | Indet | Thyroid, 2.7% | Indet | Indet | Select investigations may be of benefit |
| Willett and King, 1977 (51) | N=636; females, 59%; mean age, 32; diagnoses: 25% schizophrenia, 19% personality disorder | Retrospective, 17-month review; inpatient; protocolized; evaluator, psychiatric resident | Detection or confirmation of missed nonpsychiatric illness | 2.2% | Indet | Indet | Indet | Investigations were not useful in the psychiatric care of inpatients |
TABLE 1. Characteristics of studies included in the meta-analysis and selected resultsa
Investigations showed significant heterogeneity in number and type and ranged from commonly performed tests, including complete blood count (CBC) (N=13) (27, 28, 31, 33, 35, 37, 40, 42–45, 47, 48) to lumbar puncture (N=2) (30, 37). The mean±SD number of investigations per study was 11±7.2 for both protocolized and nonprotocolized approaches, with a wide range from 3 (28) to 32 (31). No study provided detailed methodology in terms of either history or physical examination, including constituent components, although neurological examination was explicitly noted to have been undertaken in four studies (27, 28, 35, 45).
Quality and Bias
Study quality was evaluated by the AXIS tool (18) (see table in online supplement). All studies clearly outlined their objective. None of the studies met all the criteria stipulated for “Methods,” “Results,” and “Discussion,” including studies that did not adequately describe their basic data (30, 32, 33, 37, 39, 43, 44) and that did not explore study limitations (32–34, 36, 37, 39, 41, 51). Details of ethical approval or participant consent were missing in 14 studies (29, 31–36, 39, 41, 42, 47, 48, 50, 51). None of the studies was of sufficiently low quality to warrant its exclusion. Egger’s test for asymmetry of the funnel plot (24) was significant (p<0.01), although on visual inspection, several smaller studies appeared to be associated with low yield, suggesting limited likelihood of publication bias. Limited numbers did not allow for analysis by subgroups. Meta-regression indicated that the later the year of publication, the smaller the yield of investigations; although significant, this was a small association (β=−0.06, df=1, p=0.03), and nonsignificant small associations were noted with yield of history (β=−0.04) and physical examination (β=−0.01).
Outcome
Investigations.
Heterogeneity among the studies was high (I2=96%, df=18, p<0.001). Overall yield pooled across 19 studies was 2.6% (95% CI=1.3%–5.2%) (27, 29–34, 36, 38, 40, 42–49, 51). Because of heterogeneity and because of theoretical considerations as outlined below, two subgroups were created—namely, older adults (29, 41) and agitated or disoriented patients (32, 36, 37, 45). Unless specified, subgroups were excluded and analyzed separately below. With these subgroups excluded, overall yield was 1.1% (95% CI=0.5%–2.2%, df=14, N=15, p<0.001) (Figure 1). Limited data suggested that yield was higher among the two created subgroups, compared with the overall pooled yield (df=3, p<0.001), including in the three studies with largely agitated or disoriented patients (22.0%, 95% CI=8.7%–45.7%) (32, 36, 45) and in the one study among older patients from which yield could be derived (48.5%, 95% CI=13.0%–85.5%) (29). The analysis presented here involved exclusion of these subgroups unless specified otherwise.
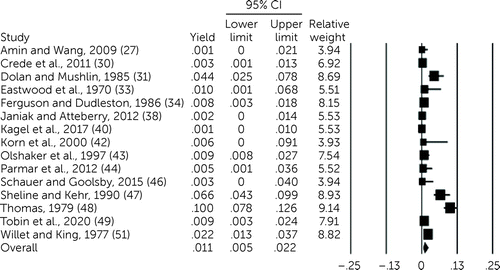
FIGURE 1. Forest plot of meta-analysis for yield of investigations among psychiatric patients undergoing medical clearancea
aI2=90%, df=14, p<.001.
