Aberrant Developmental Patterns of Gamma-Band Response and Long-Range Communication Disruption in Youths With 22q11.2 Deletion Syndrome
Abstract
Objective:
Brain oscillations play a pivotal role in synchronizing responses of local and global ensembles of neurons. Patients with schizophrenia exhibit impairments in oscillatory response, which are thought to stem from abnormal maturation during critical developmental stages. Studying individuals at genetic risk for psychosis, such as 22q11.2 deletion carriers, from childhood to adulthood may provide insights into developmental abnormalities.
Methods:
The authors acquired 106 consecutive T1-weighted MR images and 40-Hz auditory steady-state responses (ASSRs) with high-density (256 channel) EEG in a group of 58 22q11.2 deletion carriers and 48 healthy control subjects. ASSRs were analyzed with 1) time-frequency analysis using Morlet wavelet decomposition, 2) intertrial phase coherence (ITPC), and 3) theta-gamma phase-amplitude coupling estimated in the source space between brain regions activated by the ASSRs. Additionally, volumetric analyses were performed with FreeSurfer. Subanalyses were conducted in deletion carriers who endorsed psychotic symptoms and in subgroups with different age bins.
Results:
Deletion carriers had decreased theta and late-latency 40-Hz ASSRs and phase synchronization compared with control subjects. Deletion carriers with psychotic symptoms displayed a further reduction of gamma-band response, decreased ITPC, and decreased top-down modulation of gamma-band response in the auditory cortex. Reduced gamma-band response was correlated with the atrophy of auditory cortex in individuals with psychotic symptoms. In addition, a linear increase of theta and gamma power from childhood to adulthood was found in control subjects but not in deletion carriers.
Conclusions:
The results suggest that while all deletion carriers exhibit decreased gamma-band response, more severe local and long-range communication abnormalities are associated with the emergence of psychotic symptoms and gray matter loss. Additionally, the lack of age-related changes in deletion carriers indexes a potential developmental impairment in circuits underlying the maturation of neural oscillations during adolescence. The progressive disruption of gamma-band response in 22q11.2 deletion syndrome supports a developmental perspective toward understanding and treating psychotic disorders.
Synchronization between neuronal oscillations enables coordinated brain activity subserving different functions that are altered in schizophrenia, including perceptual and higher cognitive processes (1–4). In particular, gamma-band oscillations have been proposed to render neural communication effective and precise (5). Thus, abnormalities of synchronized neural activity may lead to impaired temporal coordination within and across networks, resulting in disturbed neural communication at different temporal and spatial scales (6–8).
A fundamental mechanism for communication between neural ensembles is phase-amplitude coupling (PAC), with high-frequency oscillations being nested into the phase of low oscillations. Assuming that low frequencies modulate brain activity over distant brain regions in long temporal windows while high frequencies modulate the activity locally, theta-gamma PAC provides a means to integrate neural activity across different spatial and temporal scales (1, 2). However, so far, studies exploring PAC in patients within the psychosis spectrum have yielded contrasting results (9, 10).
On the other hand, a large body of research has reported decreased power and phase synchronization of gamma-band response during auditory and visual perception and in the context of working memory and attention paradigms in patients with schizophrenia (11–13). In particular, there is consistent evidence for an impairment of the 40-Hz auditory steady-state responses (ASSRs), reflecting the failure of periodic auditory stimulation to induce resonant neuronal oscillations in patients with schizophrenia (11, 14). Reduced ASSRs have been identified across all the stages of psychotic disorders, from individuals at clinical high risk for psychosis to patients with chronic schizophrenia (15). Similar abnormalities have been found in first-degree relatives of patients with schizophrenia (16), suggesting that reduced gamma-band response is a heritable trait and a putative endophenotype of schizophrenia (17).
22q11.2 deletion syndrome (22q11DS) is a neurogenetic disorder with high penetrance for psychiatric disorders, conferring a 30%−40% lifetime risk of developing a psychotic disorder (18, 19). Several genes within the 22q11.2 region, such as DGCR8, PRODH, and CXCR4, have been linked to glutamatergic and GABAergic neural transmission (20) and disrupted migration and placement of cortical interneurons (21), which are essential for the generation of gamma-band oscillations (22). Additionally, previous research showed decreased ASSRs in a small sample of deletion carriers without psychosis (23). However, to the best of our knowledge, no study has investigated whether the observed impairment in gamma-band response is a stable trait or a consequence of aberrant development. Volumetric studies in 22q11DS have highlighted deviant developmental trajectories of cortical and subcortical areas during late adolescence (24–26). Since there is a correlation between gray matter volume and gamma-band response (27–30), it is possible that the development of brain oscillations may be likewise compromised.
Interestingly, developmental data in healthy subjects suggest that there is a significant increase in high-frequency brain oscillation power between adolescence and adulthood (31–34), reflecting the physiological maturation of cortical inhibitory circuits (35, 36). This period has thereby been proposed to represent a vulnerable time window, during which maladaptive development may lead to long-lasting functional abnormalities of gamma-band response and the emergence of psychotic symptoms (7, 32, 35). Because of the early genetic diagnosis, 22q11DS provides an ideal model to characterize the maturation of brain oscillations over time. Furthermore, studying deletion carriers allows clarification of whether the emergence of psychotic symptoms influences gamma-band response and large-network coordination, as well as exploration of the relationship between gamma-band response and gray matter volume.
Accordingly, our primary objective in this study was to compare age-related changes in gamma-band response between deletion carriers and healthy control subjects. We decided to employ a 40-Hz ASSR task, based on robust findings of reduced ASSRs in patients with schizophrenia (11) and established developmental trajectories of ASSRs in healthy subjects (31).
