Double-Blind, Sham-Controlled Randomized Trial Testing the Efficacy of fMRI Neurofeedback on Clinical and Cognitive Measures in Children With ADHD
Abstract
Objective:
Functional MRI neurofeedback (fMRI-NF) could potentially be a novel, safe nonpharmacological treatment for attention deficit hyperactivity disorder (ADHD). A proof-of-concept randomized controlled trial of fMRI-NF of the right inferior frontal cortex (rIFC), compared to an active control condition, showed promising improvement of ADHD symptoms (albeit in both groups) and in brain function. However, comparison with a placebo condition in a larger trial is required to test efficacy.
Methods:
This double-blind, sham-controlled randomized controlled trial tested the effectiveness and efficacy of fMRI-NF of the rIFC on symptoms and executive functions in 88 boys with ADHD (44 each in the active and sham arms). To investigate treatment-related changes, groups were compared at the posttreatment and 6-month follow-up assessments, controlling for baseline scores, age, and medication status. The primary outcome measure was posttreatment score on the ADHD Rating Scale (ADHD-RS).
Results:
No significant group differences were found on the ADHD-RS. Both groups showed similar decreases in other clinical and cognitive measures, except for a significantly greater decrease in irritability and improvement in motor inhibition in sham relative to active fMRI-NF at the posttreatment assessment, covarying for baseline. There were no significant side effects or adverse events. The active relative to the sham fMRI-NF group showed enhanced activation in rIFC and other frontal and temporo-occipital-cerebellar self-regulation areas. However, there was no progressive rIFC upregulation, correlation with ADHD-RS scores, or transfer of learning.
Conclusions:
Contrary to the hypothesis, the study findings do not suggest that fMRI-NF of the rIFC is effective in improving clinical symptoms or cognition in boys with ADHD.
Attention deficit hyperactivity disorder (ADHD) is defined as persistent, age-inappropriate, and impairing symptoms of inattention and/or hyperactivity-impulsiveness (1), and is highly prevalent (5%–7%) (2). Meta-analytic evidence shows underactivation in fronto-striato-thalamic and fronto-parieto-cerebellar networks (3–5), particularly in the right inferior frontal cortex (rIFC), which mediates cognitive control and attention functions (4–6). Psychostimulant medication, the first-line treatment for ADHD (7, 8), most consistently increases/normalizes IFC activation (9) but is not indicated for all patients and has side effects (10) and poor adherence in adolescents (8, 11). Furthermore, evidence for long-term efficacy is limited (12), possibly due to brain adaptation (13).
Functional MRI neurofeedback (fMRI-NF), which enables self-regulation of brain activation in specific regions or networks by providing feedback of brain activity in real time (14, 15), could be a novel alternative to pharmacological treatment. fMRI-NF can target areas associated with ADHD, such as the opercular rIFC or basal ganglia, that are not accessible with electroencephalography neurofeedback (EEG-NF). Moreover, EEG-NF has shown small effect sizes in improving ADHD symptoms in the latest meta-analyses, and self-regulation learning is faster with fMRI-NF (6, 16).
In the first proof-of-concept single-blind randomized controlled trial of fMRI-NF in ADHD (17), boys with ADHD successfully learned progressive increase of activation in the rIFC (active group; N=18) or the left parahippocampal gyrus (active control group; N=13) after 11 runs in four 1-hour fMRI-NF sessions, which was associated with improved ADHD symptoms in both groups relative to baseline, with no side effects. At follow-up, improvement was more pronounced in the rIFC group (Cohen’s d ∼1) but no longer significant in the left parahippocampal gyrus group, suggesting potential delayed consolidation or plasticity effects. Cognitively, only the rIFC group showed improved sustained attention, which fell short of significance (Cohen’s d=2) and successfully upregulated rIFC during a “transfer” run (regulation without feedback [14]), which correlated with reduced ADHD symptoms (17). In an fMRI stop-signal task, the rIFC group compared to the left parahippocampal gyrus group showed significantly increased fronto-striatal activation during inhibition and error monitoring after treatment compared to before treatment (17, 18), and functional connectivity increases in IFC-cingulo-striatal networks, but decreases between rIFC and default-mode network areas (19). However, these promising findings were limited by small sample sizes, a single-blind design, and no placebo (sham) control condition.
To address these limitations, in the largest-to-date double-blind, sham-controlled randomized controlled trial in boys with ADHD, we tested the effectiveness and efficacy of 15 runs of active versus sham rIFC fMRI-NF over four 1-hour sessions using a range of clinical, cognitive, and fMRI measures. Based on our previous findings, we hypothesized that the active compared to the sham fMRI-NF group would show significant improvements in ADHD symptoms at the posttreatment assessment, covarying for baseline, improvements in clinical and cognitive measures at the posttreatment assessment, and sustained clinical and/or cognitive improvements at 6-month follow-up, with no side effects or adverse effects, and progressively increased rIFC activation across sessions/runs and a transfer effect, in correlation with reduced ADHD symptoms.
Methods
Trial Design
In this preregistered (ISRCTN14491589) double-blind, sham-controlled, parallel randomized controlled trial, participants were block-randomized in a 1:1 ratio to an active or sham intervention group and varying block sizes, stratified by medication status (nonmedicated or on stable ADHD medication) and by age group (under or over 14 years 6 months). Randomization was conducted independently by the King’s Clinical Trial Unit.
Families and researchers involved in data collection were blind to group allocations. Once a participant was allocated to a treatment arm, one researcher was unblinded to administer the treatment to participants via a shielded computer terminal. This unblinded researcher had no direct interaction with the participants and families and was prohibited from sharing the information with other team members. Blinding integrity was examined from the blind participants’, caregivers’, and researchers’ guesses of group allocation at the posttreatment assessment.
The trial was approved by the U.K. National Health Service Health Research Authority, London Bromley Research Ethics Committee (Ref. No. 17/LO/1368), was conducted in accordance with the 1975 revision of the Declaration of Helsinki, and is reported following the CONSORT guidelines.
