Neural Correlates of the Dual-Pathway Model for ADHD in Adolescents
Abstract
Objective:
The dual-pathway model has been proposed to explain the heterogeneity in symptoms of attention deficit hyperactivity disorder (ADHD) by two independent psychological pathways based on distinct brain circuits. The authors sought to test whether the hypothesized cognitive and motivational pathways have separable neural correlates.
Methods:
In a longitudinal community-based cohort of 1,963 adolescents, the neuroanatomical correlates of ADHD were identified by a voxel-wise association analysis and then validated using an independent clinical sample (99 never-medicated patients with ADHD, 56 medicated patients with ADHD, and 267 healthy control subjects). The cognitive and motivational pathways were assessed by neuropsychological tests of working memory, intrasubject variability, stop-signal reaction time, and delay discounting. The associations were tested between the identified neuroanatomical correlates and both ADHD symptoms 2 years later and the polygenic risk score for ADHD.
Results:
Gray matter volumes of both a prefrontal cluster and a posterior occipital cluster were negatively associated with inattention. Compared with healthy control subjects, never-medicated patients, but not medicated patients, had significantly lower gray matter volumes in these two clusters. Working memory and intrasubject variability were associated with the posterior occipital cluster, and delay discounting was independently associated with both clusters. The baseline gray matter volume of the posterior occipital cluster predicted the inattention symptoms in a 2-year follow-up and was associated with the genetic risk for ADHD.
Conclusions:
The dual-pathway model has both shared and separable neuroanatomical correlates, and the shared correlate in the occipital cortex has the potential to serve as an imaging trait marker of ADHD, especially the inattention symptom domain.
Attention deficit hyperactivity disorder (ADHD) is one of the most common neurodevelopmental disorders, affecting 5.9%–7.1% children and adolescents worldwide (1), with 50%–66% of cases persisting into adulthood (2, 3). This disorder has been characterized by its significant heterogeneity, as patients receiving the diagnosis often present neuropsychological impairments in distinct domains (4). Therefore, identification of the neural abnormalities underlying these heterogeneous impairments may improve both the diagnostic accuracy and the treatment efficiency of this disorder.
To account for such heterogeneity, a dual-pathway model has suggested two separable pathophysiological pathways leading to the symptoms of ADHD (5–7), including cognitive dysfunctions, such as deficits in working memory (8), attention regulation (intrasubject variability) (9), and response inhibition (stop-signal reaction time) (10), and motivational dysfunction, such as preferring small immediate rewards over larger delayed rewards (delay discounting) (11). As frontostriatal dysfunction has frequently been associated with ADHD in neuroimaging studies (12), one hypothesis has been proposed that these two pathways can be dissociated into the fronto-dorsal striatal circuit, responsible for cognitive dysfunction, and the fronto-ventral striatal circuit, responsible for motivational dysfunction (5). Previous behavioral studies have reported that children with ADHD have cognitive and motivational deficits (13, 14), both of which independently contribute to ADHD symptoms (15–17). However, it is still under debate whether the cognitive and motivational deficits are independent from (18, 19) or associated with each other (20, 21). Recent studies seem to suggest a functional overlap between these two pathways; for example, working memory training could also improve delay discounting (22, 23). In neuroimaging studies, a large-scale brain system beyond the frontostriatal model has also been discussed (24); for example, a 2016 meta-analysis (25) reported structural abnormalities in ADHD patients in both the right basal ganglia/insula and prefrontal cortex as well as in the left occipital lobe.
Our main goal in the present study, then, was to test whether these two pathways are linked to ADHD symptoms by shared and/or separable neuroanatomical correlates. Given that ADHD has been considered an extreme of a quantitative trait (26), we first analyzed a large-scale population-based sample to identify its neuroanatomical correlates, and then validated the findings using an independent clinical sample. With both medicated and never-medicated patients with ADHD in this clinical sample, we were also able to assess the effects of medication on these neuroanatomical correlates. This is the first study, to our knowledge, to assess the independent associations of the identified neuroanatomical correlates with both cognitive deficits (i.e., working memory, intrasubject variability, stop-signal reaction time) and motivational deficits (i.e., delay discounting). To demonstrate the potential of our findings to be an intermediate phenotype of ADHD (27), we further tested whether the identified neuroanatomical correlates contributed to explaining ADHD symptoms 2 years later and whether these correlates were associated with genetic risks for the disorder.
Methods
Participants
Population-based cohort.
IMAGEN is a community-based longitudinal study of adolescent brain development. Details on the recruitment procedure have been published elsewhere (28). Written informed consent was obtained from all participants and their legal guardians. A total of 1,963 participants (952 [49%] of them male) who had completed psychometric assessments and for whom baseline (i.e., at age 14) quality-controlled neuroimaging data were available were included in the analysis (Table 1).
| Characteristic or Measure | Baseline (N=1,963) | 2-Year Follow-Up (N=1,518) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Male | 952 | 48.5% | 728 | 48.0% |
| Mean | SD | Mean | SD | |
| Age (years) | 14.43 | 0.40 | 16.47 | 0.57 |
| Hyperactivity-inattention subscale on parent SDQ | ||||
| Total score | 2.97 | 2.29 | 2.39 | 2.05 |
| Hyperactivity-impulsivity score | 0.70 | 1.05 | 0.47 | 0.87 |
| Inattention score | 2.27 | 1.65 | 1.92 | 1.57 |
| N | % | N | % | |
| ADHD categories by hyperactivity-inattention total scoreb | ||||
| Normal | 1,690 | 86.1 | 1,394 | 91.8 |
| Borderline | 107 | 5.5 | 64 | 4.1 |
| Abnormal | 166 | 8.5 | 62 | 4.1 |
| Mean | SD | |||
| Delay discounting | –1.98 | 0.61 | ||
| Working memory | 19.45 | 14.00 | ||
| Intrasubject variabilityc | 119.38 | 30.96 | ||
| Stop-signal reaction timec | 186.43 | 61.90 | ||
TABLE 1. Characteristics of the study population in the IMAGEN cohorta
Clinical cohort.