In addition, we analyzed measures of local (gamma-band response) and long-range coordination (PAC between distant brain regions) and their relationship to psychotic symptoms and gray matter volume. Based on previous studies, we expected to find major developmental abnormalities in deletion carriers, with a lack of gamma-band response maturation during late adolescence and a more severe phenotype in individuals endorsing psychotic symptoms.
Methods
Recruitment and Assessment
Individuals with 22q11DS and control subjects were recruited in the context of the 22q11DS Swiss Cohort (24–26). Inclusion criteria were age between 7 and 30 years and presence of a 22q11.2 microdeletion for patients confirmed by quantitative fluorescent polymerase chain reaction.
Exclusion criteria included presence of auditory impairments documented by audiometry screening and, for control subjects, any past or present neurological or psychiatric disease, use of psychotropic medications, psychopathology, learning difficulties, or premature birth.
The occurrence of attenuated psychotic symptoms was assessed in deletion carriers by the Structured Interview for Prodromal Syndromes (SIPS) (37). Deletion carriers were divided into subgroups according to the presence of moderate to severe positive symptoms of psychosis, using a cutoff score of 3 or higher in at least one of the corresponding items for positive symptoms of the SIPS. Because the youngest deletion carrier with psychotic symptoms in this sample was 14 years old, we selected an age-matched control group of deletion carriers without psychotic symptoms.
Written informed consent was obtained from participants and/or their parents. The study was approved by the cantonal ethics committee for research and conducted according to the Declaration of Helsinki.
EEG Data Acquisition During Auditory Task and Preprocessing
EEG data were continuously recorded with a sampling rate of 1000 Hz using a 256-electrode HydroCel cap (EGI-Philips Healthcare) referenced to the vertex (Cz) during the ASSR task. The impedance was kept below 30 kΩ for all electrodes and below 10 kΩ for the reference and ground electrodes. One hundred 40-Hz amplitude-modulated sounds (ripple tones: 1000 Hz carrier tones, duration 2 seconds) and 10 semirandomly intermixed identical, but non–amplitude modulated sounds (flat tones; see Figure S1 in the online supplement) were presented binaurally using intra-aural insert earphones (Etymotic Research, Elk Grove Village, Ill.). The interstimulus interval (ISI) was 2 seconds on average (1.5–2.5 seconds, equal distribution). In order to ensure attentional engagement, participants were asked to detect the flat tones by button press and ignore the 40-Hz ripple tones that entrained the gamma-band responses (14). As in previous studies, the preprocessing steps were performed with Cartool (https://sites.google.com/site/cartoolcommunity/home) (38). More details on EEG preprocessing are provided in the online supplement.
MRI Acquisition
We acquired T1-weighted images with a 3-T Siemens Prisma. The parameters for the acquisition of structural images for the T1-weighted MPRAGE sequence were as follows: TR=2500 ms, TE=3 ms, flip angle=8°, acquisition matrix=256×256, field of view=23.5 cm, voxel size=0.9×0.9×1.1 mm, and 192 slices.
MRI Analysis
T1-weighted images underwent fully automated image processing with FreeSurfer, version 6, comprising skull stripping, intensity normalization, reconstruction of the internal and external cortical surface, and parcellation of subcortical brain regions. Then average measures of volume were extracted from 84 cortical and subcortical regions based on the Desikan-Killiany parcellation (39).
EEG Analysis
Time-frequency analysis was performed by using the Morlet transform in MATLAB, release 2018b, both at sensor level, selecting a cluster of fronto-central electrodes around FCz, and in the source space. Time epochs were averaged from −1.5 to +1.5 seconds relative to the stimulus onset, and then the event-related spectral perturbation (ERSP) was computed, correcting the poststimulus period by the baseline period (40). Intertrial phase coherence (ITPC) amplitudes were also estimated, reflecting the phase consistency across trials (41).
For whole brain source analysis, the inverse solution model was computed based on individual T1-weighted images, by using the “locally spherical model with anatomical constraints” method for lead field and a distributed linear inverse solution (local autoregressive average) to determine the inverse solution transformation matrix (38). The individual Desikan-Killiany parcellation was used to label the 5,000 solution points from the inverse solution model.
For the analysis of theta-gamma phase-amplitude coupling (PAC), preprocessed EEG data were filtered into the frequency bands of interest (4–8 Hz for theta and 38–42 Hz for gamma) and then transformed to the singular value decomposition of the signal for each frequency band and each region of interest with the PyCartool toolbox (https://github.com/Functional-Brain-Mapping-Laboratory/PyCartool). The phase angle and amplitude envelope of the signals were obtained with the Hilbert transform. Coupling between selected regions of interest (i.e., regions of interest exhibiting a task-related increase of at least 15% of the power with respect to the baseline in theta and gamma frequency) was estimated with the modulation index, as described by Tort and colleagues (42) (see the online supplement for further details).
Statistical Analysis
Nonparametric Monte Carlo–based permutations (N=500; 1–100 Hz; 0–1.5 sec; alpha=0.05, two-tailed) implemented in Fieldtrip (43) were employed for the statistical testing of group differences in ERSP and ITPC.
Unpaired two-tailed t tests were performed to compare PAC between groups and subgroups. Pearson’s correlation coefficient was employed to investigate correlations between clinical measures and EEG data, and between age and EEG data. When appropriate, correlation coefficients were compared between groups by using the Fisher Z transformation.