Participants
Participants were 88 boys (10–18 years old) who met DSM-5 diagnostic criteria for ADHD, confirmed with the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) (20), and had a t score ≥60 on the Conners Rating Scales, 3rd ed., Parent Report (Conners 3-P) (21) DSM-5 inattention and/or hyperactivity-impulsivity domains. Participants were medication naive or on stable ADHD medication from at least 2 weeks before baseline until posttreatment assessment. Participants using stimulant medications were requested to abstain from taking the medication 24 hours before each assessment but could remain on medication throughout the study if preferred. Exclusion criteria were IQ <80 (22); co-occurring psychiatric disorders, except oppositional defiant disorder and conduct disorder; neurological conditions; and contraindication to MRI. Parents and participants gave informed consent or assent and received £180 plus travel cost reimbursement.
Sample Size Calculation
A priori power analysis (23) assuming a Cohen’s d value of 0.60 based on the change of ADHD-RS scores from baseline to posttreatment assessment during rIFC fMRI-NF in our proof-of-concept study (17) suggested an N of 45 per group. Five participants were added per group to allow for 10% attrition (17, 24, 25) (see Supplementary Methods in the online supplement).
Procedure
Participants were invited for seven visits (Figure 1), including eligibility screening and baseline assessment (visit 1), fMRI-NF interventions (visits 2–5), and posttreatment and 6-month follow-up assessments (visits 6 and 7).
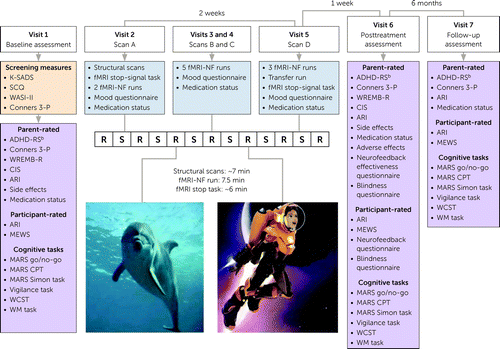
FIGURE 1. Schematic representation of a randomized controlled trial of fMRI neurofeedback in ADHDa
aADHD-RS=ADHD Rating Scale; ARI=Affective Reactivity Index; CIS=Columbia Impairment Scale; Conners 3-P=Conners 3rd Edition–Parent Report; CPT=continuous performance task; fMRI-NF=functional MRI neurofeedback; K-SADS=Schedule for Affective Disorders and Schizophrenia for School-Age Children; MARS=Maudsley Attention and Response Suppression task battery; MEWS=Mind Excessively Wandering Scale; SCQ=Social Communication Questionnaire; WASI-II=Wechsler Abbreviated Scale of Intelligence, 2nd ed.; WCST=Wisconsin card sorting task; WM=working memory; WREMB-R=Weekly Rating of Evening and Morning Behavior–Revised. Each fMRI-NF run contains seven rest blocks (R, 30 seconds) presented alternatingly with six self-regulation blocks (S, 40 seconds).
bADHD-RS total score, measured at the baseline and posttreatment assessments, is the primary outcome for the study.
fMRI-NF
Intervention.
Treatment comprised 15 active or sham fMRI-NF runs over four 1-hour scan sessions, to replicate the successful proof-of-concept study design and maximize run numbers across 4-hour MRI scans. Each run had seven 30-second “rest” and six 40-second “self-regulation” blocks. During the self-regulation blocks, neurofeedback was given to participants via a video of a rocketeer flying up from ground level into space (https://osf.io/fz2y7/), projected on a screen. The speed of the rocketeer was determined by the participant’s brain activity. Increased or decreased rIFC activation led to upward or downward movement of the rocketeer. Performance scores were based on the percentage of the maximum video length displayed (0–10 points for 0%–100%) (Figure 1) and were shown at the end of each run. No specific instructions were given, but participants were told that concentrating might help to self-upregulate brain activation. After the last fMRI-NF run of the last session, a transfer run was completed, which was identical to the previous runs except without feedback (see Supplementary Methods in the online supplement). Participants also completed an fMRI stop-signal task before and after neurofeedback training, which will be presented elsewhere.
Sham condition.
The sham intervention group underwent identical procedures but received sham neurofeedback, in which the rocketeer video was simulated using data from the last active participant who completed a minimum of eight fMRI-NF runs (see Supplementary Methods in the online supplement).
Acquisition and real-time processing of fMRI-NF data.
Imaging data were acquired at the Centre for Neuroimaging Sciences, King’s College London, on a GE Discovery MR750 3-T scanner (GE Medical Systems, Chicago) with a 12-channel head coil receiver. fMRI-NF scans were T2*-weighted echo planar imaging sequence, interleaved from top to bottom (see Supplementary Methods in the online supplement).
Game control was enabled by real-time transfer and analyses of fMRI data, facilitated by a custom fMRI interface and the AFNI software suite (26), which preprocessed and corrected head motion in real time. Data were acquired from a region of interest in the rIFC opercular and triangular parts, coregistered to a structural localizer, the AFNI CA_N27_ML/TT_N template (14,138 voxels in Talairach space). The fMRI-NF signal was the mean signal of the region of interest. A detailed description of the fMRI-NF signal, along with its formula, is presented in the Supplementary Methods section in the online supplement.
Outcomes
Figure 1 lists the study outcome measures and visits. The primary outcome was score on the parent-rated ADHD Rating Scale (ADHD-RS) (27) at the posttreatment assessment. Secondary outcomes included ADHD-RS score at 6-month follow-up and parent- or participant-rated clinical outcomes, including the Conners 3-P ADHD index (21), the parent- and participant-rated Affective Reactivity Index (28), and the participant-rated Mind Excessively Wandering Scale (29) at the baseline, posttreatment, and follow-up assessments and the parent-rated Weekly Rating of Evening and Morning Behavior–Revised (30) and Columbia Impairment Scale (31) at the baseline and posttreatment assessments. Secondary cognitive outcomes at all three assessments included measures from the adult Maudsley Attention and Response Suppression (MARS) (32) task battery—motor inhibition (go/no-go task; probability of inhibition), interference inhibition (Simon task; Simon reaction time effect), and sustained attention (continuous performance task; omission and commission errors). Also included were measures of vigilance (the Mackworth clock vigilance task [33]; omission and commission errors); cognitive flexibility (the computerized Wisconsin Card Sorting Task; perseverative and nonperseverative errors) (34); working memory (the list sorting task from the NIH Toolbox [35]; total score); and composite response prematurity, processing speed, and intrasubject response variability from the MARS go/no-go, Simon, and continuous performance tasks combined (see Supplementary Methods in the online supplement). Mood questionnaires (36) assessing the participants’ mood before and during MRI scans, motivational state, performance, and liking of scans were administered after each scan. Feedback from participants and parents about their experience and the effectiveness of fMRI-NF (17), respectively, was taken at the posttreatment assessment (see Supplementary Methods in the online supplement). Parent-rated safety measures included a questionnaire on side effects at the baseline and posttreatment assessments (37) and a questionnaire on adverse events (adapted from reference 38) at the posttreatment assessment (see Supplementary Methods in the online supplement).
