Associations of Benzodiazepines, Z-Drugs, and Other Anxiolytics With Subsequent Dementia in Patients With Affective Disorders: A Nationwide Cohort and Nested Case-Control Study
Abstract
Objective:
Benzodiazepines and Z-drugs are two of the most prescribed agents worldwide. However, because of their cognitive side effects, the question of their influence on the risk of dementia has been raised. The authors examined the association of benzodiazepines, Z-drugs, and other anxiolytics with incident dementia in patients with affective disorders.
Methods:
The authors conducted a cohort and nested case-control study of 235,465 patients over age 20 who were identified in the Danish National Patient Registry as having had a first-time hospital contact for an affective disorder between 1996 and 2015. From the Danish National Prescription Registry, information was obtained on all prescriptions for benzodiazepines, Z-drugs, and other anxiolytics, and patients were followed for incident dementia (defined by hospital discharge diagnosis or acetylcholinesterase inhibitor use). Cox proportional hazards and conditional logistic regression models were used to calculate hazard ratios and odds ratios with adjustment for sociodemographic and clinical variables.
Results:
A total of 75.9% (N=171,287) of patients had any use of benzodiazepines or Z-drugs, and during the median follow-up of 6.1 years (interquartile range, 2.7–11), 9,776 (4.2%) patients were diagnosed with dementia. Any use of benzodiazepines or Z-drugs showed no association with dementia after multiple adjustments in either the cohort analysis or a nested case-control design. In the cohort analysis, the number of prescriptions and the cumulated dose of benzodiazepines or Z-drugs at baseline were not associated with dementia. In the nested case-control study, where prescriptions were counted from 1995 until 2 years before the index date, there was a slightly higher odds ratio of dementia in patients with the lowest use of benzodiazepines or Z-drugs (odds ratio=1.08, 95% CI=1.01, 1.15) compared with no lifetime use. However, patients with the highest use had the lowest odds of developing dementia (odds ratio=0.83, 95% CI=0.77, 0.88).
Conclusions:
This large cohort study did not reveal associations between use of benzodiazepines or Z-drugs and subsequent dementia, even when exposures were cumulated or divided into long- and short-acting drugs. Some results were compatible with a protective effect.
Benzodiazepines have been available primarily as anxiolytics and hypnotics since 1960, when they gradually replaced more toxic drugs, such as barbiturates, meprobamate, and chloral hydrate (1). After some time, it was realized that some patients began to develop addiction or tolerance to benzodiazepines, and in recent decades, prescriptions for benzodiazepines have been restricted in many countries. In 1980, benzodiazepine-related drugs referred to as Z-drugs, assumed to be less addictive than benzodiazepines, were introduced and approved for insomnia (2). However, because benzodiazepines are effective drugs for patients with acute anxiety and severe sleeping disturbances, they are along with Z-drugs among the most prescribed agents in many countries (2, 3). Consequently, the potential short- and long-term side effects of benzodiazepines have received considerable attention, and several studies on potential health hazards associated with both benzodiazepines and Z-drugs have been published (4). Because benzodiazepines and Z-drugs are associated with cognitive side effects in many patients (2, 5), the question of their influence on the risk of dementia in the longer term has been raised (6). Reviews and meta-analyses have examined the risk of dementia associated with benzodiazepines (7, 8). Recently published meta-analyses based on five cohort studies and 10 case-control studies found higher odds ratios (pooled odds ratio=1.38, 95% CI=1.07, 1.77) of dementia associated with any use of benzodiazepines (7). The risk estimate was attenuated but remained significant after accounting for reverse causality and confounding by indication from comorbid depression and anxiety. However, results from the few studies that have explored any dose relationship have been inconsistent, and the mechanism by which benzodiazepine use may increase the risk of dementia is far from obvious (6).
Current evidence on benzodiazepines and dementia risk suggests that larger studies with enough power to infer differences between long-acting and short-acting benzodiazepines, and between various exposure loads (duration and dose), are needed (4, 6, 7). However, the largest limitation of previous observational studies is inappropriate adjustment for factors associated with selection into treatment (confounding by indication). Affective disorders are closely associated with dementia risk and often prompt initiation of treatment with benzodiazepines, and when affective disorders are not considered, a flawed association between initiation of benzodiazepines and dementia may be present. In addition, although most previous cohort studies have been based on older populations and benzodiazepines are used in terminal care (9), no previous study has accounted for competing mortality.