Two studies that excluded persons with either “medical complaints” (42) or abnormal physical examination or vital signs (40) showed a nonsignificant trend toward lower yield, compared with all other studies (0.3%, p=0.24), although this was not the case for two others with similar exclusion criteria (overall yield unavailable) (35, 37). Utility varied by individual investigation, including with tests such as CBC (1.7%, 95% CI=0.8%–3.6%, N=11 studies), urinalysis (1.0%, 95% CI=0.4%–2.8%, N=6), thyroid (0.8%, 95% CI=0.3%–1.9%, N=9), electrocardiogram (ECG) (2.3%, 95% CI=0.1%–28.5%, N=2), creatinine 0% (N=2), and syphilis 0% (N=2). No significant differences were noted, compared with the older adult subgroup, with respect to ECG (4.7%, p=0.37) or CBC (7.8%, p=0.16), but urinalysis was found to be significantly higher (15.5%, 95% CI=4.0%–33.8%, df=1, p=0.001); yield of CBC was significantly higher among agitated or disoriented patients (8.8%, 95% CI=2.2%–29.1%, df=1, p=0.04).
A nonsignificant difference in yield was noted between protocolized approaches (27, 30, 31, 33, 38, 42, 43, 46, 48, 51) (1.6%, 95% CI 0.8–3.1, N=10), compared with nonprotocolized approaches (34, 40, 44) approaches (0.4%, 95% CI=0.1–1.9, N=3). In studies of evaluations of patients presenting to the ER (27, 30, 33, 40, 42–44, 46), a significantly lower yield (df=1, p=0.003) of investigations was noted (0.5%, 95% CI=0.2%–1.3%, N=9), compared with studies in which evaluations were conducted in the inpatient setting (31, 34, 38, 47, 48, 51) (2.9%, 95% CI=1.3%–6.1%, N=6).
History and physical examination.
Heterogeneity across studies was high for both history (I2=90%, df=3, p<0.001) and physical examination (I2=93%, df=6, p<0.001). In the four studies for which utility of history could be gleaned (28, 31, 35, 43), yield was 15.6% (95% CI=9.1%–25.6%, df=3, p<0.001) (Figure 2), and for physical examination, yield was 14.9% (95% CI=8.1%–25.9%, df=4, N=5, p<0.001) (28, 33, 35, 39, 43) (Figure 3). Among 16 studies reporting on the qualitative utility of history or physical examination, 11 reported a benefit (27, 30, 34, 38, 40–42, 44–47). Among agitated or disoriented patients, yield of history was available in only one study (27.0%, 95% CI=9.6%–56.2%) (37), with two reporting a combined yield for history and physical examination (4.0%, 95% CI=1.3%–11.8%) (36, 37). Neither of the studies of older patients allowed for calculation of yield of either history or physical examination (29, 41).
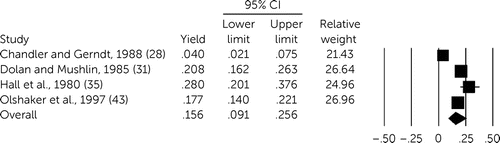
FIGURE 2. Forest plot of meta-analysis for yield of history taking among psychiatric patients undergoing medical clearancea
aI2=90%, df=3, p<.001.
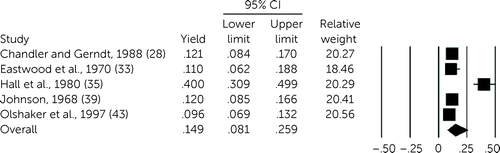
FIGURE 3. Forest plot of meta-analysis for yield of a physical examination among psychiatric patients undergoing medical clearancea
aI2=93%, df=4, p<.001.
Only one study explored history with psychiatrists as evaluators (28); it found a lower yield (4.0%, 95% CI=2.0%–8.0%), compared with the three studies in which nonpsychiatrists were evaluators (21.0%, 95% CI=17.0%–27.0%, df=1, p<0.001) (31, 35, 43), although with no demonstrable differences by physical examination: three studies with psychiatrists as evaluators (12.0%, 95% CI=5.0%–26.0%) (28, 33, 39), compared with two studies with nonpsychiatrists as evaluators (21.0%, p=0.37) (35, 43).