ADHD-200 is a multicenter clinical study (29) approved by the local research ethics review boards at each center. A total of 233 patients with ADHD and 267 typically developed control subjects (141 [53%] of them male; mean age, 11.98 years [SD=3.04]) for whom quality-controlled MRI data were available were included in the analysis (see eMethods 1 and Table S1 in the online supplement). Of the ADHD patients, 129 had the combined subtype, 96 the inattentive subtype, and eight the hyperactive/impulsive subtype; 56 were medicated, 99 were never medicated, and medication information was missing for 78 patients. A full-scale IQ score >80 was an inclusion criterion (see Table S2 in the online supplement).
Measurements
ADHD.
The Strengths and Difficulties Questionnaire (SDQ), administered at both baseline and follow-up in IMAGEN, is a validated assessment tool for mental health problems in children and adolescents (30) and has been demonstrated in IMAGEN to be a promising assessment for ADHD symptoms (31–34). The hyperactivity-inattention subscale is composed of five items covering three key symptom domains for ADHD; the subscale’s internal consistency (Cronbach’s alpha=0.75) is at an acceptable level (35). The ADHD total score was the total score of all five items; the inattention score was calculated using two items (“poor concentration” and “good attention”), and the hyperactivity-impulsivity score was estimated using the other three items (“restless,” “fidgety,” and “reflective”). As used in nationwide epidemiological studies (36), a three-band classification was established for the SDQ using a cut-off score of 6 (normal: scores <6, 80%; borderline: score of 6, 10%; abnormal: scores >6, 10%). We used the parent-report SDQ because it is more reliable than the child self-report version, and the parent-report SDQ also has a stronger association with clinical assessments (reported odds ratios of 32.3 and 5 for ADHD) (30, 36). Participants who had abnormal scores at both ages 14 and 16 were classified into the persistent ADHD group, and those with normal scores at both ages were classified into the typically developed control group.
Delay discounting.
The Monetary Choice Questionnaire (37), an efficient and reliable measurement of delay discounting that has been validated in adolescents (38), was administered at baseline. It contains 27 dichotomous-choice items pitting a smaller immediate reward against a larger delayed reward for three levels of reward magnitude (small, medium, and large). Higher k coefficients in a hyperbolic discounting equation for each reward level represent greater preference for small immediate rewards and higher impulsivity (see eMethods 2 in the online supplement). The geometric mean was calculated and logarithmically transformed to use in our analyses.
Working memory.
Spatial working memory, as assessed by the Cambridge Neuropsychological Testing Automated Battery (39), was measured at baseline. This self-ordered searching task to measure participants’ ability to preserve spatial information (40) is widely used in studies of ADHD in children and adolescents (41). The number of errors was used as an index of working memory.
Intrasubject variability and stop-signal reaction time.
Intrasubject variability and stop-signal reaction time were obtained by behavioral data for the stop-signal functional MRI (fMRI) task (42) (N=1,846). Intrasubject variability was estimated by the standard deviation of reaction time in successful go trials. Stop-signal reaction time was estimated by subtracting the mean stop-signal latency from the mean correct go response time. Participants who had less than 50% correct hits and who had negative stop-signal reaction time were excluded.
Structural MRI
The MRI acquisition protocols and quality controls in IMAGEN have been described in detail (28). A high-resolution T1-weighted magnetization-prepared gradient echo sequence was collected using 3-T scanners and preprocessed using the VBM8 toolbox, as reported previously (43) (see eMethods 3 in the online supplement).
Genetic Data
Genotyping was carried out from blood drawn from IMAGEN participants (28). Genotype information was collected at 582,982 markers using the Illumina Human Genotyping BeadChip. After quality control, 1,790 cases were included in our sample, totaling 506,932 single-nucleotide polymorphisms available for establishing the polygenic risk score (PRS) for ADHD (see eMethods 4 in the online supplement).
Statistical Analysis
Voxel-wise brain-wide association analysis.
A whole-brain analysis was conducted at the voxel level using the general linear model in SPM12 to identify clusters with gray matter volume associated with the ADHD total score at baseline in IMAGEN. Age, sex, handedness, total intracranial volume, and site were considered as covariates. IQ is not recommended as a variable to be controlled in cognitive studies of neurodevelopmental disorders, since it is often affected by the disorder (44). An uncorrected p threshold of 0.001 at voxel level, with a cluster-level family-wise error (FWE) corrected p threshold of 0.05, was applied to identify significant clusters (45).
Neuropsychological association analysis.
Separate partial correlation analyses were conducted between neuropsychological measures (working memory, intrasubject variability, stop-signal reaction time, and delay discounting) and both ADHD symptoms and gray matter volumes of the significant clusters, controlling for age, sex, handedness, total intracranial volume, and site. Confidence interval was given by 5,000 bootstraps. Next, we included other variables as covariates for the association analysis of one variable. If a significant association becomes insignificant after controlling for other variables, this association is not independent of other variables but is contributed by some common factor shared between cognitive and motivational deficits.
Prospective association analysis.
We extended our analysis to ADHD symptoms at age 16 in the IMAGEN participants. Hierarchical multiple regression was applied to identify significant associations between the baseline features and ADHD symptoms 2 years later. In these regression models with covariates and corresponding baseline symptoms, the behavioral variables and gray matter volumes of the significant clusters were entered one by one. A variable was retained in the model if it significantly elevated the model performance (i.e., a significant ΔR2 with p<0.05).