Our aim in this study was to examine whether treatment with benzodiazepines and Z-drugs was associated with increased rates of subsequent dementia while minimizing the risk of confounding by indication. We analyzed prescription registry data for a cohort of patients with affective disorders, which is assumed to be more homogeneous regarding the severity of psychopathology and thereby limits confounding by indication. In addition, we explored whether the rates of dementia differed in relation to drug type (benzodiazepines, Z-drugs, and long-acting and medium- and short-acting drugs) and the timing and duration of treatment. Finally, we assessed confounding by indication by including drugs also used for anxiety (i.e., pregabalin, buspirone, hydroxyzine) as a negative control exposure (i.e., with similar confounding but no assumed shared mechanistic pathogenesis).
Methods
Study Population and Follow-Up
Patients were citizens of Denmark with a first-time hospital contact due to an affective disorder from January 1996 through December 2015. A total of 268,218 inpatients and outpatients were identified in the Danish National Patient Registry (10) using ICD-10 codes F30.0–F39.9. A total of 22,677 individuals under age 20 or with missing information pertaining to their birth date were excluded from this patient cohort. The cohort was followed from 1969 until 2016 for a dementia diagnosis or prescriptions for dementia medication as documented in the Danish Psychiatric Central Research Register, the Danish National Patient Registry, or the Danish National Prescription Registry. During the follow-up, 19,852 patients with incident dementia were identified. Among these, 10,076 had a diagnosis before or at study entry and were excluded from the main analyses.
Based on this information, a case-control study was nested within the cohort using risk set sampling generated by the procedure sttocc in Stata. Briefly, we randomly selected control subjects among members of the cohort who remained dementia free and matched them on follow-up time with case subjects (N=9,776) who reached the same age at the time of dementia diagnosis in a 1:4 ratio (N=39,104). They represented a subsample of the followed person-time (Figure 1).
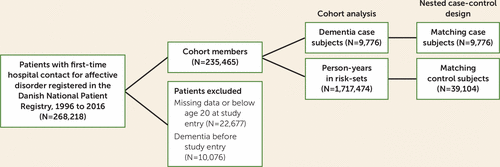
FIGURE 1. Flow diagram of cohort selection in a study of dementia and benzodiazepines, Z-drugs, and other anxiolytics
The study was approved by the regional Danish Data Inspection. Individual-level consent was not required, and all data were anonymized.
Outcome Definition
Dementia was defined as a first inpatient or outpatient hospital discharge diagnosis of dementia (ICD-8 codes 290.00–290.99; ICD-10 codes F00.00–F03.9 and G30.0–G30.9) as documented in the Danish Psychiatric Central Research Register and the Danish National Patient Registry. The validity of dementia diagnoses obtained from the Danish National Patient Registry has been assessed in two studies (11, 12) reporting that 70% and 83%, respectively, of dementia cases diagnosed by an external rater according to ICD-10 criteria conformed with the diagnosis in the register, with the lowest validity in younger patients. We supplemented our definition of dementia with data on individual patient refills of at least one redeemed prescription of an acetylcholinesterase inhibitor identified by the Anatomic Therapeutic Chemical (ACT) classification system codes (N06D) in the Danish National Prescription Registry. This register holds information on all prescribed and redeemed drugs sold at Danish pharmacies since 1995 (13).
Exposure Definition (Benzodiazepines and Related Drugs)
All refills of prescriptions for medication were identified in the Danish National Prescription Registry, where the quantity dispensed for each prescription is expressed by the defined daily dose. We used ACT codes to define benzodiazepines (N05BA, N05CD, and N03AE01), Z-drugs (N05CF), and other anxiolytics (N05BB01, N05BE01, and N03AX16). Benzodiazepines and Z-drugs were also classified into short- and medium-acting (N05BA04, 06, 12; N05CD04–09, 11; and N05CF01–03) and long-acting (N03AE01; N05BA01–03, 05, 08, 09, 11, 16; and N05CD01–03), as recommended by other investigators (3, 14). To assess dose dependence, we calculated the number of prescriptions, the sum of all prescribed defined daily doses registered in the Danish National Prescription Registry, and the intensity of use as the cumulative amount divided by the duration of use. Because benzodiazepine use can be either chronic or episodic, we assigned each prescription an exposure period of 105 days based on an analysis of waiting-time distributions used in previous Danish register-based studies (15). Because we found no evidence for specific cut-points for cumulative use, it was categorized on the basis of the distribution of exposure. In addition, we modeled restricted cubic splines with four knots equally spaced over the variables to examine whether the results were influenced by the cut-points chosen for exposure categories.