Discussion
Our comprehensive meta-analysis explored the utility of investigations, history, and physical examination among adult patients with psychiatric presentations who were being “medically cleared.” Pooled results for investigations suggested relatively low utility, especially in the ER. Overall yield was higher among some subgroups, including older patients and agitated or disoriented patients. Results of individual investigations varied but were similarly relatively low; as an example, CBC, which has been suggested to be “commonly overused” in some clinical settings (52), demonstrated a pooled yield of 1.7%. Yield of history and physical examination was high, compared with yield of investigations, and recommended by most authors, although nonprotocolized approaches, in which history and physical examination are typically conducted, were not found to be of higher utility than were protocolized approaches. Limited data suggested lower yield of history taking by psychiatrists, compared with nonpsychiatrists, although this did not apply to physical examination.
Our findings suggest some consistency across time, compared with findings from prior reviews. With respect to laboratory investigations, Gregory et al. (13) also reported limited utility among patients, with pooled estimates ranging from 0.3% to 6.9%, and Anfinson and Kathol (15) noted the yield of clinically significant results to be “small,” ranging from 0.8% to 4.0%. Compared with our analysis, Conigliaro et al. (14), in a more recent review, reported a somewhat lower range, with only 0%–0.4% of tests changing patient disposition, although this range was based on only three studies, and, in addition, the outcome measure used in the review was only that of change in patient disposition, whereas our study also included change in treatment or diagnosis. In the review by Conigliaro et al. (14), yield of history and physical examination was not undertaken. Chennapan et al. (17), by contrast, did not provide quantitative estimates but similarly suggested relatively higher yield of history and physical examination, compared with investigations. Broadening our findings to investigations performed in the ER among patients not specifically undergoing the process of “medical clearance,” one study reported that at most 4% of investigations influenced diagnosis, management, or disposition; common tests, such as creatinine, liver function tests, and serum electrolytes, influenced only between 0.3% and 3.4% of cases (53). Other specialties may also provide some guidance. In a recent study of preoperative evaluation, less than 1% of abnormal “routine” tests resulted in any change in perianesthetic management (54).
Our finding of the relative benefit of history and physical examination is important. No study provided detailed information on components, such as vital signs or mental status examination. The absence of such information may reflect clinical practice. Szpakowicz and Herd (55) found that vital signs were documented in only 52% of cases, and Riba and Hale (56) noted that only 8% had a full neurological examination. Others have suggested that the neurological examination may be of especially high utility among patients with psychiatric symptoms (28, 35, 57). We note a report by Tintinalli et al. (58) on a sample of 298 ER patients with psychiatric chief complaints; these authors found that 12 patients (4%) received “acute medical treatment” after “clearance” in the ER, and of these, 10 had findings that were “easily demonstrated on essential portions of the examination.” At least one survey indicated that only a minority of psychiatrists conducted their own physical examination (59), but there are certainly arguments for doing so (57, 60). The authors of one study remarked that “psychiatry is a medical specialty and to abrogate responsibility for physical evaluation has implications for the profession as a whole” (61). Although based on a small number of studies, our analysis suggested no difference of yield of physical examination by evaluator. Therefore, at minimum, we suggest that chart review be undertaken by the consultant psychiatrist to ensure that an examination has in fact taken place and for which components.
Given our finding that the pooled yield of investigations was only 1.1%, when should such tests be undertaken for patients presenting with psychiatric complaints? Our review does not lend itself well to addressing such a question. We note that two studies that excluded patients with either “medical complaints” (42) or abnormal physical examination or vital signs (40) reported low overall yields of laboratory investigations, although we were not able to demonstrate statistical significance. We speculate that such patients have a low likelihood of pathology and associated findings. However, two studies that had similar exclusion criteria—“significant medical illness” (35) or “medical complaints” (37)—showed a nonsignificant trend toward higher yields, albeit with samples of largely disoriented patients with new psychiatric symptoms (37) or involuntary patients with limited preadmission care (35). Despite the importance of history and physical examination, a nonprotocolized approach—one in which history and physical examination guide ordering of investigations—was not demonstrated to result in a higher yield, compared with a protocolized approach, although data were limited—for example, only three studies using nonprotocolized approaches were included (34, 40, 44). The single study that directly compared protocolized and nonprotocolized approaches reported greater utility for the latter (47).