Analysis of covariance was performed between the persistent ADHD group and the typically developed control group in IMAGEN while controlling for sex, handedness, total intracranial volume, and site. Significance of the results was given by 10,000 random permutations (reported as p-perm) and was validated by the comparisons between well-matched samples (healthy control subjects were selected by the R package MatchIt to match the sample size with the persistent ADHD group) (46).
Polygenic analysis.
The latest genome-wide association meta-analysis of 20,183 patients with ADHD and 35,191 control subjects was used as the discovery data set (47); the summary statistics were downloaded from the Psychiatric Genomics Consortium (http://www.med.unc.edu/pgc/results-and-downloads). The primary analyses are based on the threshold of p<0.50, since it maximally captures phenotypic variance (48), using PRS software (PRSice; http://prsice.info/) (49). Associations of PRS with the neuropsychological variables were tested by partial correlation analyses while controlling for age, sex, and site, and its associations with gray matter volumes of the significant clusters were assessed by additionally controlling for handedness and total intracranial volume.
Validation.
We applied the same preprocessing pipeline of structural neuroimaging data as that used in IMAGEN to the ADHD-200 clinical sample. Using a mask of the significant clusters identified in IMAGEN, the gray matter volume of each cluster was extracted for analyses. We tested 1) whether patients with ADHD had lower gray matter volumes of the significant clusters by comparing patients with control subjects; 2) which ADHD patient subtype had the lowest gray matter volumes of the significant clusters by comparing between two ADHD subtypes (hyperactive/impulsive subtype was excluded because of a small sample size of eight) and control subjects; and 3) whether medication had any remedial effect on the reduced gray matter volumes of the significant clusters by group comparisons of never-medicated patients, medicated patients, and control subjects. All analyses were controlled for age, sex, handedness, total intracranial volume, and site.
Results
Descriptive Statistics
In the IMAGEN cohort at baseline (Table 1), working memory, intrasubject variability, and delay discounting were positively associated with ADHD symptoms, and the correlations were not confounded by one another (Table 2). There was no significant correlation between ADHD symptoms and stop-signal reaction time (p>0.05), and therefore it was not included in further analyses. Delay discounting rate was positively associated with working memory errors (r=0.13, df=1952, p<0.001, 95% CI=0.08, 0.17) and increased intrasubject variability (r=0.09, df=1835, p<0.001, 95% CI=0.04, 0.13).
| ADHD Symptoms | Gray Matter Volume | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Score | Hyperactivity-Impulsivity | Inattention | Prefrontal Cluster | Posterior occipital Cluster | ||||||
| Measure | r | 95% CI | r | 95% CI | r | 95% CI | r | 95% CI | r | 95% CI |
| Working memoryb | 0.19*** | 0.15, 0.24 | 0.09*** | 0.05, 0.14 | 0.21*** | 0.16, 0.25 | −0.04 | −0.08, 0.01 | −0.08*** | −0.12, −0.03 |
| Delay discountingb | 0.13*** | 0.08, 0.17 | 0.06** | 0.02, 0.11 | 0.14*** | 0.09.0.18 | −0.07** | −0.11, −0.02 | −0.06* | −0.10, −0.01 |
| Intrasubject variabilityc | 0.14*** | 0.10, 0.19 | 0.09*** | 0.04, 0.14 | 0.14*** | 0.10, 0.19 | −0.05* | −0.10, −0.01 | −0.06** | −0.11, −0.01 |
| Working memory corrected for delay discounting and intrasubject variability | 0.16*** | 0.12, 0.21 | 0.07** | 0.03, 0.12 | 0.18*** | 0.13, 0.22 | −0.04 | −0.09, 0.005 | −0.07** | −0.11, −0.02 |
| Delay discounting corrected for working memory and intrasubject variability | 0.10*** | 0.05, 0.15 | 0.05* | 0.001, 0.10 | 0.11*** | 0.06, 0.15 | −0.05* | −0.09, −0.002 | −0.05* | −0.09, −3.3e–4 |
| Intrasubject variability corrected for working memory and delay discounting | 0.12*** | 0.08, 0.17 | 0.08** | 0.03, 0.13 | 0.12*** | 0.07, 0.16 | −0.04 | −0.09, 0.005 | −0.05* | −0.10, −0.01 |
TABLE 2. Associations of neuropsychological variables with ADHD symptoms and gray matter volumes of the significant clustersa
Neuroanatomical Correlates of Inattention in a Population-Based Cohort
In IMAGEN at baseline, we found that higher ADHD total score was associated with lower gray matter volumes of two brain clusters in both the prefrontal cortex (x=−19.5, y=49.5, z=3; 3,357 voxels; peak t=−4.29, df=1950, cluster-level pFWE<0.001) and the posterior occipital cortex (x=−1.5, y=91.5, z=15; 1,295 voxels; peak t=−4.32, df=1950, cluster-level pFWE=0.025). The prefrontal cluster covered the left ventromedial prefrontal cortex, dorsal anterior cingulate cortex, and anterior insula, and the posterior occipital cluster was mainly in the left cuneus and extended to the left calcarine cortex (Figure 1). These associations were not confounded by either site (see Figure S1 in the online supplement) or IQ (see eResults 2 in the online supplement). These associations became insignificant after controlling for the inattention score but remained significant after controlling for the hyperactivity-impulsivity score (prefrontal cluster: r=−0.08, df=1949, p<0.001, 95% CI=−0.03, −0.13; posterior occipital cluster: r=−0.07, df=1949, p=0.002, 95% CI=−0.03, −0.11).