Statistical Analysis
As prespecified (39), primary intention-to-treat analyses of treatment effectiveness were conducted with the randomized participants who underwent fMRI scanning. We conducted a series of 2×2 repeated-measures analyses of covariance (ANCOVAs) with outcomes as dependent variables, covarying for their baseline values, age, and medication status, with group (active/sham), time (posttreatment/follow-up), and group by time as fixed effects. Equivalent univariate ANCOVAs were used for outcomes measured at the baseline and posttreatment assessments (the Weekly Rating of Evening and Morning Behavior–Revised, the Columbia Impairment Scale, and side effects) or at the posttreatment assessment only (adverse effects). Significant group-by-time interactions were explored using simple-effect analyses. The two-tailed repeated-measures ANCOVAs were run using the Mixed command with restricted maximum likelihood estimator and exchangeable covariance structure using SPSS, version 26 (IBM, Armonk, N.Y.). Data were assumed to be missing at random. False discovery rate (FDR) corrections for multiple comparisons were applied per fixed effect for secondary clinical and cognitive domains separately; simple-effect analyses were uncorrected for multiple testing. Secondary ANOVAs assessed changes of scores within groups across time points, uncorrected for multiple testing. Treatment efficacy, estimated using complier average causal effect (40), and sensitivity analyses exploring impacts of medication changes and of nationally implemented COVID-19 lockdown on follow-up findings were conducted in STATA, version 16 (StataCorp, College Station, Tex.) (see Supplementary Methods in the online supplement). Fisher’s exact test was used to test the association between treatment group allocation and guesses by researchers, participants, and their parents to test blinding effectiveness.
For analysis of fMRI images, structural MRI images were reoriented and skull-stripped. All functional images were corrected for head motion and were coregistered to the structural image and a standard template. Data were high-pass filtered (100 s) and smoothed with a Gaussian kernel of 5 mm full width at half maximum.
Individual BOLD activations representing the rest and self-regulation blocks were contrasted, and the resulting images were entered into group analyses (see Supplementary Methods in the online supplement).
Group-by-session ANCOVAs covaried for age, medication status, and movement. Analyses were at the whole-brain level and with small-volume correction for region of interest (with pre-threshold masking), within the same regions used for neurofeedback (the opercular and triangular rIFC), exploring group differences in final versus baseline run activation, in linear regression across all runs, and in transfer activation.
All fMRI analyses used a cluster threshold of alpha <0.05 and a family-wise error rate correction.
Associations between brain activation and clinical symptoms were analyzed within the active fMRI-NF group. For each participant, the average BOLD activation extracted from the significant group difference cluster (i.e., rIFC) was averaged across the last two runs and compared with the baseline value to compute an fMRI-NF learning score (15, 41). Pearson’s correlations were used to test for associations between fMRI-NF learning scores and ADHD-RS total score changes from baseline to posttreatment assessment. Similar exploratory correlations were run for secondary measures (the Affective Reactivity Index, go/no-go inhibition) within the sham fMRI-NF group (see Supplementary Results in the online supplement).
Results
Between January 2, 2018, and March 11, 2020, a total of 122 families completed baseline assessments, and 94 participants (77.0%) were randomized into active or sham intervention groups. Six participants (6.4%) declined scanning and dropped out of the study, leaving 44 participants per group (see Figure S1 in the online supplement). The trial was discontinued prematurely because of the COVID-19 lockdown. The two groups did not significantly differ at baseline (see Table S1 in the online supplement), except that the active intervention group had more combined-type ADHD presentations than the sham intervention group (χ2=6.47, df=1, N=88, p=0.011).
Clinical and Cognitive Outcomes
Primary outcome.
The repeated-measures ANCOVAs showed no significant group-by-time interaction, nor a group effect on ADHD-RS total scores, as primary posttreatment or secondary 6-month follow-up outcomes. Time effect showed significantly increasing ADHD-RS scores from posttreatment assessment to follow-up assessment (F=8.44, df=1, 82.7, p=0.005) (Table 1, Figure 2). Within-group ANOVAs showed significantly reduced scores for both groups, relative to baseline, at the posttreatment (p values <0.001) and follow-up (p values <0.009) assessments (see Table S2 in the online supplement).