Covariates
We selected the following covariates, assumed to be associated with both benzodiazepine use and dementia risk, from the Danish National Patient Registry and the Danish National Prescription Registry: depression subtype, year of diagnosis, history of alcohol or mixed substance abuse, diabetes or cardiovascular disease, and prescriptions for antipsychotic (ACT code N05A) and antidepressant (ACT code N06A) medications. Data on the highest achieved educational level were obtained from the Danish Population Education Register and categorized as low, middle, high, and unknown. Information on gender, age, and marital status was obtained from the Danish Civil Registration System.
The ACT codes used for definitions of exposure, outcome, and covariates are listed in Table S1 in the online supplement.
Statistical Analysis
For comparison with previous studies, we analyzed our data using both a cohort design and a nested case-control design. The nested case-control design is suitable for analysis of time-varying exposures, such as benzodiazepines. In the cohort study, we analyzed the associations between any use of benzodiazepines (overall and divided according to type of medication (benzodiazepines, Z-drugs, and other anxiolytics and short- or medium-acting and long-acting drugs) and subsequent dementia by using Cox proportional hazards regression (hazard ratios and 95% confidence intervals), with age as an underlying time scale. Person-years of follow-up were accumulated from age at study entry (date of the first registered affective disorder) and terminated at age at first registration of dementia or death or at the end of follow-up (October 31, 2016), whichever came first. Because the mechanism linking benzodiazepines to the development of dementia is unknown (6), we used different approaches to model benzodiazepine exposure. For comparison with previous studies, we first analyzed benzodiazepine exposure (any use) at study entry (baseline) and further evaluated any interactions between the different types of benzodiazepines using the likelihood-ratio test. Second, we included benzodiazepines initiated during follow-up. Therefore, to account for immortal time bias, benzodiazepine exposure was entered as a time-varying variable in these analyses. This measure of benzodiazepine exposure included current and former (prevalent) users as well as new (incident) users, and we evaluated the effect of timing of benzodiazepine use on dementia by defining a supplementary time-varying exposure matrix including both pre- and postdiagnostic benzodiazepine use, categorized into four categories: no prior postdiagnostic use (no lifetime use, reference group), prediagnostic but no postdiagnostic use (former use), pre- and postdiagnostic use (continuing use), and postdiagnostic but no prediagnostic use (new use). Finally, we analyzed the associations with cumulated use of benzodiazepines at baseline as exposure. Long-term users may represent a selected group of patients. To address this potential bias, we repeated the analyses for benzodiazepine use cumulated 2 years before study entry, which may reveal more immediate effects (e.g., whether benzodiazepines influence the course of presymptomatic dementia). However, prodromal symptoms of dementia may be diagnosed as depression. Therefore, to account for reverse causality (protopathic bias), we delayed follow-up for 2 years using the stsplit option in Stata to divide the follow-up time into 0–2 years and >2 years. Physicians may interpret cognitive deficits in patients treated with benzodiazepines as side effects and thus be less likely to diagnose these patients with dementia the first time after hospital admission for an affective disorder. In our analyses, any such bias may be accounted for by the 2-year latency period. The assumption of proportional hazards was assessed by inspection of cumulative hazard plots and trends in scaled Schoenfeld residuals. The analyses for any benzodiazepine use and cumulated benzodiazepine use were repeated in a nested case-control design, where odds ratios for benzodiazepine and related drug use estimated from 1995 until 2 years before the index date (date of dementia diagnosis or control assignment) were calculated using conditional logistic regression. The odds ratio in the nested case-control analysis is largely equivalent to the hazard ratio in the cohort study. We accounted for confounding by selection of patients who received benzodiazepines by using multiple adjustment in models with all covariates mentioned above included. The analyses were performed using Stata, release 15.