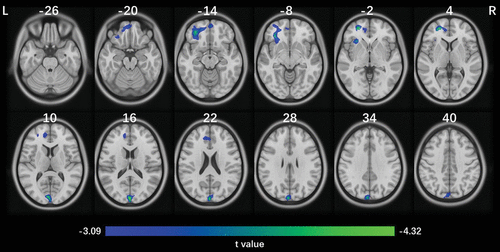
FIGURE 1. Significant brain clusters associated with ADHD total score in a population-based cohorta
a The results were given by a voxel-wise whole brain analysis using the IMAGEN cohort at age 14 (N=1,963). Age, sex, handedness, total intracranial volume, and site were used as covariates. An uncorrected p threshold of 0.001 at voxel level, with a cluster-level family-wise error (FWE) corrected p threshold of 0.05, was applied to identify significant clusters. Two clusters were found to be negatively associated with the ADHD total score: the prefrontal cluster (x=−19.5, y=49.5, z=3; 3,357 voxels; peak t=−4.29, df=1950, cluster-level pFWE<0.001) and the posterior occipital cortex (x=−1.5, y=91.5, z=15; 1,295 voxels; peak t=−4.32, df=1950, cluster-level pFWE=0.025). No clusters were found to be positively associated with the ADHD total score.
Neuroanatomical Correlates of Inattention Selectively Associated With Working Memory, Intrasubject Variability, or Delay Discounting
In IMAGEN at baseline, we found that a larger number of working memory errors was associated with lower gray matter volume of the posterior occipital cluster even after controlling for intrasubject variability and delay discounting (r=−0.07, df=1831, p=0.005) (Table 2). Similar to working memory, increased intrasubject variability was associated with lower gray matter volume of the posterior occipital cluster even after controlling for working memory and delay discounting (r=−0.05, df=1831, p=0.027) (Table 2). Greater delay discounting rate was associated with lower gray matter volumes of both clusters even after controlling for working memory and intrasubject variability (prefrontal cluster: r=−0.05, df=1831, p=0.042; posterior occipital cluster: r=−0.05, df=1831, p=0.049) (Table 2).
Prospective Associations With Inattention 2 Years Later
After controlling for the corresponding ADHD symptom at age 14, working memory and delay discounting at age 14 were selectively associated with inattention (t=2.35, df=1505, p=0.019) and hyperactivity-impulsivity (t=2.24, df=1505, p=0.025) at age 16. In the multivariate regression model, we found that both working memory (t=2.04, df=1404, ΔR2=0.002, p=0.042) and gray matter volume of the posterior occipital cluster (t=−3.55, df=1404, ΔR2=0.005, p<0.001) at age 14 were associated with inattention 2 years later (Table 3).
| Step | Category | Independent Variable | R2 | ΔR2 | pea | βfb | tfc | pfd |
|---|---|---|---|---|---|---|---|---|
| Step 1 | Covariates | Sex | 0.412 | 0.412 | <0.001 | 0.073 | 2.86 | 0.004 |
| Handedness | 0.016 | 0.79 | 0.430 | |||||
| Total intracranial volume | 0.010 | 0.375 | 0.708 | |||||
| Inattention at age 14 | 0.608 | 28.45 | <0.001 | |||||
| Site 1 | 0.018 | 0.66 | 0.508 | |||||
| Site 2 | 0.050 | 1.80 | 0.072 | |||||
| Site 3 | 0.068 | 2.57 | 0.010 | |||||
| Site 4 | 0.065 | 2.47 | 0.014 | |||||
| Site 5 | 0.010 | 0.36 | 0.721 | |||||
| Site 6 | 0.038 | 1.44 | 0.151 | |||||
| Site 7 | 0.036 | 1.37 | 0.170 | |||||
| Step 2 | Behavior | Working memory | 0.414 | 0.002 | 0.017 | 0.044 | 2.04 | 0.042 |
| Step 3 | Delay discounting | 0.414 | 0.000 | 0.593 | 0.007 | 0.35 | 0.724 | |
| Step 4 | Intrasubject variability | 0.415 | 0.001 | 0.164 | 0.028 | 1.31 | 0.190 | |
| Step 5 | Brain structure | Gray matter volume in prefrontal cluster | 0.415 | 0.000 | 0.685 | 0.047 | 1.88 | 0.060 |
| Step 6 | Gray matter volume in posterior occipital cluster | 0.42 | 0.005 | <0.001 | –0.083 | –3.55 | <0.001 |
TABLE 3. Hierarchical multiple regression of working memory, delay discounting, intrasubject variability, and gray matter volumes of the significant clusters on inattention at age 16 (N=1,421)
Adolescents with persistent ADHD symptoms (N=29) had reduced gray matter volumes of both the prefrontal cluster as compared with the typically developed control subjects (N=1,278; 5.63 mL [SD=1.03] compared with 6.23 mL [SD=1.19]; F=6.37, df=1, 1295, p-perm=0.012; partial eta-squared [η2p]=0.005) and the posterior occipital cluster (2.06 mL SD=0.35] compared with 2.19 mL [SD=0.28]; F=5.12, df=1, 1295, p-perm=0.022; η2p=0.004; see Figure S2 in the online supplement). Significant results with even larger effect sizes were found using matched-group comparisons (29 compared with 58; see eResults 3 in the online supplement).
Associations of Neuropsychological and Neuroanatomical Intermediate Phenotypes With Polygenic Risk for ADHD
In the IMAGEN sample, we found that higher PRS for ADHD was associated with higher ADHD total score at baseline (r=0.14, df=1779, p<0.001, 95% CI=0.097, 0.188), more working memory errors ( r=0.07, df=1779, p=0.002, 95% CI=0.026, 0.121), greater delay discounting rate (r=0.06, df=1779, p=0.007, 95% CI=0.021, 0.109), and lower gray matter volume of the posterior occipital cluster only (r=−0.06, df=1777, p=0.009, 95% CI=−0.106, −0.015).