| EMM, Posttreatment | EMM, Follow-Up | Fixed Effects | Simple Effects | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham | Active | Sham | Active | Group | Time | Group×Time | Posttreatment | Follow-Up | ||||||||||
| Measure | Mean | SE | Mean | SE | Mean | SE | Mean | SE | F | p | F | p | F | p | F | p | F | p |
| Primary clinical outcome | ||||||||||||||||||
| ADHD-RS total (P) | 28.7 | 1.48 | 30.6 | 1.48 | 32.7 | 1.53 | 33.2 | 1.48 | 0.48 | 0.49 | 8.44 | 0.005 | 0.37 | 0.55 | 0.84 | 0.36 | 0.06 | 0.80 |
| Secondary clinical outcomes | ||||||||||||||||||
| Conners 3-P ADHD index (P) | 11.7 | 0.76 | 11.8 | 0.76 | 12.0 | 0.78 | 12.1 | 0.77 | 0.01 | 0.93 | 0.20 | 0.66 | 0.001 | 0.98 | 0.004 | 0.95 | 0.008 | 0.93 |
| ARI (P) | 0.63 | 0.07 | 0.83 | 0.07 | 0.91 | 0.07 | 0.81 | 0.07 | 0.34 | 0.56 | 6.50 | 0.013 | 7.73 | 0.007b | 4.04 | 0.046 | 1.03 | 0.31 |
| ARI (C) | 0.63 | 0.06 | 0.63 | 0.06 | 0.63 | 0.06 | 0.63 | 0.06 | 0.03 | 0.86 | 0.28 | 0.60 | 0.031 | 0.86 | 0.063 | 0.80 | <0.001 | 0.99 |
| MEWS (C) | 16.4 | 1.01 | 15.2 | 1.04 | 15.2 | 1.04 | 13.5 | 1.02 | 1.78 | 0.19 | 2.32 | 0.13 | 0.06 | 0.82 | 0.75 | 0.39 | 1.32 | 0.25 |
| CIS (P) | 18.4 | 1.19 | 21.0 | 1.19 | — | — | — | — | 2.42 | 0.12 | — | — | — | — | — | — | — | — |
| WREMB-R (P) | 17.2 | 0.95 | 19.8 | 0.95 | — | — | — | — | 3.66 | 0.06 | — | — | — | — | — | — | — | — |
| Side effects (P) | 12.5 | 1.08 | 12.5 | 1.08 | — | — | — | — | 0.22 | 0.64 | — | — | — | — | — | — | — | — |
| Adverse effects (P) | 16.7 | 0.56 | 15.2 | 0.56 | — | — | — | — | 3.75 | 0.06 | — | — | — | — | — | — | — | — |
| Secondary cognitive outcomes | ||||||||||||||||||
| Go/no-go probability of inhibition | 55.0 | 2.15 | 48.5 | 2.01 | 50.6 | 2.1 | 54.5 | 2.03 | 0.12 | 0.73 | 0.58 | 0.45 | 8.78 | 0.004c | 4.45 | 0.037 | 2.12 | 0.15 |
| Simon incompatibility RT effect | 61.1 | 3.91 | 62.1 | 3.66 | 52.0 | 3.82 | 59.6 | 3.7 | 1.79 | 0.19 | 1.89 | 0.17 | 1.12 | 0.29 | 0.24 | 0.63 | 2.76 | 0.10 |
| CPT omission errors | 9.46 | 1.24 | 10.6 | 1.16 | 10.6 | 1.16 | 12.1 | 1.17 | 0.95 | 0.33 | 1.76 | 0.19 | 0.03 | 0.87 | 0.82 | 0.37 | 0.43 | 0.51 |
| CPT commission errors | 1.37 | 0.30 | 1.53 | 0.28 | 1.42 | 0.29 | 1.76 | 0.28 | 0.09 | 0.76 | 1.96 | 0.17 | 0.67 | 0.42 | 0.03 | 0.86 | 0.46 | 0.50 |
| MCT omission errors | 29.5 | 2.19 | 32.1 | 2.07 | 32.1 | 2.15 | 31.2 | 2.09 | 0.11 | 0.74 | 0.28 | 0.60 | 1.29 | 0.26 | 0.10 | 0.76 | 0.72 | 0.40 |
| MCT commission errors | 5.99 | 0.70 | 5.13 | 0.66 | 4.63 | 0.69 | 4.36 | 0.67 | 0.34 | 0.56 | 0.10 | 0.76 | 0.001 | 0.97 | 0.28 | 0.60 | 0.22 | 0.64 |
| WCST perseverative errors | 6.22 | 0.54 | 7.7 | 0.51 | 6.07 | 0.53 | 7.23 | 0.52 | 0.27 | 0.61 | 8.93 | 0.004d | 0.17 | 0.68 | 0.03 | 0.86 | 0.42 | 0.52 |
| WCST nonperseverative errors | 7.14 | 0.81 | 9.64 | 0.78 | 7.72 | 0.80 | 8.63 | 0.78 | 0.02 | 0.89 | 8.81 | 0.004e | 0.34 | 0.56 | 0.05 | 0.83 | 0.18 | 0.67 |
| Working memory total score | 28.2 | 1.94 | 26.5 | 1.83 | 30.5 | 1.91 | 27.5 | 1.85 | 0.56 | 0.46 | 2.47 | 0.12 | 0.18 | 0.67 | 0.56 | 0.46 | 2.47 | 0.12 |
| Composite response prematurity | 2.63 | 0.35 | 3.5 | 0.33 | 2.97 | 0.34 | 2.7 | 0.33 | 1.34 | 0.25 | 2.69 | 0.11 | 5.58 | 0.02 | 5.65 | 0.019 | 0.18 | 0.68 |
| Composite processing speed | 375.0 | 4.88 | 382.6 | 4.63 | 362.5 | 4.81 | 381.5 | 4.68 | 1.16 | 0.29 | 16.9 | <0.001f | 4.25 | 0.04 | 0.002 | 0.97 | 3.60 | 0.06 |
| Composite response variability | 0.28 | 0.01 | 0.29 | 0.01 | 0.28 | 0.01 | 0.28 | 0.01 | 0.49 | 0.48 | 1.35 | 0.25 | 3.82 | 0.05 | 3.00 | 0.09 | 0.27 | 0.60 |
TABLE 1. Clinical and neurocognitive outcome estimated marginal means at posttreatment and follow-up assessments, with fixed-effect and simple-effect statistics, in a randomized controlled trial of fMRI neurofeedback in ADHDa
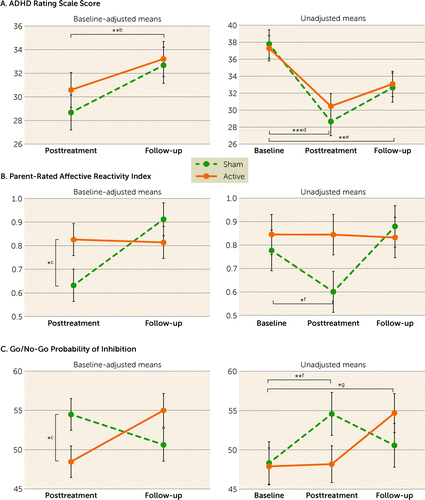
FIGURE 2. Primary outcomes and outcomes with significant effects of group at posttreatment assessment in a randomized controlled trial of fMRI neurofeedback in ADHDa
aIn panels A–C, the graphs on the left show posttreatment and follow-up data for scores for the ADHD Rating Scale, the Affective Reactivity Index, and the go/no-go probability of inhibition, covarying for their baseline values, medication status, and age, and the graphs on the right show the unadjusted mean values of these outcome measures at the baseline, posttreatment, and 6-month follow-up assessments. Error bars indicate 95% confidence intervals. Indicated on the panels are the significant effects of time (footnote b); the simple effects of group at posttreatment assessment (footnote c); the effects of time between baseline and posttreatment assessments (footnote d) and between baseline and follow-up assessments (footnote e) within each treatment group; the simple effect of time between baseline and posttreatment assessments within the sham group (footnote f); and the simple effect of time between baseline and follow-up assessments within the active group (footnote g).