Sensitivity Analysis
We performed two sensitivity analyses to explore the robustness of our results. First, the exclusion of patients who have a history of the event under study (dementia in this study) before study entry may cause bias in a prevalent user design (16). This exclusion may have resulted in a reference population representing patients who were less likely to develop dementia during the period of exposure. We explored this potential selection by comparing premorbid use of benzodiazepines among the 9,671 patients who developed dementia between 1995 and study entry with benzodiazepine use among control subjects with no dementia at the same age and time before inclusion. Second, because mortality in this cohort was considerable and appeared to be related to exposure (see Table S2 in online supplement), competing mortality risk may have influenced the risk estimates, since patients who die are no longer at risk of developing dementia. Thus, to account for competing mortality risk, we used the Fine-Gray model for the cumulative incidence of dementia to estimate subdistribution hazard ratios for benzodiazepine use.
All data were obtained from administrative registers.
Results
A total of 75.9% (N=171,287) of patients with affective disorders had any use of benzodiazepines or Z-drugs (Table 1), and 63.1% (N=148,620) had at least one prescription before study entry (prevalent users). Most patients (55.7%) had used both benzodiazepines and a Z-drug, and the most frequently prescribed drugs were zopiclone, oxazepam, and diazepam (see Table S3 in the online supplement). Use of benzodiazepines and Z-drugs was most frequent among middle-aged patients with severe single-episode or recurrent depression and patients treated with antipsychotics or antidepressants at study entry (see Table S4 in the online supplement). There was considerable overlap in use of both benzodiazepines and Z-drugs, as well as between long- and short-acting drugs (see Table S5 in the online supplement). Thirteen percent of patients (N=31,431) had any use of other anxiolytics, but most had also used benzodiazepines or Z-drugs.
| Total Cohort | Case Subjects With Dementia | Density-Sampled Control Subjects | ||||
|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % |
| 235,465 | 9,776 | 39,104 | ||||
| Female | 144,002 | 61.2 | 6,414 | 65.6 | 26,883 | 68.8 |
| Marital status at baseline | ||||||
| Unmarried | 75,449 | 32.0 | 657 | 6.7 | 2,988 | 7.7 |
| Married | 92,504 | 39.3 | 4,106 | 42.1 | 17,075 | 43.8 |
| Divorced | 33,687 | 14.3 | 1,278 | 13.1 | 5169 | 13.3 |
| Widowed | 32,048 | 13.6 | 3,716 | 38.1 | 13,774 | 35.3 |
| Unknown | 1,777 | 0.8 | 19 | 0.2 | 58 | 0.4 |
| Educational level at baseline | ||||||
| Primary only (0–7 years of school) | 88,226 | 37.5 | 4,191 | 42.8 | 16,973 | 43.3 |
| Middle school | 88,724 | 37.7 | 2,450 | 25.1 | 10,281 | 26.3 |
| High school | 34,339 | 14.6 | 926 | 9.5 | 4,142 | 10.6 |
| Unknown | 24,176 | 10.3 | 2,209 | 22.6 | 7,708 | 19.7 |
| Depression subtype | ||||||
| Manic or bipolar disorder | 13,548 | 5.8 | 351 | 3.6 | 1,653 | 4.3 |
| Single or recurrent depression, mild | 31,898 | 13.6 | 1,374 | 14.1 | 5,631 | 14.4 |
| Single or recurrent depression, moderate | 65,771 | 27.9 | 2,206 | 22.6 | 9,357 | 23.9 |