Validation Using an ADHD Clinical Cohort
In the ADHD-200 sample, we confirmed that patients had lower gray matter volumes in both the prefrontal (3.86 mL [SD=1.67] compared with 4.40 mL [SD=1.56]; F=12.18, df=1, 491, p<0.001; η2p=0.024) (Figure 2A) and the posterior occipital clusters (1.21 mL [SD=0.30] compared with 1.28 mL [SD=0.29]; F=9.28, df=1, 491, p=0.002; η2p=0.019) (Figure 2B). These volumetric reductions were nonsignificant in patients with the combined subtype (N=129) and significant only in patients with the inattentive subtype (N=96; prefrontal cluster: F=12.92, df=1, 354, p<0.001; η2p=0.035; posterior occipital cluster: F=7.29, df=1, 354, p=0.007; η2p=0.20) (Figure 2C–D).
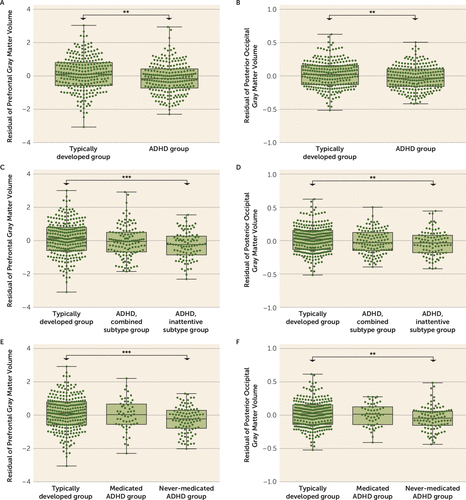
FIGURE 2. Group comparisons of gray matter volumes of the identified clusters in a clinical cohorta
a The results were based on the ADHD-200 cohort. The y-axis in all panels is the residual of gray matter volumes of the identified clusters regressed on age, sex, handedness, total intracranial volume, and site. The bottom and top of the boxes are the minimum and maximum, and the band near the middle of the box is the median. Significant group comparisons are indicated with asterisks. Panel A depicts the difference in gray matter volume of the prefrontal cluster between typically developed control subjects (N=267) and ADHD patients (N=233). Panel B depicts the difference in gray matter volume of the posterior occipital cluster between the typically developed group (N=267) and ADHD patients (N=233). Panel C depicts the difference in gray matter volume of the prefrontal cluster among the typically developed group (N=267), the ADHD group with the combined subtype (N=129), and the ADHD group with the inattentive subtype (N=96). Panel D depicts the difference in gray matter volume of the posterior occipital cluster among the typically developed group (N=267), the ADHD group with the combined subtype (N=129), and the ADHD group with the inattentive subtype (N=96). Panel E depicts the difference in gray matter volume of the prefrontal cluster among the typically developed group (N=267), the medicated ADHD group (N=56), and the never-medicated ADHD group (N=99). Panel F depicts the difference in gray matter volume of the posterior occipital cluster among the typically developed group (N=267), the medicated ADHD group (N=56), and the never-medicated ADHD group (N=99).
**p<0.01. ***p<0.001.
Medication Effects
In the ADHD-200 sample, we found that the typically developed control subjects (N=267) had the highest gray matter volumes of both clusters, the medicated patients (N=56) had intermediate gray matter volumes, and the never-medicated patients (N=99) had the lowest gray matter volumes. Compared with the control subjects, the never-medicated patients had significant group differences in both clusters (prefrontal cluster: F=12.37, df=1, 357, p<0.001; η2p=0.033; posterior occipital cluster: F=8.50, df=1, 357, p=0.004; η2p=0.023) (Figure 2E–F). However, the group differences between the typically developed control subjects and the medicated patients became nonsignificant. The corresponding effect sizes were significantly decreased compared with that for the never-medicated patients (prefrontal cluster: η2p(control vs. medicated)−η2p(control vs. never-medicated)=−0.031, 95% CI=−0.068, −0.004; posterior occipital cluster: −0.022, 95% CI=−0.055, 0.000 [given by 5,000 bootstraps]). Therefore, these findings were unlikely to be explained by group differences in either demographic characteristics or symptom severity between the medicated and the never-medicated patients (see eMethods 1 and Table S3 in the online supplement).
Discussion
To our knowledge, this is the first study to differentiate the neuroanatomical basis for the cognitive and motivational pathways of ADHD in a large population-based cohort of adolescents. The neuroimaging finding of a common neuroanatomical correlate, namely, gray matter volume of the posterior occipital cluster, shared by both cognitive and motivational deficits suggests an overlapping neuroanatomical basis for the dual-pathway model of ADHD. Intriguingly, the study also revealed associations of gray matter volume of this cluster with both future symptoms of and polygenic risk for ADHD in the population-based cohort. Compared with typically developed control subjects, never-medicated patients with ADHD had the lowest gray matter volume of this cluster and medicated patients had an intermediate gray matter volume. These findings demonstrate that this neuroanatomical feature has the potential to serve as an intermediate phenotype of ADHD.