Select populations may benefit from investigations. In the one study of older adults for which the yield of investigations could be derived (29), almost half (48.5%) had a change in diagnosis or management resulting from investigations. Both studies of older adults (29, 41) cited urinalysis as the investigation of highest yield, although only one study (29) indicated whether bacteriuria was associated with symptoms. Higher yield among older patients, compared with younger patients, might reflect higher comorbidity (62) or, broadly, higher rates of laboratory abnormalities (63). Further support for testing among older individuals comes from Chandler and Gerndt (28), who reported that 27% of those over age 60 had a change in psychiatric diagnosis or treatment as a result of their evaluation, whereas only 4.5% of those under age 30 had a change in outcome. In our analysis, investigations among largely agitated patients, including “severely disturbed” (36) and aggressive patients requiring sedation (45), along with largely disoriented patients (32, 37), were also found to be of relatively higher yield. Higher yield might reflect dissimilar etiology, compared with other study samples—for example, a number of “severely disturbed” (36) patients were dehydrated, with associated laboratory abnormalities, and “disoriented” may in many instances reflect cases of delirium. In addition, we speculate that higher yield may reflect degree of cooperation, whereby uncooperative patients might have lower relative yield of history and physical examination, although we were unable to explore this hypothesis given limited data. We note that one study that compared agitated patients requiring intramuscular medication and patients not requiring psychotropics found a higher incidence of abnormal laboratory findings among the former (64).
Differences in disease prevalence, as observed in at least one study (11), might account for some of the differences observed in yield by site (ER or inpatient). Others have suggested possible benefit to investigations among other groups, such as a chest X-rays for those at elevated risk of tuberculosis (13). In addition, limited access to health care may also modify the approach (35), such as in obtaining baseline measurements when psychotropic initiation is planned or in monitoring disease. Starting or monitoring medications—e.g., a psychotropic—may also influence the ordering of investigations. Cultural or geographic differences may also be of interest (45).
The finding of limited utility of investigations suggests caution for reasons of both resource allocation and patient care. Cost was not well explored in the studies reviewed, although two studies provided quantitative estimates. Parmar et al. (44) estimated a cost for mandatory ER laboratory testing of US$37,682 among 191 patients (approximately $197 per patient). Schauer and Goolsby (46) reported a somewhat lower estimate of $146 per patient for laboratory screening for “medical clearance.” Of note, in neither study did testing have a significant effect on disposition. Costs less easily measured include time for specimen collection and review of results (47), in addition to those associated with delayed discharge or admission (65). Patient harm may also result from “investigative cascades,” including those stemming from false positives (52). Psychological sequelae are also an important consideration of unnecessary testing (66), including anxiety over pending results, stigma around diagnosis, or misplaced reassurance from false negatives. To be sure, there is growing awareness of the need for judicious use of investigations, for example, as evidenced by the initiative Choosing Wisely (5).
Our finding of limited yield of investigations, coupled with variable clinical approach, suggests that few presumptions should be made at the time of referral to psychiatry. Nonetheless, it is common for the consulting psychiatrist to assume “medical clearance.” Such an assumption may amplify potential for error in diagnosis and management. For example, Anderson et al. (6) suggested that “ED [emergency department] staff should consider nonpsychiatric diagnoses that mimic psychiatric conditions, such as hypothyroidism causing depressive symptoms.” If so, should the consulting psychiatrist assume that the patient has been assessed for, in this case, hypothyroidism? The interpretation of “nonpsychiatric” is also likely to vary; thyroid pathology may manifest in psychiatric illness, and many psychiatrists would accept its consideration to lie within their scope. Broadly, the consultant should have expectations regarding presentation not etiology: the respirologist can expect a referral for dyspnea but not necessarily one for respiratory disease. Indeed, use of the term “medical clearance” may hamper patient care, and its use, with its connotation of medical and nonmedical approaches, may further reinforce the divide between psychiatry and other specialties in medicine.