| Single or recurrent depression, severe | 25,995 | 8.1 | 939 | 9.6 | 3,856 | 9.9 |
| Single or recurrent depression, other | 87,828 | 37.3 | 4,519 | 46.2 | 16,810 | 43.0 |
| Persistent or unspecified affective disorder | 10,425 | 4.4 | 387 | 4.0 | 1,797 | 4.6 |
| Psychotropic medication at baseline | ||||||
| Antipsychotic drugs | 54,051 | 24.0 | 2,479 | 25.4 | 9,870 | 25.3 |
| Antidepressant drugs | 175,307 | 74.5 | 7,258 | 74.2 | 29,620 | 75.8 |
| Comorbidity at baseline | ||||||
| Anxiety | 37,697 | 16.0 | 1,189 | 12.6 | 4,176 | 10.6 |
| Drug and alcohol abuse | 25,221 | 10.7 | 716 | 7.3 | 2,499 | 6.4 |
| Diabetes mellitus | 13,750 | 5.8 | 782 | 8.0 | 2,412 | 6.2 |
| Cardiovascular disease | 23,134 | 10.3 | 2,040 | 20.9 | 7,769 | 19.9 |
| Benzodiazepine and related drug useb | ||||||
| Any use | 179,249 | 76.1 | 7,942 | 81.2 | 33,666 | 86.1 |
| Any benzodiazepine use | 150,334 | 63.8 | 7,961 | 72.3 | 30,273 | 77.7 |
| Any Z-drug use | 128,683 | 54.7 | 5,170 | 52.9 | 23,691 | 60.6 |
| Any other anxiolytic use | 31,428 | 13.4 | 700 | 7.2 | 5,066 | 12.6 |
| Any long-acting drug use | 93,151 | 39.6 | 4,611 | 47.2 | 19,869 | 50.8 |
| Any short- and medium-acting drug use | 165,662 | 70.4 | 7,132 | 72.0 | 30,883 | 79.0 |
TABLE 1. Demographic and clinical characteristics of patients with affective disorders in a study of dementia and benzodiazepines, Z-drugs, and other anxiolyticsa
During the median follow-up of 6.1 years (interquartile range, 2.7–11 years), 9,776 patients (4.2%) were diagnosed with dementia (incidence rate: 57.1 per 10,000 person-years, 95% CI=56.0, 58.2). Patients who developed dementia were more often over age 70, less educated, widowed, and had a diagnosis of diabetes or cardiovascular disease (Table 1).
Any Use of Benzodiazepines, Related Drugs, and Dementia
Any use of benzodiazepines, Z-drugs, or other anxiolytics was not associated with subsequent dementia after adjustment for sociodemographic and clinical variables in the cohort analysis or the nested case-control design (Table 2). However, in the cohort study, benzodiazepine use was associated with lower risk of dementia during the first 2 years after study entry. The estimates for benzodiazepine use or long-acting drug use were slightly attenuated when adjusting for any use of Z-drugs or short-acting drugs, respectively. In addition, any use of benzodiazepines did not appear to influence the effect of Z-drugs on dementia. Similarly, use of long-acting drugs did not modify the association of short-acting drugs with dementia (Figure 2; also see Table S6 in the online supplement). Second, in our analyses evaluating the timing of benzodiazepine use, continued and new users showed a reduction in dementia rates compared with patients with no lifetime use, whereas the association was closer to unity among former users (see Table S7 in the online supplement).
| Variable | Age- and Gender-Adjusted Model | Fully Adjusted Modela | Fully Adjusted Model Plus Other Drugsb | |||
|---|---|---|---|---|---|---|
| Cohort study | ||||||
| Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | |
| Baseline | ||||||
| 0–2 years of follow-up, number of outcomes (N=5,085) | ||||||
| Any use | 0.69 | 0.66, 0.74 | 0.70 | 0.65, 0.75 | NA | |
| Benzodiazepine use | 0.72 | 0.67, 0.76 | 0.72 | 0.67, 0.76 | 0.74 | 0.69, 0.78 |