Our findings in this neuroimaging study support an involvement of the visual attention network and emphasize the importance of the large-effect cognitive impairment seen in previous behavioral studies in visual attention specifically, as compared with auditory attention (50, 51). First, both identified regions, the prefrontal and posterior occipital clusters, are located in the visual attention network, and second, a prospective association of gray matter volume of the posterior occipital clustered selectively with the inattention score assessed 2 years later. Abnormal brain activities of the visual attention network have been associated with ADHD in functional neuroimaging studies (52), which constitute findings that are complementary to those of the present structural neuroimaging study. In this functional network, the occipital cortex interacts with the dorsal attention network to maintain visual attention (53) and suppress attention to irrelevant visual stimuli by a top-down modulation of the prefrontal cortex (54). These brain regions are also structurally wired together, and the inferior fronto-occipital fasciculus in particular is a direct pathway that connects the frontal and occipital lobes as well as the parietal and posterior temporal cortices (55). A 2016 meta-analysis of diffusion tensor imaging studies on ADHD reported consistent white matter differences in the left inferior fronto-occipital fasciculus (56), which had been implicated in attention (57, 58). The hypothesis is that these regions may be modulated by dopamine activity. In addition to the well-known effect of dopamine on the prefrontal region (59–62), a 2018 study found that the dopamine transporter gene (DAT1)-related reduction of gray matter volume in the left posterior occipital region may contribute to visual memory performance in children with ADHD (63).
Our comparison between medicated and never-medicated patients with ADHD may suggest a positive effect of medication on gray matter atrophy in patients with ADHD. Stimulant medication for ADHD affects the brain in manifold ways, including in its structure (64, 65) and function (66) and in neurotransmission (67). Therefore, lower gray matter volumes of the identified clusters in never-medicated patients with ADHD may exclude one alternative explanation, namely, that lower gray matter volumes were caused secondarily by medication, while comparable gray matter volumes between medicated patients and typically developed control subjects suggest that medication may have a remedial effect on brain structure in ADHD, which provides a possible neuroanatomical basis for the behavioral improvement in visual attention by stimulant treatment for ADHD (68).
The dual-pathway model of ADHD has been a valuable model for our understanding of the neuropsychopathology of this disorder (5–7). Our findings do not support the independent-pathways model (i.e., the cognitive circuit between dorsolateral prefrontal cortex and dorsal striatum and the motivational circuit between orbitofrontal cortex and ventral striatum) (5) but instead demonstrate an interaction between cognition and motivation in ADHD. This interaction not only is supported by the associations between working memory and delay discounting and between intrasubject variability and delay discounting in the IMAGEN sample but also is supported by previous reports of both monetary incentive–enhanced cognition and cognitive bias–enhanced avoidance motivation (69). Our findings further suggest that this interaction may have its neural basis within the visual attention network, especially the left cuneus in the posterior occipital cortex, and hyperactivation of this region has previously been reported in task-based fMRI experiments (70). Its association with delay discounting is not completely surprising given that both hyperactivation (71, 72) and higher gray matter volume (73) of the posterior occipital cortex have already been associated with choosing delayed gain over immediate reward. Its association with working memory performance (i.e., 2-back accuracy) has also been observed in an fMRI experiment (74). Together, these findings may suggest that the dual pathways in ADHD are likely related to dysfunction of these cognitive and motivational processes, particularly in the visual attentional system, which supports the top-down selection of relevant information from the environment during goal-directed tasks (75).
It has been reported that even a small improvement in memory score (10%) can make a significant difference in school performance (76). The effect sizes of the identified neural associations in our study were small to medium, partially owing to the multifactorial nature of ADHD. However, as shown in the results, these findings were statistically robust and empirically replicable using an independent clinical sample. Therefore, the findings may improve the accuracy of the diagnosis by using gray matter volume of the posterior occipital cluster as an intermediate phenotype of ADHD, especially the inattention symptoms. This neuroanatomical feature 1) is associated with inattention in the general population; 2) is associated with neuropsychological endophenotypes of ADHD; 3) is associated with genetic risk for ADHD; 4) contributes to the explanation of future inattention symptoms; and 5) is preserved in clinical patients with ADHD and cannot be simply explained by a confounding effect of medication. These are exactly the lines of evidence that are required for the identification of an intermediate phenotype of a mental health disorder (27). The occipital cluster develops early in life and functionally matures during childhood (77), and therefore it may also be used as a neuroimaging biomarker of disrupted brain development for the early diagnosis of ADHD. As expected, we also found that the ADHD-associated prefrontal cluster was indeed correlated with delay discounting, which is consistent with the frontostriatal model of ADHD (78). However, given that this prefrontal area is under significant development during adolescence (79), with a significant degree of individual variation (80), it may be difficult to use gray matter volume of this prefrontal cluster as an imaging trait marker for ADHD.
Several limitations of this study should be mentioned. Because both parent and teacher ratings are needed for clinical diagnosis, our study using parent ratings may not have fully captured ADHD symptoms. This study identified a common neural correlate for working memory—attention regulation and delay discounting—which represents particular aspects of the broader cognitive and motivational deficits in ADHD. However, the study did not identify any significant association of response inhibition with the ADHD symptoms. Our findings are in adolescents, but the development and maturation of the posterior occipital cortex are believed to be largely completed during childhood. Longitudinal neuroimaging in an ADHD cohort during childhood will be necessary to confirm whether any abnormal development of this occipital cortex can be observed leading to the manifestation of ADHD symptoms.
Conclusions
Using a comprehensive approach, we revealed a common neuroanatomical correlate of both cognitive and motivational pathways for the development of ADHD. Given that the posterior occipital region develops and matures much earlier than the prefrontal areas previously focused on in the frontostriatal model of ADHD, these results may provide new clues to discovering novel imaging markers for early diagnosis and preemptive intervention strategies for ADHD.