*p<0.05. **p<0.01. ***p<0.001.
Secondary outcomes.
There were no significant effects on any clinical measures, but there was a significant group-by-time interaction in parent-rated Affective Reactivity Index (F=7.73, df=1, 83.1, p=0.028), explained by simple effects of lower Affective Reactivity Index in sham than active fMRI-NF at the posttreatment assessment (F=4.04, df=1, 136.5, p=0.046) but not the follow-up assessment. Cognitively, there were no significant effects, but a group-by-time interaction was observed in go/no-go probability of inhibition (F=8.78, df=1, 75.8, p=0.048); simple-effect analyses indicated lower go/no-go probability of inhibition in the active relative to the sham intervention group at the posttreatment assessment (F=4.45, df=1, 142.7, p=0.037) but not the follow-up assessment.
Within-group ANOVAs showed reductions in Conners 3-P ADHD index from baseline to posttreatment assessment and to follow-up assessment for both groups (p values <0.033) and in Affective Reactivity Index at posttreatment relative to baseline assessment in the sham intervention group only (p=0.018). Reduction of go/no-go inhibition was found from baseline to posttreatment assessment in the sham intervention group only, and from baseline to follow-up in the active intervention group only (p values <0.01). Reductions in continuous performance task omission/commission errors, Mackworth clock vigilance task omission errors, and Wisconsin card sorting task perseverative errors were found for both groups from baseline to posttreatment assessment and to follow-up assessment (p values, 0.001–0.027) (see Table S2 in the online supplement).
Secondary complier average causal effect analyses showed similar findings for ADHD-RS score and go/no-go probability of inhibition (see Table S3 in the online supplement). Sensitivity analyses revealed no significant effects of changing medication from posttreatment to follow-up assessment or of COVID-19 lockdown (see Supplementary Results in the online supplement).
Neuroimaging Outcomes
All 88 participants were included in the final fMRI analyses, but 17% of runs in each group were excluded due to excessive head motion (relative mean displacement >0.9 mm [42]). The first fMRI-NF run for each participant was excluded, a common practice since it is often used for familiarizing participants with neurofeedback training (43), and since there were unusually high widespread brain activation patterns in this run (see Supplementary Methods in the online supplement).
Group-by-Session ANCOVA.
fMRI-NF sessions
Whole-brain analyses, but not region-of-interest analyses, showed significant group-by-session interaction. The sham relative to the active intervention group showed higher BOLD activation in session 3 relative to session 4 in the left thalamus (p=0.009; Montreal Neurological Institute peak coordinates [x, y, z], −20, −26, 6; cluster size [k]=317 voxels) (Figure 3).
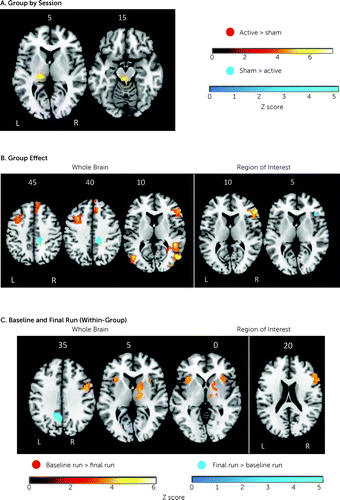
FIGURE 3. Brain activation findings in a randomized controlled trial of fMRI neurofeedback in ADHDa
aAxial slices showing brain activation at false positive error–corrected cluster-level p<0.05. Panels A and B show the group-by-session interaction effect and the main effect of group, at the whole-brain and region-of-interest levels, from the analyses of variance of group-by-session and group effects. Clusters in red correspond to active versus sham contrasts, and clusters in blue correspond to sham versus active contrasts. In panel C, axial slices show within-group analyses of brain activation from the baseline and final fMRI-NF runs, within the active group and within the sham group. Clusters in red correspond to baseline run (i.e., run 2) versus final run (i.e., run 11) contrasts, and clusters in blue correspond to final versus baseline run. Montreal Neurological Institute z-coordinates are shown with z-statistic images overlaid. fMRI-NF=functional MRI neurofeedback.
Group effect was significant across sessions. The active relative to the sham intervention group had greater whole-brain activation in the rIFC, right dorsomedial and left middle frontal gyri, right middle and superior temporal gyri, and left and right middle occipital gyrus, cerebellum, and occipital lobes (Table 2, Figure 3), and higher region-of-interest activation in two rIFC clusters (cluster 1: p<0.001; peak coordinates, 56, 40, 14, k=337, Brodmann area [BA] 45; cluster 2: p=0.032; peak coordinates, 42, 10, 36, k=29, BA44). Conversely, the sham relative to the active intervention group showed higher whole-brain activation in the right middle/posterior cingulate/precuneus (Table 2, Figure 3) and higher region-of-interest activation in the ventral rIFC (p=0.040; peak coordinates, 40, 28, 4, k=22, BA47). Within-session group differences are presented in the Supplementary Results section in the online supplement.