| Z-drug use | 0.77 | 0.73, 0.82 | 0.79 | 0.74, 0.84 | 0.82 | 0.78, 0.88 |
| Long-acting drug use | 0.80 | 0.75, 0.85 | 0.80 | 0.75, 0.84 | 0.82 | 0.77, 0.87 |
| Short- and medium-acting drug use | 0.73 | 0.69, 0.77 | 0.74 | 0.70, 0.78 | 0.75 | 0.71, 0.80 |
| Other anxiolytic use | 0.79 | 0.68, 0.91 | 0.85 | 0.73, 0.98 | 0.88 | 0.76, 1.02 |
| 2–20.1 years of follow-up, number of outcomes (N=4,691) | ||||||
| Any use | 0.99 | 0.92, 1.06 | 0.95 | 0.88, 1.02 | NA | |
| Benzodiazepine use | 1.01 | 0.95, 1.09 | 0.97 | 0.91, 1.04 | 0.97 | 0.92, 1.05 |
| Z-drug use | 0.95 | 0.89, 1.00 | 0.96 | 0.90, 1.02 | 0.96 | 0.90, 1.02 |
| Long-acting drug use | 1.03 | 0.98, 1.04 | 0.99 | 0.94, 1.05 | 0.99 | 0.93, 1.05 |
| Short- and medium-acting drug use | 0.98 | 0.92, 1.04 | 0.97 | 0.91, 1.03 | 0.97 | 0.91, 1.03 |
| Other anxiolytic use | 0.95 | 0.80, 1.10 | 1.00 | 0.85, 1.18 | 1.01 | 0.86, 1.18 |
| Nested case-control study | ||||||
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| 2 years before index date (1995) | ||||||
| Any use | 1.04 | 0.99, 1.10 | 0.97 | 0.92, 1.02 | NA | |
| Benzodiazepine use | 1.11 | 1.06, 1.16 | 1.03 | 0.98, 1.08 | 1.04 | 0.98, 1.09 |
| Z-drug use | 1.01 | 0.97, 1.06 | 0.96 | 0.91, 1.01 | 0.95 | 0.91, 1.00 |
| Long-acting drug use | 1.10 | 1.05, 1.15 | 1.02 | 0.97, 1.08 | 1.02 | 0.97, 1.02 |
| Short- and medium-acting drug use | 1.07 | 1.02, 1.12 | 1.01 | 0.96, 1.06 | 1.01 | 0.95, 1.06 |
| Other anxiolytic use | 0.98 | 0.88, 1.09 | 0.91 | 0.81, 1.01 | 0.91 | 0.81, 1.01 |
TABLE 2. Hazard and odds ratios for associations of any use of benzodiazepines and related drugs at baseline with subsequent dementia in patients with affective disorder in the cohort and nested case-control studies
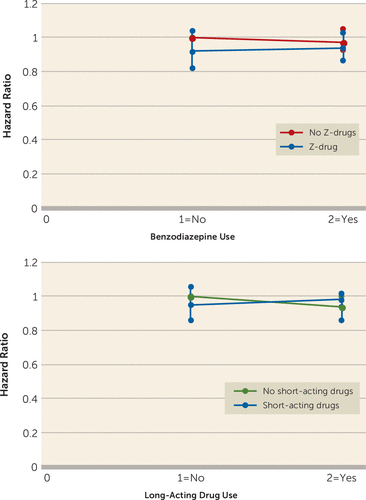
FIGURE 2. Adjusted hazard ratios and 95% confidence intervals for the interaction between drug types in a study of dementia and benzodiazepines, Z-drugs, and other anxiolyticsa
a The plot diagrams show whether use or no use of one drug type (Z-drugs or short-acting drugs) changed the effect of other drug types (benzodiazepines or long-acting drugs) on dementia in patients with affective disorders.
Cumulated Duration and Dose of Benzodiazepines and Dementia
In the cohort analysis, number of prescriptions and cumulated dose of benzodiazepines or Z-drugs at baseline were not associated with rates of dementia (Table 3). Similar results were obtained for recent prolonged use. In the nested case-control design, where prescriptions were counted from 1995 until 2 years before the index date, the odds of developing dementia were slightly higher among patients with the lowest rate of benzodiazepine use compared with patients with no lifetime use (cumulated defined daily dose [DDD]: odds ratio=1.08, 95% CI=1.01, 1.15), while those with highest use had lower odds (cumulated DDD: odds ratio=0.83, 95% CI=0.77, 0.88). This pattern was observed for all drug types (benzodiazepines, Z-drugs, and long-acting and medium- and short-acting drugs). The estimates for drug intensity (DDD/duration) were similar to those for cumulated dose. The results from exposure modeled as a continuous variable with restricted cubic splines are presented in Figure S1 in the online supplement. These curves revealed no indication of any dose-response relation.