1 : The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics 2012; 9:490–499Crossref, Medline, Google Scholar
2 : The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol 2002; 111:279–289Crossref, Medline, Google Scholar
3 : Childhood predictors of adult attention-deficit/hyperactivity disorder: results from the World Health Organization World Mental Health Survey Initiative. Biol Psychiatry 2009; 65:46–54Crossref, Medline, Google Scholar
4 : Heterogeneity in ADHD: neuropsychological pathways, comorbidity, and symptom domains. J Abnorm Child Psychol 2009; 37:551–564Crossref, Medline, Google Scholar
5 : The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neurosci Biobehav Rev 2003; 27:593–604Crossref, Medline, Google Scholar
6 : Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry 2005; 57:1231–1238Crossref, Medline, Google Scholar
7 : Psychological heterogeneity in AD/HD: a dual pathway model of behaviour and cognition. Behav Brain Res 2002; 130:29–36Crossref, Medline, Google Scholar
8 : A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2005; 44:377–384Crossref, Medline, Google Scholar
9 : Reaction time variability in ADHD: a meta-analytic review of 319 studies. Clin Psychol Rev 2013; 33:795–811Crossref, Medline, Google Scholar
10 : Response inhibition and ADHD traits: correlates and heritability in a community sample. J Abnorm Child Psychol 2013; 41:497–507Crossref, Medline, Google Scholar
11 : Hyperactivity and delay aversion, I: the effect of delay on choice. J Child Psychol Psychiatry 1992; 33:387–398Crossref, Medline, Google Scholar
12 : “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biol Psychiatry 2011; 69:e69–e87Crossref, Medline, Google Scholar
13 : Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2010; 49:345–355Medline, Google Scholar
14 : A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making, and variability in attention deficit hyperactivity disorder: advancing beyond the three-pathway models. Psychol Med 2014; 44:1989–2001Crossref, Medline, Google Scholar
15 : Do executive deficits and delay aversion make independent contributions to preschool attention-deficit/hyperactivity disorder symptoms? J Am Acad Child Adolesc Psychiatry 2003; 42:1335–1342Crossref, Medline, Google Scholar
16 : Cool and hot executive functions in medication-naive attention deficit hyperactivity disorder children. Psychol Med 2011; 41:2593–2602Crossref, Medline, Google Scholar
17 : Do delay aversion and executive function deficits make distinct contributions to the functional impact of ADHD symptoms? A study of early academic skill deficits. J Child Psychol Psychiatry 2007; 48:1061–1070Crossref, Medline, Google Scholar
18 : The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. J Abnorm Child Psychol 2001; 29:215–228Crossref, Medline, Google Scholar
19 : Greater delay discounting among girls, but not boys, with ADHD correlates with cognitive control. Child Neuropsychol 2018; 24:1026–1046Crossref, Medline, Google Scholar
20 : Examining relationships between executive functioning and delay aversion in attention deficit hyperactivity disorder. J Clin Child Adolesc Psychol 2011; 40:837–847Crossref, Medline, Google Scholar
21 : Visuospatial working memory underlies choice-impulsivity in boys with attention-deficit/hyperactivity disorder. Res Dev Disabil 2015; 38:134–144Crossref, Medline, Google Scholar
22 : Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry 2011; 69:260–265Crossref, Medline, Google Scholar
23 : Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biol Psychiatry 2014; 75:435–448Crossref, Medline, Google Scholar
24 : Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci 2012; 16:17–26Crossref, Medline, Google Scholar
25 : Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: a comparative meta-analysis. JAMA Psychiatry 2016; 73:815–825Crossref, Medline, Google Scholar
26 : Discoveries on the genetics of ADHD in the 21st century: new findings and their implications. Am J Psychiatry 2018; 175:943–950Link, Google Scholar
27 : Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci 2006; 7:818–827Crossref, Medline, Google Scholar
28 : The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry 2010; 15:1128–1139Crossref, Medline, Google Scholar
29 : a model to advance the translational potential of neuroimaging in clinical neuroscience. Front Syst Neurosci 2012; 6:62Medline, Google Scholar
30 : The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry 1997; 38:581–586Crossref, Medline, Google Scholar
31 : White matter microstructure is associated with hyperactive/inattentive symptomatology and polygenic risk for attention-deficit/hyperactivity disorder in a population-based sample of adolescents. Neuropsychopharmacology 2019; 44:1597–1603Crossref, Medline, Google Scholar
32 : Ventromedial prefrontal volume in adolescence predicts hyperactive/inattentive symptoms in adulthood. Cereb Cortex 2019; 29:1866–1874Crossref, Medline, Google Scholar
33 : Inattention and reaction time variability are linked to ventromedial prefrontal volume in adolescents. Biol Psychiatry 2017; 82:660–668Crossref, Medline, Google Scholar
34 : Distinct brain structure and behavior related to ADHD and conduct disorder traits. Mol Psychiatry (Epub ahead of print, August 14, 2018)Google Scholar
35 : Measurement reliability and agreement in psychiatry. Stat Methods Med Res 1998; 7:301–317Crossref, Medline, Google Scholar
36 : Psychometric properties of the Strengths and Difficulties Questionnaire. J Am Acad Child Adolesc Psychiatry 2001; 40:1337–1345Crossref, Medline, Google Scholar
37 : Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen 1999; 128:78–87Crossref, Medline, Google Scholar
38 : Self-discipline outdoes IQ in predicting academic performance of adolescents. Psychol Sci 2005; 16:939–944Crossref, Medline, Google Scholar
39 : Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med 1992; 85:399–402Medline, Google Scholar
40 : Practitioner review: computerized assessment of neuropsychological function in children: clinical and research applications of the Cambridge Neuropsychological Testing Automated Battery (CANTAB). J Child Psychol Psychiatry 2003; 44:649–663Crossref, Medline, Google Scholar