False discovery rate (FDR) correction for multiple comparisons with the Benjamini-Hochberg method was applied to correct for the number of regions of interest and time points within a given frequency band (theta, 4–8 Hz, and gamma, 38–42 Hz, based on surface results and previous studies [9]) for source analyses. FDR was also applied for correction of modulation index between regions of interest in PAC analyses and of SIPS subitems and brain regions in the correlation analyses (i.e., brain regions with different gamma-band response between deletion carriers and control subjects). All the reported p values are corrected for multiple comparisons.
Results
Participants
Of 120 potential participants, six deletion carriers were not included in the study because of auditory impairment. Data for eight participants (five deletion carriers and three control subjects) were additionally excluded from the study because the number of accepted clean epochs in EEG preprocessing was insufficient to ensure reliable analyses (N<40). A total of 106 participants, comprising 58 individuals with 22q11DS (mean age, 17.6 years [SD=6.9]; 26 females) and 48 control subjects (mean age, 17.7 years [SD=6.2]; 24 females), were included in the results of this study. The participants of each group were then evenly divided into age bins for the analysis on development: childhood (7–13 years), adolescence (14–18 years), and adulthood (≥19 years). The participants’ demographic and clinical characteristics are summarized in Table 1. Clinical evaluation of deletion carriers identified 16 participants who endorsed moderate to severe positive psychotic symptoms (mean age, 18.6 years [SD=7]; eight females).
| Characteristic | Control Subjects | Deletion Carriers | No Psychotic Symptoms | Psychotic Symptoms | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | ||||||||||
| N | % | N | % | p | N | % | N | % | p | |
| Number of subjects | 48 | 58 | 28 | 16 | ||||||
| Female | 26 | 54.2 | 28 | 48.3 | 0.76 | 13 | 46.4 | 8 | 50.0 | 0.81 |
| Mean | SD | Mean | SD | p | Mean | SD | Mean | SD | p | |
| ASSR task performance (% correct answers) | 95.8 | 8.9 | 95.0 | 10.7 | 0.24 | 96.9 | 6.5 | 96.7 | 6.2 | 0.92 |
| Age (years)b | 17.7 | 6.2 | 17.6 | 6.9 | 0.96 | 19.3 | 6.5 | 18.6 | 7.0 | 0.78 |
| Full-scale IQ | 110.5 | 11.9 | 73.9 | 11.9 | <0.01 | 74 | 9.5 | 71.2 | 15.5 | 0.14 |
| Children | ||||||||||
| N | % | N | % | p | ||||||
| Number of subjects | 11 | 14 | ||||||||
| Female | 7 | 63.6 | 8 | 57.1 | 0.37 | |||||
| Mean | SD | Mean | SD | p | ||||||
| Age (years) | 10.4 | 2.4 | 10.3 | 1.7 | 0.76 | |||||
| Adolescents | ||||||||||
| N | % | N | % | p | ||||||
| Number of subjects | 18 | 21 | ||||||||
| Female | 10 | 55.6 | 9 | 42.8 | 0.33 | |||||
| Mean | SD | Mean | SD | p | ||||||
| Age (years) | 15.7 | 0.8 | 15.7 | 1.2 | 0.97 | |||||
| Adults | ||||||||||
| N | % | N | % | p | ||||||
| Number of subjects | 19 | 23 | ||||||||
| Female | 9 | 47.4 | 11 | 47.8 | 0.40 | |||||
| Mean | SD | Mean | SD | p | ||||||
| Age (years) | 26.2 | 2.7 | 25.4 | 3.9 | 0.36 | |||||
| N | % | N | % | N | % | p | ||||
| Medicated | 27 | 46.5 | 15 | 53.6 | 9 | 56.2 | 0.86 | |||
| Psychostimulants | 16 | 27.6 | 11 | 39.3 | 6 | 37.5 | 0.90 | |||
| Antidepressants | 15 | 25.9 | 9 | 32.1 | 5 | 31.2 | 0.95 | |||
| Antipsychotics | 5 | 8.6 | 0 | 0.0 | 5 | 31.2 | <0.01 | |||
| Met criteria for psychiatric diagnosis | 34 | 58.6 | 18 | 64.3 | 10 | 66.7 | 0.90 | |||
| ADHD | 28 | 48.3 | 19 | 67.8 | 9 | 56.2 | 0.44 | |||
| Anxiety disorders | 17 | 29.3 | 11 | 39.3 | 6 | 37.5 | 0.90 | |||
| Mood disorders | 9 | 15.5 | 6 | 21.4 | 3 | 18.7 | 0.83 | |||
| Psychosis spectrum disorders | 5 | 8.6 | 0 | 0.0 | 5 | 31.2 | <0.01 | |||
| Mean | SD | Mean | SD | Mean | SD | p | ||||
| SIPS positive symptom score | 5.1 | 5.3 | 2.5 | 2.4 | 11.0 | 5.1 | <0.01 | |||
| SIPS negative symptom score | 12.7 | 6.0 | 11.5 | 4.7 | 13.7 | 4.2 | 0.16 | |||
TABLE 1. Demographic and clinical characteristics of control subjects and 22q11.2 deletion carriers, with and without psychosisa
ERSP and ITPC Differences Between Control Subjects and Deletion Carriers
Clusters of sustained decrease of gamma power and ITPC amplitude in deletion carriers as compared with control subjects were found over fronto-central electrodes (ERSP: 38–42 Hz, tcluster=1.6e5, p=0.002, 95% CI=<0.001, 0.006; ITPC: 38–44 Hz, tcluster=3.1e5, p=0.002, 95% CI=<0.001, 0.006). Additionally, a widespread cluster of reduced early-latency theta response was identified (ERSP: 2–8 Hz, tcluster=9.9e3, p=0.008, 95% CI=0.002, 0.016; ITPC: 6–8 Hz, tcluster=2.4e4, p=0.004, 95% CI=<0.001, 0.009) (Figure 1). The results remained significant even after removing deletion carriers with psychotic symptoms (ERSP: 38–42 Hz, tcluster=1.2e5, p=0.002, 95% CI=<0.001, 0.006; 2–8 Hz, tcluster=1.7e4, p=0 0.014, 95% CI=0.024, 0.004) (see Figure S2 in the online supplement).