| Peak MNI Coordinates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cluster | Region | Side | BA | Size (voxels) | z | p | x | y | z |
| Group effect | |||||||||
| Active > sham | |||||||||
| 1 | Middle/superior temporal gyrus | R | 21/37/22 | 891 | 5.93 | 0.00001 | 66 | −54 | 4 |
| 2 | Middle/inferior occipital gyrus/middle temporal gyrus | R | 19/37 | 572 | 6.81 | 0.0004 | 42 | −88 | 4 |
| 3 | Middle frontal gyrus/precentral gyrus | L | 46/44/9/6 | 413 | 5.25 | 0.003 | −32 | 16 | 38 |
| 4 | Inferior frontal gyrus | R | 45/44 | 375 | 5.3 | 0.004 | 56 | 40 | 14 |
| 5 | Cerebellum/lingual gyrus/inferior occipital gyrus/fusiform gyrus | L | 19/18 | 363 | 5.82 | 0.005 | −42 | −78 | −20 |
| 6 | Middle occipital gyrus | L | 37/39 | 347 | 5.7 | 0.006 | −44 | −70 | 10 |
| 7 | Dorsomedial frontal gyrus | R | 8/9/32 | 302 | 5.08 | 0.011 | 6 | 36 | 52 |
| 8 | Cerebellum/lingual gyrus | R | 30 | 280 | 4.96 | 0.016 | 12 | −38 | −10 |
| 9 | Middle temporal gyrus | R | 21 | 276 | 5.17 | 0.017 | 70 | −34 | −2 |
| Sham > active | |||||||||
| 1 | Middle/posterior cingulate cortex/precuneus | R | 23 | 206 | 5.18 | 0.034 | 14 | −32 | 40 |
| Baseline and final run | |||||||||
| Active group | |||||||||
| Baseline > final run | |||||||||
| 1 | Thalamus/putamen | R | — | 430 | 4.48 | 0.002 | 14 | 0 | 6 |
| 2 | Precentral gyrus | R | 6/44 | 288 | 4.25 | 0.012 | 48 | 4 | 32 |
| 3 | Insula | L | 48/47 | 260 | 4.38 | 0.019 | −36 | 16 | −4 |
| 4 | Insula | R | 47 | 260 | 4.08 | 0.019 | 34 | 20 | −12 |
| Final > baseline run | |||||||||
| 1 | Precuneus | L | 7/23 | 729 | 4.74 | 0.00001 | −6 | −54 | 36 |
| Sham group | |||||||||
| Baseline > final run | |||||||||
| 1 | Inferior frontal gyrus | R | 44/45 | 426 | 4.24 | 0.002 | 58 | 18 | 18 |
TABLE 2. Whole-brain analysis results, showing the group effect from the group-by-session ANCOVA, and comparing brain activation differences within groups between baseline and final fMRI-NF runsa
Baseline and final fMRI-NF run
Between-group differences were nonsignificant for the final versus baseline run contrast, or vice versa, at the whole-brain level and at the region-of-interest level.
The active group had increased activation in the final run relative to baseline in the precuneus (Table 2, Figure 3) and decreased whole-brain activation in the right thalamus/putamen, premotor cortex, left and right insula, and the rIFC region of interest (Table 2, Figure 3). The sham intervention group had significantly reduced activation in the rIFC in the final run relative to baseline at both the whole-brain and region-of-interest levels (Table 2, Figure 3). The reverse contrast revealed no significant findings.
Transfer run
Twenty-nine participants (10 in the active and 19 in sham intervention group) were excluded from analysis because of motion. Only the sham intervention group showed a significant activation increase, in the left supramarginal gyrus (p=0.046; peak coordinates, −58, −26, 24, k=227). Neither between-group nor within-group differences were significant at the region-of-interest level.
Linear regression
No significant between- or within-group effects were found in linear increase of activation across the 15 runs at the whole-brain or region-of-interest levels.
Correlation between outcomes and rIFC activation.
No significant correlation was found between rIFC activation changes (final vs. baseline run) and ADHD-RS total score changes (posttreatment vs. baseline) (Pearson’s r=0.001, p=0.99 [two-tailed], N=41) (see the online supplement for other correlations).
Influences of covariates.
Among the covariates (medication, age, and relative mean displacement of head motion), motion was most strongly correlated with whole-brain activation, and relative mean displacement was most correlated with the rIFC region-of-interest activation, which differed between groups (r=0.59, p<0.0001; triangular part: r=0.61, p<0.0001; opercular part: r=0.64, p<0.0001).
Side Effects and Adverse Effects
Side effects did not differ between groups at the posttreatment assessment (F=0.22, df=1, 81, p=0.64). The adverse events anxiety/worry (14%) and distractibility (20.9%) were higher in the sham relative to the active intervention group, but not significantly (2.3% and 4.7%, respectively; F=3.75, df=1, 82, p=0.06). In the sham group exploratory analyses, rIFC activation was correlated with neither anxiety/worry (Spearman’s ρ=−1.7, p=0.28, two-tailed) nor distractibility (ρ=−1.4, p=0.40, two-tailed).
Blinding Integrity
Actual group allocation corresponded with the researchers’ guesses (p=0.018, Fisher’s exact test; two-tailed) but not with the participants’ (p=0.55) or parents’ guesses (p=0.51, Fisher’s exact test; two-tailed).
Discussion
In the largest double-blind, sham-controlled randomized controlled trial to date of fMRI-NF of the rIFC compared with sham fMRI-NF in boys with ADHD, we found, contrary to our hypothesis, no improvement in ADHD-RS total scores or other clinical and cognitive measures. Instead, relative to the active intervention group, the sham intervention group showed reduced irritable mood and improved motor inhibition at the posttreatment assessment only, the latter of which could be a training effect that was unobserved in the active group. No significant side effects or adverse events were found. At the fMRI level, the active relative to sham fMRI-NF group showed overall increased activation in the rIFC (alongside other dorsomedial frontal and temporo-occipital-cerebellar self-regulation regions) across all sessions. However, there was neither progressively increasing upregulation across sessions or runs, nor correlations between changes in rIFC activation and ADHD-RS scores, nor transfer of learning, indicating no progressive training effects. The findings do not suggest that fMRI-NF of rIFC is an effective treatment for ADHD.