As with any review, we were limited by the studies available. Although none were of sufficiently low quality to warrant exclusion, none of the 25 studies met all the 20 assessment criteria (18). We attempted to mitigate heterogeneity with subgroup analysis and the use of mixed-effects modeling, although limitations remained. As an example, definition of our primary outcome measure varied by study and may have also evolved over the years. Kolman (41), for instance, treated almost all older patients who had a positive urine culture, whereas current practice is to not treat asymptomatic bacteriuria (67). Other considerations may have affected outcomes. For one, studies were cross-sectional, and longitudinal observation may have resulted in different outcomes. Outcome is associated with number and type of tests undertaken, in addition to disease prevalence, but we were unable to adjust for these considerations. Similarly, measures including sensitivity, specificity, and predictive value could not be calculated. The utility of findings that did not necessarily lead to clinical change, such as those that offered diagnostic reassurance, was unknown, and if it were considered, it could increase yield. In some cases, we assumed that every patient received a given test, an assumption that would tend to underestimate yield when not true. Conversely, yield might be inflated if a given outcome were represented by more than one abnormal investigation. It might also be true that some pathology is best diagnosed or managed by multiple investigations, a truth difficult to reflect with single-test yields. Of note, underutilization—tests indicated but not ordered—has been cited to be of concern (68), although missed diagnoses in our included studies appeared to be relatively low, ranging from 0.8% to 3%; they included thyroid disease and HIV (3%) (37); “mild” hypokalemia (0.6%) (43); subdural hematoma, hyperthyroidism, pulmonary tuberculosis, and meningitis (1.6%) (45); and urinary tract infection (0.8%) (34). Finally, for reasons of heterogeneity and scope, we did not explore UDS and cannot comment on its effectiveness, although one review found it to be of low utility (69).
Future studies should help to better define the components of evaluation of psychiatric patients in the ER. Designs are many but could include a diagnostic before-after study (70), such as one in which the anticipated effect of history, physical examination, or investigation on outcome is prospectively recorded and evaluated against actual outcome, such as change in diagnosis or management (71). Outcomes would ideally be referenced against objective measures (72), including ones obtained longitudinally—e.g., hospital admission over a defined period (73). Reasons for ordering investigations, such as patient reassurance, diagnostic uncertainty, or disease monitoring, could be recorded (74). Subsequent regression modeling, including exploration of goodness of fit, could delineate quantitative contribution, including contributions of individual investigations; thresholds could be explored with decision curve or similar analysis (75). Subgroup analyses would help refine approach. Cost-effectiveness analysis would better inform conclusions about overall utility.
Conclusions
Our study is to our knowledge the most comprehensive review—and the only meta-analysis—to have addressed the important clinical question of the utility of investigations, history, and physical examination among patients presenting with psychiatric complaints. Investigations were of limited utility. They did not significantly alter diagnosis or management of most patients undergoing “medical clearance,” especially in the ER setting. When weighed against potential harm and cost, investigations should not be routinely conducted. Limited data suggest that some subgroups, including agitated, disoriented, or older patients, may benefit from such investigations. Our findings support the undertaking of physical examination and history for all patients, ideally with participation of the consultant psychiatrist. The term “medically cleared” should not be used: for one, as we have shown, investigations are of limited utility, and for another, with any investigations, history, or physical examination, it is often unclear what was undertaken and for which indications. Use of the term “medically cleared” detracts from patient care and, arguably, from the psychiatrist-nonpsychiatrist relationship. A shift in the practice of both referring physicians and consulting psychiatrists is needed.