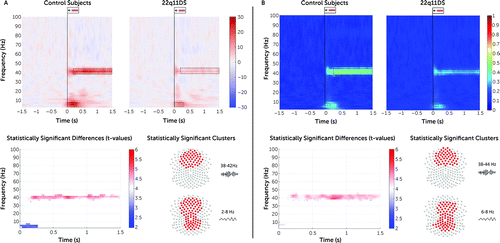
FIGURE 1. Comparison of ERSP and ITPC between control subjects and 22q11.2 deletion carriersa
a The top plots in panel A are time-frequency plots displaying the average pre- and poststimulus event-related spectral perturbation (ERSP) in control subjects and deletion carriers, and the top plots in panel B display intertrial phase coherence (ITPC) amplitude in control subjects and deletion carriers. The outlined dotted boxes highlight the time window of statistically significant group differences in gamma and theta power. At the bottom, panel A shows significant delta ERSP and panel B shows significant delta ITPC, with t-values for statistically significant differences in theta and gamma band. Topographical maps display the clusters of electrodes showing statistically significant differences in the gamma and theta bands represented in the time-frequency plots. Power values are expressed in percent.
Further analyses in the source space revealed that the left and right anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), and thalamus and the right primary auditory cortex showed a decreased gamma-band response in the deletion carriers (Figure 2A,C). In contrast, brain areas with lower theta response in deletion carriers were widespread across the cortex, comprising prefrontal, lateral parietal, temporal, and occipital areas (Figure 2B).
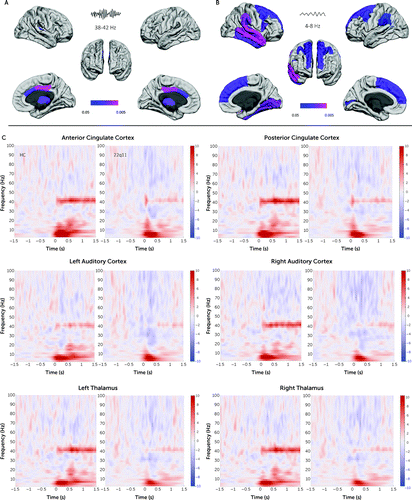
FIGURE 2. Results in the source space of the time-frequency decomposition in control subjects and 22q11.2 deletion carriersa
a The brain map in panel A shows regions with a statistically significant lower gamma response (38–42 Hz) in deletion carriers during the first 1.5 sec of the response, and in panel B, with a statistically significant lower theta response (4–8 Hz) in deletion carriers during the first 0.5 sec of the response. Panel C shows time-frequency plots for brain regions with decreased gamma response in deletion carriers. Power values are expressed in percent.
Psychotic Symptoms and Brain Oscillations
Sixteen deletion carriers with psychotic symptoms were compared with a group of age-matched nonpsychotic individuals with 22q11DS (i.e., older than 14 years; N=28). On the sensor level, decreased early-latency gamma-band response and ITPC were found in deletion carriers with psychosis (ERSP: approximately 38–46 Hz, tcluster=4.5e4, p=0.019, 95% CI=0.001, 0.037; ITPC: approximately 38–46 Hz, tcluster=5.1e4, p=0.002, 95% CI=<0.001, 0.009) (Figure 3; for ITPC, see Figure S3 in the online supplement). Source analysis revealed that the brain areas underlying this decreased response were the left and right ACC and superior frontal gyrus and the right auditory cortex. However, the results in the source space were not significant after correction for multiple comparisons. Additionally, on the sensor level, we found a negative correlation (r=−0.4, p=0.03) (Figure 3A) between the averaged gamma power (0–1.5 sec, cluster of fronto-central electrodes) and the P4 subscale of the SIPS (i.e., hallucinations): the higher the severity of hallucinations, the lower the gamma power response. No correlation was found with the other subitems of the SIPS.
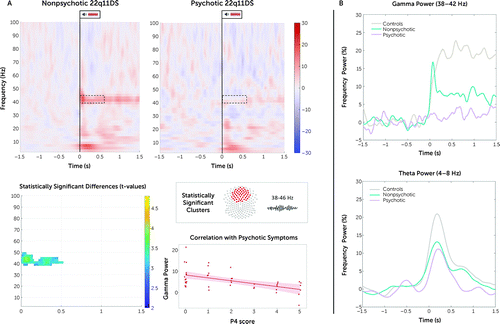
FIGURE 3. Comparison of ERSP between 22q11.2 deletion carriers with and without psychosisa
a In panel A, the time-frequency plots on top display the average pre- and poststimulus event-related spectral perturbation (ERSP) in deletion carriers with and without psychosis. The outlined dotted boxes highlight the time window of statistically significant group differences in gamma and theta power. At the bottom in panel A, the graph on the left, of delta ERSP, shows statistically significant differences in theta and gamma band. On the right, a topographical map displays the cluster of electrodes showing statistically significant differences in the gamma band represented in the time-frequency plot, and the graph shows the correlation between averaged gamma power and P4 score from the Structured Interview for Prodromal Syndromes (hallucinations). Power values are expressed in percent. The graphs in panel B show group comparisons for averaged gamma and theta powers over a fronto-central cluster of electrodes between control subjects, deletion carriers with psychosis, and deletion carriers without psychosis, older than 14 years old.