The absence of clinical or cognitive effects of active versus sham fMRI-NF of the rIFC extends the findings from our proof-of-concept trial of no superior clinical or cognitive effects of rIFC fMRI-NF compared to an active (i.e., parahippocampal) fMRI-NF control condition (19). As in the previous trial, both groups improved in clinical and most cognitive measures. In the previous trial, such improvements could be attributed to region-nonspecific brain self-regulation effects in both groups. Such self-regulation was not expected for sham treatment, but recent evidence suggests that attempts to self-regulate and concentrate on stimuli during sham neurofeedback could lead to activation in self-control regions (44). In our study, such focus and self-regulation attempts likely explain the overall increased activation in the sham group in rIFC self-control and in posterior parietal visual spatial attention areas, which may have exerted unintended clinical or cognitive effects and diminished group differences. Further, the parahippocampal control condition in the previous study (17) may have been a greater contrast, leading to more positive findings. Thus, our findings contribute to the ongoing debate on whether sham fMRI-NF is the most appropriate control for neurofeedback studies (as opposed to alternative regions, mental rehearsal, or bidirectional neurofeedback controls) (15, 45, 46). They also raise the question of whether regions not involved in self-control and feedback monitoring might be better targets for sham-controlled fMRI-NF.
Also unlike in the previous trial (17), there was neither progressive linear upregulation of rIFC activation across runs or sessions nor correlation between rIFC activation changes with ADHD symptom improvements, despite overall increases of rIFC activation across sessions in the active versus the sham condition. Therefore, the differential rIFC engagement between groups alone may have been insufficient, in the absence of progressive training effects, to produce clinical or cognitive benefits. While such findings are not encouraging, several factors could have mitigated effects. Most participants (∼65%) were current medication users, which could mask potential neurofeedback-related clinical or cognitive effects or limit potential rIFC upregulation effects, given that stimulants already increase activation in this region (4, 5, 7). Replication in a medication-naive cohort would clarify this. Further, our cohort was younger than the one in the proof-of-concept study, and the more severe ADHD symptoms typical of younger children could hamper neurofeedback learning (47).
The parallel improvement of ADHD symptoms and other clinical and cognitive measures in both groups echoes similar observations from other neurotherapies (e.g., EEG-NF, brain stimulation) (16) and could reflect nonspecific psychosocial or placebo effects of the neurotechnology-based intervention (48).
Motion had a significant effect on neurofeedback-related brain activation, raising queries on the suitability of neurofeedback for patient groups with high motion artifacts, such as patients with ADHD.
The use of a rigorous double-blind, sham-control randomized controlled trial design with a prespecified analysis plan (39) constitutes a substantial methodological advance from previous fMRI-NF ADHD trials (17, 49). However, our study had limitations. The inclusion of only boys in the study and the use of only parent reports limit the generalizability of our findings to other populations and contexts. The inclusion of mostly medicated participants could have masked fMRI-NF effects. The study could have been underpowered for detecting smaller effect sizes. Despite randomization, there were significant differences in ADHD presentation between the sham and active intervention groups, albeit on interview assessments only and not on other clinical ADHD measures (i.e., the ADHD-RS and the Conners 3-P). Finally, the significant convergence between treatment condition and guesses from researchers might indicate compromised blinding, but this is unlikely to have influenced outcomes, given that the blinding integrity was maintained among participants and parents.
In summary, this double-blind, sham-controlled randomized controlled trial of fMRI-NF in ADHD failed to provide evidence that rIFC fMRI-NF is more effective than sham fMRI-NF in improving clinical symptoms or cognition in boys with ADHD. Future studies should investigate whether fMRI-NF of alternative regions of interest or networks implicated in ADHD may be more effective in improving clinical and cognitive problems. Optimal protocols for fMRI-NF in ADHD, including choice of target region, number of runs/sessions, neurofeedback stimuli and appropriate control conditions, medication, and potential brain saturation effects, should be systematically tested, perhaps with the use of neuroadaptive Bayesian optimization methods (50). In addition, identification of ADHD subgroups or individuals through normative modeling of multivariate brain activation or functional connectivity patterns (51) could potentially provide better neurofeedback targets (52, 53).
1. : Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association, 2013 Crossref, Google Scholar
2. : Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics 2015; 135:e994–1001Crossref, Medline, Google Scholar
3. : Cognitive neuroscience of attention deficit hyperactivity disorder (ADHD) and its clinical translation. Front Hum Neurosci 2018; 12:100Crossref, Medline, Google Scholar
4. : Comparative meta-analyses of brain structural and functional abnormalities during cognitive control in attention-deficit/hyperactivity disorder and autism spectrum disorder. Psychol Med 2020; 50:894–919Crossref, Medline, Google Scholar
5. : Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: a comparative meta-analysis. JAMA Psychiatry 2016; 73:815–825Crossref, Medline, Google Scholar
6. : What do meta-analyses have to say about the efficacy of neurofeedback applied to children with ADHD? Review of previous meta-analyses and a new meta-analysis. J Atten Disord 2021; 25:473–485Crossref, Medline, Google Scholar
7. : Attention deficit hyperactivity disorder: diagnosis and management [NG87]. March 14, 2018. https://www.nice.org.uk/guidance/ng87/resources/attention-deficit-hyperactivity-disorder-diagnosis-and-management-pdf-1837699732933 Google Scholar
8. : Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry 2018; 5:727–738Crossref, Medline, Google Scholar
9. : Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol Psychiatry 2014; 76:616–628Crossref, Medline, Google Scholar
10. : Using stimulants for attention-deficit/hyperactivity disorder: clinical approaches and challenges. Prim Care Companion CNS Disord 2013; 15:PCC.12f01472Medline, Google Scholar
11. : Efficacy, safety, and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl) 2016; 233:187–197Crossref, Medline, Google Scholar