1. : Mental health and emergency medicine: a research agenda. Acad Emerg Med 2009; 16:1110–1119Crossref, Medline, Google Scholar
2. : Temporal trends in mental health service utilization across outpatient and acute care sectors: a population-based study from 2006 to 2014. Can J Psychiatry 2018; 63:94–102Crossref, Medline, Google Scholar
3. : Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry 2017; 16:30–40Crossref, Medline, Google Scholar
4. : ED utilization of medical clearance testing for psychiatric admission: National Hospital Ambulatory Medical Care Survey analysis. Am J Emerg Med 2018; 36:745–748Crossref, Medline, Google Scholar
5. . Philadelphia, American Board of Internal Medicine Foundation. www.choosingwisely.org. Accessed Feb 16, 2021Google Scholar
6. : American Association for Emergency Psychiatry task force on medical clearance of adults part I: introduction, review and evidence-based guidelines. West J Emerg Med 2017; 18:235–242Crossref, Medline, Google Scholar
7. : Medical clearance in the psychiatric emergency setting: a call for more standardization. Healthc Q 2010; 13:77–82Crossref, Medline, Google Scholar
8. : A screening tool to medically clear psychiatric patients in the emergency department. J Emerg Med 2012; 43:871–875Crossref, Medline, Google Scholar
9. : Prospective medical clearance of psychiatric patients. Prim Psychiatry 2008; 15:60–66Google Scholar
10. : “Medical clearance” of patients with acute mental health needs in the emergency department: a literature review and practice recommendations. WMJ 2019; 118:156–163Medline, Google Scholar
11. : A medical algorithm for detecting physical disease in psychiatric patients. Hosp Community Psychiatry 1989; 40:1270–1276Abstract, Google Scholar
12. : Emergency room medical clearance: an educational problem. Am J Psychiatry 1979; 136:787–790Link, Google Scholar
13. : Medical screening in the emergency department for psychiatric admissions: a procedural analysis. Gen Hosp Psychiatry 2004; 26:405–410Crossref, Medline, Google Scholar
14. : Protocolized laboratory screening for the medical clearance of psychiatric patients in the emergency department: a systematic review. Acad Emerg Med 2018; 25:566–576Crossref, Medline, Google Scholar
15. : Screening laboratory evaluation in psychiatric patients: a review. Gen Hosp Psychiatry 1992; 14:248–257Crossref, Medline, Google Scholar
16. : American Association for Emergency Psychiatry task force on medical clearance of adult psychiatric patients. part II: controversies over medical assessment, and consensus recommendations. West J Emer Med 2017; 18:640–646Crossref, Medline, Google Scholar
17. : Medical screening of mental health patients in the emergency department: a systematic review. J Emerg Med 2018; 55:799–812Crossref, Medline, Google Scholar
18. : Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016; 6:e011458Crossref, Medline, Google Scholar
19. : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62:e1–e34Crossref, Medline, Google Scholar
20. : Morbidity and rate of undiagnosed physical illnesses in a psychiatric clinic population. Arch Gen Psychiatry 1979; 36:414–419Crossref, Medline, Google Scholar
21. : Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560Crossref, Medline, Google Scholar
22. : Subgroup analyses; in Introduction to Meta-Analysis. Chichester, UK, Wiley, 2009Crossref, Google Scholar
23. : Analysis of categorical moderators in mixed-effects meta-analysis: consequences of using pooled versus separate estimates of the residual between-studies variances. Br J Math Stat Psychol 2017; 70:439–456Crossref, Medline, Google Scholar
24. : Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634Crossref, Medline, Google Scholar
25. : Heterogeneity of psychosis risk within individuals at clinical high risk: a meta-analytical stratification. JAMA Psychiatry 2016; 73:113–120Crossref, Medline, Google Scholar