Divergent Developmental Trajectories of Gamma-Band Response in Deletion Carriers
ERSP comparison between control subjects and deletion carriers was repeated for each age bin. No statistically significant differences between groups were found during childhood or adolescence at sensor or source space. Conversely, adult control subjects had a higher gamma (38–42 Hz, tcluster=3.5e5, p=0.002, 95% CI=<0.001, 0.006) and theta response (4–8 Hz, tcluster=2.9e4, p=0.005, 95% CI=0.002, 0.006) with respect to adults with 22q11DS. Additionally, there was a significant linear increase of gamma power from childhood to adulthood in control subjects (r=0.75, p<0.001) but not in deletion carriers (r=0.13, p=0.62) (Figure 4). Even after removing participants with psychotic symptoms from the group of deletion carriers (N=16), there was no significant correlation with age (r=0.25, p=0.11). Comparison of correlation coefficients for gamma power between control subjects and deletion carriers was statistically significant (control subjects compared with deletion carriers: p<0.001, Z=4.2; control subjects compared with deletion carriers without psychotic symptoms: p=0.001, Z=3.28). Similar results were found for ITPC, with an increase of phase consistency from childhood to adulthood in control subjects (r=0.71, p<0.001) but not in deletion carriers (r=0.19, p=0.09) or in deletion carriers when individuals with psychotic symptoms were excluded (r=0.21, p=0.33). Comparison of correlation coefficients for ITPC between control subjects and deletion carriers was statistically significant (control subjects compared with deletion carriers: p=0.002, Z=3.45; control subjects compared with deletion carriers without psychotic symptoms: p=0.002, Z=3.1).
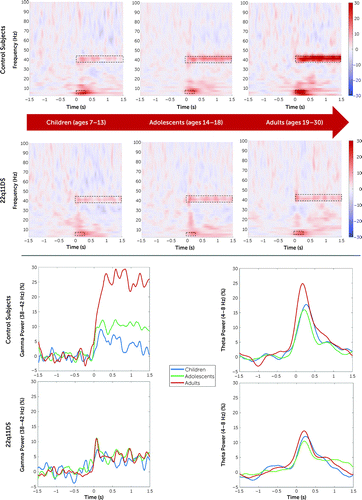
FIGURE 4. Developmental trajectories of gamma and theta bands in control subjects and 22q11.2 deletion carriersa
a The top panel shows time-frequency plots for each age bin (childhood, adolescence, and adulthood) in the two groups compared. The outlined dotted boxes highlight gamma and theta frequency bands according to previous analyses throughout the entire time window. Statistically significant differences were found only between adult subgroups and between the control adult group and the children and adolescent control groups. The bottom panel presents an age subgroup comparison for averaged gamma and theta power over a fronto-central cluster of electrodes in control subjects and deletion carriers. Power values are expressed in percent.
Theta-Gamma Phase Amplitude Coupling
When differences in theta-gamma PAC between control subjects and deletion carriers were examined, no result survived after FDR correction. However, deletion carriers with psychosis exhibited a pattern of decreased modulation of gamma-band amplitude in sensory regions and increased modulation in the cingulate cortex when compared with deletion carriers without psychosis (Figure 5). A cluster of regions survived FDR correction with decreased PAC in deletion carriers with psychosis between the right auditory cortex and the left and right superior frontal gyrus (left: t=−2.6, p=0.02; right: t=−2.2, p=0.03; df=42 for all t values reported in this paragraph) and pars triangularis (left: t=−2, p=0.04; right; t=−2.1, p=0.03), the right pars opercularis (t=−2.5, p=0.01), and the left superior temporal gyrus (STG) (t=−2.6, p=0.01); between the left auditory cortex and supramarginal gyrus (left: t=−3.4, p=0.001; right: t=−2.5, p=0.01); and between the thalamus and right pars opercularis (t=−2, p=0.04) and left STG (t=−3, p=0.002). Conversely, PAC was significantly higher in deletion carriers with psychosis between cingulate regions and the left pars opercularis (left ACC: t=2, p=0.04; right ACC: t=2.5, p=0.01), the right STG (t=−2.4, p=0.02), and the thalamus (left thalamus: t=2.4, p=0.02; right thalamus: t=2, p=0.04) (Figure 5).
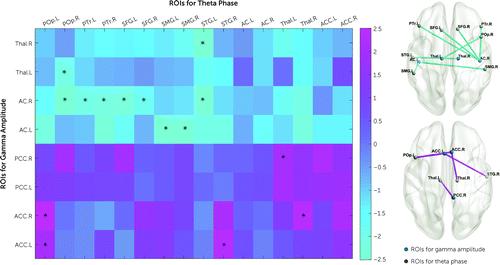
FIGURE 5. Results of phase-amplitude coupling analyses in 22q11.2 deletion carriers with and without psychosisa
a On the left is a matrix of T-values of the comparison between deletion carriers with and without psychosis between regions of interest with a task-related increase in gamma power (y-axis) and regions of interest with a task-related increase in theta power (x-axis). Cold colors correspond to lower coupling and warm colors to higher coupling for deletion carriers with psychosis. Statistically significant results are marked with an asterisk. On the right, brain maps display statistically significant decreased (top) and increased (bottom) phase-amplitude coupling in deletion carriers with psychosis. AC=auditory cortex; ACC=anterior cingulate cortex; L=left; POp=pars opercularis; PTr=pars triangularis; PCC=posterior cingulate cortex; R=right; ROIs=regions of interest; SFG=superior frontal gyrus; SMG=supramarginal gyrus; STG=superior temporal gyrus; Thal=thalamus.