12. : The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry 2009; 48:484–500Crossref, Medline, Google Scholar
13. : Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. Am J Psychiatry 2012; 169:264–272Link, Google Scholar
14. : Real-time fMRI neurofeedback: progress and challenges. Neuroimage 2013; 76:386–399Crossref, Medline, Google Scholar
15. : Neurofeedback with fMRI: a critical systematic review. Neuroimage 2018; 172:786–807Crossref, Medline, Google Scholar
16. : Neurotherapeutics for attention deficit/hyperactivity disorder (ADHD): a review. Cells 2021; 10:2156Crossref, Medline, Google Scholar
17. : Real-time fMRI neurofeedback in adolescents with attention deficit hyperactivity disorder. Hum Brain Mapp 2017; 38:3190–3209Crossref, Medline, Google Scholar
18. : Increased left inferior fronto-striatal activation during error monitoring after fMRI neurofeedback of right inferior frontal cortex in adolescents with attention deficit hyperactivity disorder. Neuroimage Clin 2020; 27:102311Crossref, Medline, Google Scholar
19. : Functional connectivity changes associated with fMRI neurofeedback of right inferior frontal cortex in adolescents with ADHD. Neuroimage 2019; 188:43–58Crossref, Medline, Google Scholar
20. : The K-SADS-PL DSM-5. Baltimore, Md, Kennedy Krieger Institute, 2016 Google Scholar
21. : Conners 3rd Edition Manual. Toronto, Multi-Health Systems, 2008 Google Scholar
22. : Wechsler Abbreviated Scale of Intelligence, 2nd Edition (WASI-II). London, Pearson, 2011 Google Scholar
23. : G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39:175–191Crossref, Medline, Google Scholar
24. : Shared and drug-specific effects of atomoxetine and methylphenidate on inhibitory brain dysfunction in medication-naive ADHD boys. Cereb Cortex 2014; 24:174–185Crossref, Medline, Google Scholar
25. : Methylphenidate normalizes fronto-striatal underactivation during interference inhibition in medication-naive boys with attention-deficit hyperactivity disorder. Neuropsychopharmacology 2011; 36:1575–1586Crossref, Medline, Google Scholar
26. : AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173Crossref, Medline, Google Scholar
27. : ADHD Rating Scale–IV: Checklists, Norms, and Clinical Interpretation. New York, Guilford, 1998 Google Scholar
28. : The Affective Reactivity Index: a concise irritability scale for clinical and research settings. J Child Psychol Psychiatry 2012; 53:1109–1117Crossref, Medline, Google Scholar
29. : Validation of the Mind Excessively Wandering Scale and the relationship of mind wandering to impairment in adult ADHD. J Atten Disord 2019; 23:624–634Crossref, Medline, Google Scholar
30. : Morning and evening behavior in children and adolescents treated with atomoxetine once daily for attention-deficit/hyperactivity disorder (ADHD): findings from two 24-week open-label studies. Child Adolesc Psychiatry Ment Health 2009; 3:5Crossref, Medline, Google Scholar
31. : The Columbia Impairment Scale (CIS): pilot findings on a measure of global impairment for children and adolescents. Int J Methods Psychiatr Res 1993; 3:167–176 Google Scholar
32. : Impaired response inhibition in obsessive compulsive disorder. Eur Psychiatry 2007; 22:404–410Crossref, Medline, Google Scholar
33. : The Mackworth Clock Test: a computerized version. J Psychol 2000; 134:153–161Crossref, Medline, Google Scholar
34. : Wisconsin Card Sorting Test–64: Computer Version 2–Research Edition (WCST-64:CV2). Lutz, Fla, Psychological Assessment Resources, 2003 Google Scholar
35. : NIH Toolbox Cognition Battery (NIHTB-CB): list sorting test to measure working memory. J Int Neuropsychol Soc 2014; 20:599–610Crossref, Medline, Google Scholar
36. : Comparing tomographic EEG neurofeedback and EMG biofeedback in children with attention-deficit/hyperactivity disorder. Biol Psychol 2014; 95:31–44Crossref, Medline, Google Scholar
37. : An auditable protocol for treating attention deficit/hyperactivity disorder. Arch Dis Child 2001; 84:404–409Crossref, Medline, Google Scholar
38. : [Attention deficit/hyperactivity disorders among three- to six-year-olds treated in medical practices: a national survey]. Z Kinder Jugendpsychiatr Psychother 2006; 34:357–365 (German)Crossref, Medline, Google Scholar
39. : ADHD fMRI Neurofeedback Imaging Study (AFNIS): a double-blind, randomised, sham-controlled trial testing the efficacy of fMRI neurofeedback (NF) on clinical, cognitive, and fMRI measures in children with ADHD: pre-registered analysis plan. 2020. https://osf.io/n8pdf/ Google Scholar
40. : Estimating psychological treatment effects from a randomised controlled trial with both non-compliance and loss to follow-up. Br J Psychiatry 2003; 183:323–331Crossref, Medline, Google Scholar
41. : Can we predict real-time fMRI neurofeedback learning success from pretraining brain activity? Hum Brain Mapp 2020; 41:3839–3854Crossref, Medline, Google Scholar
42. : Multimethod investigation of the neurobiological basis of ADHD symptomatology in children aged 9–10: baseline data from the ABCD study. Transl Psychiatry 2021; 11:64Crossref, Medline, Google Scholar
43. : Real-time fMRI neurofeedback training of the amygdala activity with simultaneous EEG in veterans with combat-related PTSD. Neuroimage Clin 2018; 19:106–121Crossref, Medline, Google Scholar
44. : Neural substrates of cognitive control under the belief of getting neurofeedback training. Front Hum Neurosci 2013; 7:914Crossref, Medline, Google Scholar
45. : The fallacy of sham-controlled neurofeedback trials: a reply to Thibault and colleagues (2018). J Atten Disord 2021; 25:448–457Crossref, Medline, Google Scholar
46. : Control freaks: towards optimal selection of control conditions for fMRI neurofeedback studies. Neuroimage 2019; 186:256–265Crossref, Medline, Google Scholar
47. : Mixed-effects modeling of neurofeedback self-regulation performance: moderators for learning in children with ADHD. Neural Plasticity 2018; 2018:2464310Crossref, Medline, Google Scholar
48. : Neurofeedback or neuroplacebo? Brain 2017; 140:862–864Crossref, Medline, Google Scholar
49. : fMRI neurofeedback training for increasing anterior cingulate cortex activation in adult attention deficit hyperactivity disorder: an exploratory randomized, single-blinded study. PLoS One 2017; 12:e0170795Crossref, Medline, Google Scholar
50. : Neuroadaptive Bayesian optimization and hypothesis testing. Trends Cogn Sci 2017; 21:155–167Crossref, Medline, Google Scholar
51. : Understanding heterogeneity in clinical cohorts using normative models: beyond case-control studies. Biol Psychiatry 2016; 80:552–561Crossref, Medline, Google Scholar
52. : Closed-loop training of attention with real-time brain imaging. Nat Neurosci 2015; 18:470–475Crossref, Medline, Google Scholar
53. : Advances in fMRI real-time neurofeedback. Trends Cogn Sci 2017; 21:997–1010Crossref, Medline, Google Scholar