26. : Unrecognized physical illness prompting psychiatric admission: a prospective study. Am J Psychiatry 1981; 138:629–635Link, Google Scholar
27. : Routine laboratory testing to evaluate for medical illness in psychiatric patients in the emergency department is largely unrevealing. West J Emerg Med 2009; 10:97–100Medline, Google Scholar
28. : The role of the medical evaluation in psychiatric inpatients. Psychosomatics 1988; 29:410–416Crossref, Medline, Google Scholar
29. : The routine use of investigations in elderly psychiatric patients. Age Ageing 1985; 14:163–167Crossref, Medline, Google Scholar
30. : Assessment of routine laboratory screening of adult psychiatric patients presenting to an emergency centre in Cape Town. S Afr Med J 2011; 101:891–894Medline, Google Scholar
31. : Routine laboratory testing for medical disorders in psychiatric inpatients. Arch Intern Med 1985; 145:2085–2088Crossref, Medline, Google Scholar
32. : Organic brain syndrome. The psychiatric imposter. JAMA 1983; 249:60–62Crossref, Medline, Google Scholar
33. : The physical status of psychiatric emergencies. Br J Psychiatry 1970; 116:545–550Crossref, Medline, Google Scholar
34. : Detection of physical disorder in newly admitted psychiatric patients. Acta Psychiatr Scand 1986; 74:485–489Crossref, Medline, Google Scholar
35. : Physical illness manifesting as psychiatric disease: II. analysis of a state hospital inpatient population. Arch Gen Psychiatry 1980; 37:989–995Crossref, Medline, Google Scholar
36. : Abnormal physiological conditions in acute schizophrenic patients on emergency admission: dehydration, hypokalemia, leukocytosis and elevated serum muscle enzymes. Eur Arch Psychiatry Clin Neurosci 1998; 248:180–188Crossref, Medline, Google Scholar
37. : Prospective evaluation of emergency department medical clearance. Ann Emerg Med 1994; 24:672–677Crossref, Medline, Google Scholar
38. : Medical clearance of the psychiatric patient in the emergency department. J Emerg Med 2012; 43:866–870Crossref, Medline, Google Scholar
39. : The evaluation of routine physical examination in psychiatric cases. Practitioner 1968; 200:686–691Medline, Google Scholar
40. : Effects of mandatory screening labs in directing the disposition of the apparently healthy psychiatric patient in the emergency department. US Army Med Dep J 2017:18–24Medline, Google Scholar
41. : Predicting the results of routine laboratory tests in elderly psychiatric patients admitted to hospital. J Clin Psychiatry 1985; 46:532–534Medline, Google Scholar
42. : “Medical clearance” of psychiatric patients without medical complaints in the Emergency Department. J Emerg Med 2000; 18:173–176Crossref, Medline, Google Scholar
43. : Medical clearance and screening of psychiatric patients in the emergency department. Acad Emerg Med 1997; 4:124–128Crossref, Medline, Google Scholar
44. : Value of mandatory screening studies in emergency department patients cleared for psychiatric admission. West J Emerg Med 2012; 13:388–393Crossref, Medline, Google Scholar
45. : Routine pre-admission screening for a medical illness in aggressive patients who required sedation in the emergency department—necessary or not? S Afr J Psychiatry 2009; 15:67–71Google Scholar
46. : A retrospective review of screening labs for medical clearance in a military population. Mil Med 2015; 180:1128–1131Crossref, Medline, Google Scholar
47. : Cost and utility of routine admission laboratory testing for psychiatric inpatients. Gen Hosp Psychiatry 1990; 12:329–334Crossref, Medline, Google Scholar
48. : The use of screening investigations in psychiatry. Br J Psychiatry 1979; 135:67–72Crossref, Medline, Google Scholar
49. : Utility of nonspecific laboratory testing for psychiatric patients undergoing medical screening in a military emergency department. Mil Med 2020; 185:e1941–e1945Crossref, Medline, Google Scholar
50. : Benefits and problems of routine laboratory investigations in adult psychiatric admissions. Br J Psychiatry 1989; 155:65–72Crossref, Medline, Google Scholar