Volumetric Differences
We additionally explored whether there were volumetric differences between deletion carriers with and without psychosis in brain regions with a reduced gamma-band response (i.e., auditory cortex, ACC, PCC, and thalamus). A statistically significant lower volume in deletion carriers with psychosis in the right auditory cortex (t=−1.88, p=0.005), ACC (t=−2.2, df=42, p=0.03), and PCC (t=−2.25, df=42, p=0.04) was found. Additionally, there was a correlation between the averaged gamma power (0–1.5 sec) and auditory cortex volume (r=0.38, p=0.02).
Discussion
We revealed a consistently reduced gamma-band response in 22q11.2 deletion carriers as compared with control subjects and more severe abnormalities of oscillatory response and long-range communication in deletion carriers who endorsed psychotic symptoms. Furthermore, to the best of our knowledge, this study is the first to show a lack of age-related increase in gamma-band response in individuals at genetic risk for psychosis.
Decreased Gamma and Theta Responses in Individuals With 22q11DS
We confirmed previous observations of decreased gamma-band response and phase synchronization during auditory processing in individuals with 22q11DS (23) and additionally found theta power decrease. Specifically, while the sustained steady-state response was impaired, the early-latency gamma-band response was relatively preserved in deletion carriers. Source analysis allowed us to localize the decreased gamma-band response found in deletion carriers in auditory cortex, thalamus, ACC, and PCC. The involvement of these regions is in agreement with previous studies aimed at identifying the brain areas underlying the ASSRs (44, 45).
On the other hand, differences in theta response were restricted to the first 0.5 seconds after stimulus but were widespread, encompassing prefrontal cortical, lateral parietal cortical, temporal, and occipital regions. Interestingly, theta and gamma are known to be part of a neural coding scheme whereby gamma oscillations are nested into theta cycles in order to promote the integration of neural activity between distant regions (1, 2). Since many areas with reduced theta oscillations belong to the attention network, we tested whether there was a reduced PAC with regions exhibiting a lower gamma response, possibly indicating a failure to modulate distant computations in the gamma range. However, investigating PAC, we did not highlight significant differences between control subjects and deletion carriers.
Overall, 22q11.2 deletion carriers display selective oscillatory abnormalities in the gamma and theta bands that cannot be directly reconducted to the disruption of long-range coordination. These findings add to the growing body of literature claiming the existence of a strong genetic substrate for the ASSRs (16, 17).
Disrupted Local and Long-Range Coordination in Deletion Carriers With Psychosis
In line with previous studies showing deficient ASSRs in individuals at clinical risk for psychosis (15), we found further reduced gamma-band response and phase synchronization in the group of deletion carriers with psychotic symptoms. Interestingly, the decrease of gamma-band response was particularly prominent in the early latencies as compared with deletion carriers without psychosis, confirming the progression from a selective decrease of the sustained steady-state response during preclinical stages to a more global impairment of gamma response after the emergence of psychotic episodes observed in previous studies (46).
With PAC analyses, we also revealed in deletion carriers with psychotic symptoms a decreased prefrontal theta modulation of gamma response in auditory cortex and thalamus and an increased modulation of cingulate cortex. Less efficient top-down coordination of auditory cortex, together with increased maladaptive modulation of cingulate cortices and saliency attribution, is in line with current theories for auditory hallucinations (47). This hypothesis is further corroborated by the negative relationship that we found between the severity of hallucinations and the magnitude of gamma-band response. An impaired top-down control may partially explain the inability to efficiently recruit the auditory network during perception and the tendency to activate it in the absence of external stimuli typically observed during auditory hallucinations.
Interestingly, previous studies showed that the brain regions with the earliest volume decline in deletion carriers experiencing hallucinations are intercalated in the auditory pathway (i.e., medial geniculate nucleus of the thalamus and superior temporal gyrus) (24, 26). Volumetric analyses revealed that brain regions exhibiting a decreased gamma-band response also had a lower volume in deletion carriers with psychosis, with a correlation between auditory cortex volume loss and impaired gamma response. These findings underline a link between the observed functional deficits and the structural architecture of the brain in 22q11DS, hinting at a common underlying circuit dysfunction. However, it remains to be determined whether aberrant synchronized network activity influenced processes of gray matter maturation (27) or gray matter atrophy, resulting in reduced synaptic connectivity, determines impaired gamma-band response (30). Early developmental abnormalities in the auditory system, together with decreased top-down control, may thereby play a pivotal role in the emergence of hallucinatory phenomena in 22q11DS.
Taken together, these findings suggest that while deletion carriers without psychosis can normally activate long-range communication—possibly allowing compensation for inefficient local gamma-band response—the additional disruption of large-scale networks in deletion carriers with psychosis may result in more severe deficits in brain oscillations and in hallucinations.
Developmental Trajectories
We further explored how brain oscillations evolve throughout development. In agreement with previous studies, gamma-band response in healthy control subjects increased from childhood to adulthood, with a major development at the transition between adolescence and adulthood (31). However, there were no significant differences across developmental stages in deletion carriers, possibly indicating a lack of maturation during adolescence.
It is widely acknowledged that adolescence is a critical developmental period for brain maturation that involves modifications in circuit mechanisms underlying the generation of gamma-band oscillations, such as parvalbuminergic interneurons (PVIs) and glutamatergic N-methyl-d-aspartate receptors (NMDARs) (36, 48), leading to increased temporal and spatial precision of information processing (32). Studies in mouse models of 22q11DS have demonstrated a reversible functional impairment of PVIs during adolescence, followed by chronically compromised activity of PVIs and impaired gamma-band response in adulthood (49). Similarly, our results support the hypothesis that during late adolescence there is a developmental impairment in circuits supporting the maturation of gamma-band oscillations in human deletion carriers.