41 : Executive function and attention deficit hyperactivity disorder: stimulant medication and better executive function performance in children. Psychol Med 1999; 29:527–538Crossref, Medline, Google Scholar
42 : Individual differences in stop-related activity are inflated by the adaptive algorithm in the stop signal task. Hum Brain Mapp 2018; 39:3263–3276Crossref, Medline, Google Scholar
43 : Association of a schizophrenia-risk nonsynonymous variant with putamen volume in adolescents: a voxelwise and genome-wide association study. JAMA Psychiatry 2019; 76:435–445Crossref, Medline, Google Scholar
44 : Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J Int Neuropsychol Soc 2009; 15:331–343Crossref, Medline, Google Scholar
45 : Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 2016; 113:7900–7905Crossref, Medline, Google Scholar
46 : MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011; 42 (https://www.jstatsoft.org/index.php/jss/article/view/v042i08/v42i08.pdf)Crossref, Google Scholar
47 : Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet 2019; 51:63–75Crossref, Medline, Google Scholar
48 : Association of genetic risk variants with attention-deficit/hyperactivity disorder trajectories in the general population. JAMA Psychiatry 2016; 73:1285–1292Crossref, Medline, Google Scholar
49 : PRSice: polygenic risk score software. Bioinformatics 2015; 31:1466–1468Crossref, Medline, Google Scholar
50 : Visual cortex abnormalities in adults with ADHD: a structural MRI study. World J Biol Psychiatry 2011; 12:260–270Crossref, Medline, Google Scholar
51 : Auditory and visual attention performance in children with ADHD: the attentional deficiency of ADHD is modality specific. J Atten Disord 2017; 21:856–864Crossref, Medline, Google Scholar
52 : Topological organization of the “small-world” visual attention network in children with attention deficit/hyperactivity disorder (ADHD). Front Hum Neurosci 2014; 8:162Crossref, Medline, Google Scholar
53 : Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. J Neurosci 2009; 29:4392–4407Crossref, Medline, Google Scholar
54 : Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J Neurosci 2009; 29:5863–5872Crossref, Medline, Google Scholar
55 : A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 2008; 44:1105–1132Crossref, Medline, Google Scholar
56 : A systematic review and meta-analysis of tract-based spatial statistics studies regarding attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev 2016; 68:838–847Crossref, Medline, Google Scholar
57 : Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 1997; 121:65–94Crossref, Medline, Google Scholar
58 : Selective frontal neurodegeneration of the inferior fronto-occipital fasciculus in progressive supranuclear palsy (PSP) demonstrated by diffusion tensor tractography. BMC Neurol 2011; 11:13Crossref, Medline, Google Scholar
59 : Effect of dopamine transporter gene (SLC6A3) variation on dorsal anterior cingulate function in attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 2010; 153B:365–375Crossref, Medline, Google Scholar
60 : Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006; 188:567–585Crossref, Medline, Google Scholar
61 : Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Mol Psychiatry 2005; 10:678–685Crossref, Medline, Google Scholar
62 : Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry 2006; 60:1111–1120Crossref, Medline, Google Scholar
63 : A haplotype of the dopamine transporter gene modulates regional homogeneity, gray matter volume, and visual memory in children with attention-deficit/hyperactivity disorder. Psychol Med 2018; 48:2530–2540Crossref, Medline, Google Scholar
64 : Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand 2012; 125:114–126Crossref, Medline, Google Scholar
65 : Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry 2011; 168:1154–1163Link, Google Scholar
66 : Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol Psychiatry 2014; 76:616–628Crossref, Medline, Google Scholar
67 : A positron emission tomography study of nigro-striatal dopaminergic mechanisms underlying attention: implications for ADHD and its treatment. Brain 2013; 136:3252–3270Crossref, Medline, Google Scholar
68 : Visual attention in adults with attention-deficit/hyperactivity disorder before and after stimulant treatment. Psychol Med 2019; 49:2617–2625Crossref, Medline, Google Scholar
69 : Relationships among cognition, emotion, and motivation: implications for intervention and neuroplasticity in psychopathology. Front Hum Neurosci 2013; 7:261Crossref, Medline, Google Scholar
70 : Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry 2013; 70:185–198Crossref, Medline, Google Scholar
71 : Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp Brain Res 2007; 179:643–653Crossref, Medline, Google Scholar
72 : Activity in dlPFC and its effective connectivity to vmPFC are associated with temporal discounting. Front Neurosci 2014; 8:50Crossref, Medline, Google Scholar
73 : Neuroanatomical foundations of delayed reward discounting decision making. Neuroimage 2017; 161:261–270Crossref, Medline, Google Scholar
74 : Distinct functional and structural neural underpinnings of working memory. Neuroimage 2018; 174:463–471Crossref, Medline, Google Scholar
75 : The attentive brain: insights from developmental cognitive neuroscience. Nat Rev Neurosci 2015; 16:606–619Crossref, Medline, Google Scholar
76 Academy of Medical Sciences Working Group (Chaired by Professor Sir Gabriel Horn): Brain Science, Addiction, and Drugs (Addiction and Drugs Project). London, Academy of Medical Sciences, 2008Google Scholar
77 : Unravelling the development of the visual cortex: implications for plasticity and repair. J Anat 2010; 217:449–468Crossref, Medline, Google Scholar
78 : Investing in the future: stimulation of the medial prefrontal cortex reduces discounting of delayed rewards. Neuropsychopharmacology 2015; 40:546–553Crossref, Medline, Google Scholar
79 : Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci Biobehav Rev 2016; 70:4–12Crossref, Medline, Google Scholar
80 : Studying individual differences in human adolescent brain development. Nat Neurosci 2018; 21:315–323Crossref, Medline, Google Scholar