51. : Implementation of laboratory screening procedures on a short-term psychiatric inpatient unit. Dis Nerv Syst 1977; 38:867–870Medline, Google Scholar
52. : Improving laboratory test utilisation at the multihospital Yale New Haven Health System. BMJ Open Qual 2019; 8:e000689Crossref, Medline, Google Scholar
53. : Analysis of blood tests in the emergency department of a tertiary care hospital. Postgrad Med J 1999; 75:662–666Crossref, Medline, Google Scholar
54. : Importance of routine laboratory investigations before elective surgery. Discoveries 2020; 8:e114Crossref, Medline, Google Scholar
55. : “Medically cleared”: how well are patients with psychiatric presentations examined by emergency physicians? J Emerg Med 2008; 35:369–372Crossref, Medline, Google Scholar
56. : Medical clearance: fact or fiction in the hospital emergency room. Psychosomatics 1990; 31:400–404Crossref, Medline, Google Scholar
57. : The role of physical examinations in psychiatry as illustrated in a case of neuroleptic malignant syndrome versus viral encephalitis: a case report and literature review. Cureus 2019; 11:e4840Medline, Google Scholar
58. : Emergency medical evaluation of psychiatric patients. Ann Emerg Med 1994; 23:859–862Crossref, Medline, Google Scholar
59. : What you should know about physical evaluations in psychiatric patients. Results of a survey. Gen Hosp Psychiatry 1987; 9:275–279Crossref, Medline, Google Scholar
60. : The psychiatric physical examination—part II: findings in 75 unselected psychiatric patients. J Clin Psychiatry 1981; 42:99–102Medline, Google Scholar
61. : Physical examination performed by psychiatrists. Int J Psychiatry Clin Pract 2004; 8:57–60Crossref, Medline, Google Scholar
62. : Mortality and medical comorbidity among psychiatric patients: a review. Psychiatr Serv 1996; 47:1356–1363Link, Google Scholar
63. : Lab test findings in the elderly. EJIFCC 2017; 28:328–332Medline, Google Scholar
64. : Laboratory findings in emergently medicated psychiatry patients. Gen Hosp Psychiatry 2004; 26:411–414Crossref, Medline, Google Scholar
65. : Effect of testing and treatment on emergency department length of stay using a national database. Acad Emerg Med 2012; 19:525–534Crossref, Medline, Google Scholar
66. : Prevention. How much harm? How much benefit? 3. Physical, psychological and social harm. CMAJ 1996; 155:169–176Medline, Google Scholar
67. : Abnormal urinalysis results in asymptomatic individuals are common. Acad Emerg Med 2021; 28:107–109Crossref, Medline, Google Scholar
68. : The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One 2013; 8:e78962Crossref, Medline, Google Scholar
69. : Drug screens for psychiatric patients in the emergency department: evaluation and recommendations. Psychosomatics 2013; 54:60–66Crossref, Medline, Google Scholar
70. : Quality assessment of diagnostic before-after studies: development of methodology in the context of a systematic review. BMC Med Res Methodol 2009; 9:3Crossref, Medline, Google Scholar
71. : Contributions of the history, physical examination, and laboratory investigation in making medical diagnoses. West J Med 1992; 156:163–165Medline, Google Scholar
72. : The evaluation of diagnostic tests: evidence on technical and diagnostic accuracy, impact on patient outcome and cost-effectiveness is needed. J Clin Epidemiol 2007; 60:1116–1122Crossref, Medline, Google Scholar
73. : Using patient management as a surrogate for patient health outcomes in diagnostic test evaluation. BMC Med Res Methodol 2012; 12:12Crossref, Medline, Google Scholar
74. : Reasons for ordering laboratory tests and relationship with frequency of abnormal results. Scand J Prim Health Care 2010; 28:18–23Crossref, Medline, Google Scholar
75. : Quantifying the added value of a diagnostic test or marker. Clin Chem 2012; 58:1408–1417Crossref, Medline, Google Scholar