Interestingly, the emergence of psychotic disorders typically occurs during late adolescence. For this reason, it has been suggested that vulnerability to psychiatric disorders, especially psychosis, may be rooted in the exacerbation of major brain changes normally taking place during adolescence (50). In our study, we observed that deletion carriers with psychotic symptoms exhibited a further decrease in gamma-band response and additional decoupling of local gamma oscillations from prefrontal top-down control. In agreement with the glutamate hypothesis of schizophrenia (51), several lines of evidence suggest that the observed long-range communication disruption may depend on NMDAR hypofunction, which in turn would lead to altered PVI function by decreasing the glutamatergic drive (4, 6).
Thus, a divergence of developmental trajectories within deletion carriers may lead one group to develop resilience mechanisms while the other progresses into the psychotic phenotype. Indeed, while many pathways could independently lead to this deviation, including the upstream disruption of signaling through NMDARs (52), aberrant maturation of PVIs may represent a critical turning point (36). Consistent with this idea, myelination of PVIs has been proposed to constitute a locus of pathophysiological convergence for psychotic disorders (53). Moreover, schizophrenia-like deficits—comprising cognitive disturbances and the underlying impaired neural synchrony—can be rescued by chemogenetic activation of PVIs only during a critical period corresponding to late adolescence (49), further supporting the existence of a direct link between maturation of PVIs, high-frequency oscillations, and psychosis.
Limitations
We must acknowledge several limitations of our study. First, we are reporting cross-sectional data, so our results on developmental trajectories should be confirmed by longitudinal studies. Second, antipsychotic treatment and IQ may be confounding factors. However, in our exploratory analysis, we did not find any difference in gamma-band response between individuals who were treated and not treated with antipsychotics and with low and high IQ scores (see the online supplement). Third, previous studies exploring PAC mechanisms during auditory processing did not identify significant abnormalities in patients with schizophrenia (9); however, such PAC disruptions were found in the context of more demanding tasks (10). One possible explanation is that individuals with 22q11DS, having a baseline cognitive dysfunction, are more likely to show a lower threshold for the disruption of long-range synchronization, even during relatively effortless tasks. Future studies employing different paradigms in this population should answer this question.
Conclusions
In this study, we demonstrated a lack of age-related increase in gamma-band response at the transition between adolescence and adulthood, suggesting a developmental impairment in 22q11.2 deletion carriers. Individuals who eventually endorsed symptoms of psychosis underwent further gamma-band response decrease and disruption of theta top-down control over gamma activity. On the other hand, the relatively preserved long-range coordination and early-latency gamma-band response in deletion carriers who did not develop psychotic symptoms may represent a neural signature of resilience that could be exploited for therapeutic purposes. Thus, our results contribute to identifying potential targets and a critical developmental window for intervention in humans, in line with studies in mice models (49). Given that molecules acting on glutamatergic and dopaminergic systems have been shown to effectively modulate gamma response (49, 54), one approach could contemplate early pharmacological intervention in individuals at risk for psychosis. Alternatively, considering that psychotic deletion carriers undergo disruption of long-range synchronization, complementary noninvasive strategies such as transcranial alternating current stimulation may be employed to restore brain communication between distant brain regions.
1 : The functional role of cross-frequency coupling. Trends Cogn Sci 2010; 14:506–515Crossref, Medline, Google Scholar
2 : High gamma power is phase-locked to theta oscillations in human neocortex. Science 2006; 313:1626–1628Crossref, Medline, Google Scholar
3 : A framework for local cortical oscillation patterns. Trends Cogn Sci 2011; 15:191–199Crossref, Medline, Google Scholar
4 : Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology 2012; 62:1504–1518Crossref, Medline, Google Scholar
5 : Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 2009; 32:209–224Crossref, Medline, Google Scholar
6 : Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 2010; 11:100–113Crossref, Medline, Google Scholar
7 : The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr Bull 2011; 37:514–523Crossref, Medline, Google Scholar
8 : Synchrony in schizophrenia: a window into circuit-level pathophysiology. Curr Opin Neurobiol 2015; 30:17–23Crossref, Medline, Google Scholar
9 : Hierarchical organization of gamma and theta oscillatory dynamics in schizophrenia. Biol Psychiatry 2012; 71:873–880Crossref, Medline, Google Scholar
10 : Impaired theta phase coupling underlies frontotemporal dysconnectivity in schizophrenia. Brain 2020; 143:1261–1277Crossref, Medline, Google Scholar
11 : The 40-Hz auditory steady-state response in patients with schizophrenia: a meta-analysis. JAMA Psychiatry 2016; 73:1145–1153Crossref, Medline, Google Scholar
12 : Association of magnetoencephalographically measured high-frequency oscillations in visual cortex with circuit dysfunctions in local and large-scale networks during emerging psychosis. JAMA Psychiatry 2020; 77:852–862Crossref, Medline, Google Scholar
13 : Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia. Biol Psychiatry 2015; 77:1010–1019Crossref, Medline, Google Scholar
14 : 40-Hz auditory steady-state responses characterize circuit dysfunctions and predict clinical outcomes in clinical high-risk for psychosis participants: a magnetoencephalography study. Biol Psychiatry 2021; 90:419–420Crossref, Medline, Google Scholar
15 : Gamma band oscillations in the early phase of psychosis: a systematic review. Neurosci Biobehav Rev 2018; 90:381–399Crossref, Medline, Google Scholar
16 : Alterations of the early auditory evoked gamma-band response in first-degree relatives of patients with schizophrenia: hints to a new intermediate phenotype. J Psychiatr Res 2011; 45:699–705Crossref, Medline, Google Scholar
17 : The early auditory gamma-band response is heritable and a putative endophenotype of schizophrenia. Schizophr Bull 2011; 37:778–787Crossref, Medline, Google Scholar
18 : Further evidence for high rates of schizophrenia in 22q11.2 deletion syndrome. Schizophr Res 2014; 153:231–236Crossref, Medline, Google Scholar
19 : Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry 2014; 171:627–639Link, Google Scholar
20 : In the line-up: deleted genes associated with DiGeorge/22q11.2 deletion syndrome: are they all suspects? J Neurodev Disord 2019; 11:7Crossref, Medline, Google Scholar
21 : Cxcr4 regulation of interneuron migration is disrupted in 22q11.2 deletion syndrome. Proc Natl Acad Sci USA 2012; 109:18601–18606Crossref, Medline, Google Scholar
22 : Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 2009; 459:663–667Crossref, Medline, Google Scholar
23 : 22q11.2 deletion syndrome is associated with impaired auditory steady-state gamma response. Schizophr Bull 2018; 44:388–397Crossref, Medline, Google Scholar
24 : Abnormal development and dysconnectivity of distinct thalamic nuclei in patients with 22q11.2 deletion syndrome experiencing auditory hallucinations. Biol Psychiatry Cogn Neurosci Neuroimaging 2020; 5:875–890Crossref, Medline, Google Scholar
25 : Positive psychotic symptoms are associated with divergent developmental trajectories of hippocampal volume during late adolescence in patients with 22q11DS. Mol Psychiatry 2020; 25:2844–2859Crossref, Medline, Google Scholar
26 : Altered cortical thickness development in 22q11.2 deletion syndrome and association with psychotic symptoms. Mol Psychiatry (Online ahead of print, July 12, 2021)Google Scholar
27 : Neural synchrony and the development of cortical networks. Trends Cogn Sci 2010; 14:72–80Crossref, Medline, Google Scholar
28 : Cortical thickness as a contributor to abnormal oscillations in schizophrenia? Neuroimage Clin 2013; 4:122–129Crossref, Medline, Google Scholar
29 : Cortical volume and 40-Hz auditory-steady-state responses in patients with schizophrenia and healthy controls. Neuroimage Clin 2019; 22:101732Crossref, Medline, Google Scholar
30 : Auditory cortex volume and gamma oscillation abnormalities in schizophrenia. Clin EEG Neurosci 2020; 51:244–251Crossref, Medline, Google Scholar
31 : Development of the 40Hz steady state auditory evoked magnetic field from ages 5 to 52. Clin Neurophysiol 2006; 117:110–117Crossref, Medline, Google Scholar
32 : The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci USA 2009; 106:9866–9871Crossref, Medline, Google Scholar
33 : Age-related changes in transient and oscillatory brain responses to auditory stimulation in healthy adults 19–45 years old. Cereb Cortex 2007; 17:1454–1467Crossref, Medline, Google Scholar
34 : Development of sensory gamma oscillations and cross-frequency coupling from childhood to early adulthood. Cereb Cortex 2015; 25:1509–1518Crossref, Medline, Google Scholar
35 : Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull 2011; 37:484–492Crossref, Medline, Google Scholar
36 : Interneuron epigenomes during the critical period of cortical plasticity: implications for schizophrenia. Neurobiol Learn Mem 2015; 124:104–110Crossref, Medline, Google Scholar
37 : Prodromal assessment with the Structured Interview for Prodromal Syndromes and the Scale of Prodromal Symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull 2003; 29:703–715Crossref, Medline, Google Scholar
38 : EEG source imaging: a practical review of the analysis steps. Front Neurol 2019; 10:325Crossref, Medline, Google Scholar
39 . An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. 2006;31:968–80.Google Scholar
40 : Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophysiol 2001; 43:41–58Crossref, Medline, Google Scholar
41 : EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 2004; 134:9–21Crossref, Medline, Google Scholar
42 : Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol 2010; 104:1195–1210Crossref, Medline, Google Scholar
43 : Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 2007; 164:177–190Crossref, Medline, Google Scholar
44 : Generators and connectivity of the early auditory evoked gamma band response. Brain Topogr 2015; 28:865–878Crossref, Medline, Google Scholar
45 : Single-trial coupling of the gamma-band response and the corresponding BOLD signal. Neuroimage 2010; 49:2238–2247Crossref, Medline, Google Scholar
46 : Differential alterations of auditory gamma oscillatory responses between pre-onset high-risk individuals and first-episode schizophrenia. Cereb Cortex 2016; 26:1027–1035Crossref, Medline, Google Scholar
47 : Auditory hallucinations in schizophrenia and nonschizophrenia populations: a review and integrated model of cognitive mechanisms. Schizophr Bull 2012; 38:683–693Crossref, Medline, Google Scholar
48 : Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology 2009; 34:2028–2040Crossref, Medline, Google Scholar
49 : Long-lasting rescue of network and cognitive dysfunction in a genetic schizophrenia model. Cell 2019; 178:1387–1402.e14Crossref, Medline, Google Scholar
50 : Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 2008; 9:947–957Crossref, Medline, Google Scholar
51 : From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 2012; 37:4–15Crossref, Medline, Google Scholar
52 : NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull 2012; 38:950–957Crossref, Medline, Google Scholar
53 : Myelination of parvalbumin interneurons: a parsimonious locus of pathophysiological convergence in schizophrenia. Mol Psychiatry 2017; 22:4–12Crossref, Medline, Google Scholar
54 : Single-dose memantine improves cortical oscillatory response dynamics in patients with schizophrenia. Neuropsychopharmacology 2017; 42:2633–2639Crossref, Medline, Google Scholar