| Measure | All Drugs | Benzodiazepines | Z-Drugs | Long-Acting Drugs | Short-Acting Drugs | Other Drugs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | Hazard ratio | 95% CI | Hazard ratio | 95% CI | Hazard ratio | 95% CI | Hazard ratio | 95% | Hazard ratio | 95% CI | |
| Cohort study (2–20.1 years of follow-up) | ||||||||||||
| Number of prescriptions | ||||||||||||
| None | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| 1–2 | 0.94 | 0.84, 1.04 | 1.00 | 0.92, 1.09 | 0.95 | 0.88, 1.03 | 0.99 | 0.91, 1.08 | 0.96 | 0.88, 1.04 | 0.94 | 0.77, 1.16 |
| 3–25 | 0.95 | 0.87, 1.03 | 0.97 | 0.90, 1.04 | 0.95 | 0.88, 1.02 | 0.98 | 0.92, 1.06 | 0.98 | 0.91, 1.05 | 1.13 | 0.86, 1.47 |
| 26 (maximum) | 0.95 | 0.87, 1.04 | 0.95 | 0.87, 1.04 | 0.98 | 0.87, 1.09 | 1.01 | 0.91, 1.11 | 0.98 | 0.89, 1.07 | 0.96 | 0.43, 2.15 |
| Total defined daily dose | ||||||||||||
| None | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Lowest third | 0.99 | 0.84, 1.03 | 0.99 | 0.90, 1.08 | 0.96 | 0.87, 1.05 | 0.95 | 0.86, 1.03 | 0.99 | 0.90, 1.08 | 0.97 | 0.76, 1.24 |
| Middle | 0.95 | 0.86, 1.03 | 0.97 | 0.89, 1.05 | 0.92 | 0.84, 1.00 | 1.02 | 0.93, 1.11 | 0.94 | 0.84, 1.02 | 1.02 | 0.78, 1.36 |
| Highest third | 0.94 | 0.86, 1.03 | 0.97 | 0.89, 1.04 | 0.98 | 0.90, 1.00 | 1.00 | 0.92, 1.08 | 0.98 | 0.89, 1.05 | 1.00 | 0.72, 1.40 |
| Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Nested case-control study (2 years before index date, 1995) | ||||||||||||
| Number of prescriptions | ||||||||||||
| None | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| 1–2 | 1.23 | 1.12, 1.35 | 1.16 | 1.06, 1.26 | 1.11 | 1.03, 1.20 | 1.07 | 0.99, 1.16 | 1.20 | 1.10, 1.31 | 1.09 | (0.94–1.26) |
| 3–25 | 1.01 | 0.95, 1.07 | 1.06 | 1.00, 1.13 | 0.98 | 0.92, 1.04 | 1.08 | 1.01, 1.15 | 1.05 | 0.99, 1.11 | 0.88 | (0.74–1.05) |
| 26 (maximum) | 0.87 | 0.82, 0.93 | 0.94 | 0.88, 1.00 | 0.84 | 0.79, 0.90 | 0.91 | 0.84, 0.97 | 0.90 | 0.84, 0.96 | 0.51 | 0.37, 0.69 |
| Total defined daily dose | ||||||||||||
| None | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Lowest third | 1.08 | 1.01, 1.15 | 1.08 | 1.01, 1.15 | 1.25 | 1.17, 1.35 | 1.05 | 0.95, –1.13 | 1.11 | (1.05–1.19) | 1.15 | 0.97, 1.36 |
| Middle | 0.99 | 0.92, 1.05 | 1.07 | 1.00, 1.14 | 1.07 | 0.98, 1.14 | 1.10 | 1.02, 1.12 | 0.97 | (0.87–1.10) | 0.86 | 0.71, 1.03 |
| Highest third | 0.83 | 0.77, 0.88 | 0.91 | 0.85, 0.91 | 0.92 | 0.86, 1.00 | 0.92 | 0.85, 0.98 | 0.86 | 0.80, 0.92 | 0.73 | 0.66, 0.88 |
TABLE 3. Adjusted hazard and odds ratios of the association between number of prescriptions and cumulated dose of benzodiazepines and related drugs and subsequent dementia in patients with affective disordersa
Sensitivity Analyses
Case subjects who were diagnosed with dementia before study entry appeared to have the same rate of benzodiazepine use as control subjects. Thus, the odds ratio for dementia among patients with any benzodiazepine use before study entry was 0.93 (95% CI=0.87, 1.00) (see Table S8 in the online supplement). The mean age of the cohort at baseline was 73.2 years (SD=11.7), and 28.9% (N=68,048) of the patients died during the follow-up period (see Table S2 in the online supplement). However, the associations reported above did not differ when the presence of competing mortality risk was considered (see Table S9 in the online supplement).
Discussion
In this nationwide cohort of patients with affective disorders, we did not find an association between any use of benzodiazepines, Z-drugs, or other anxiolytics and subsequent dementia. Similarly, no association was observed when timing of exposure was considered or drugs were divided into long- and short-acting, and we found no apparent dose-response effect. Thus, in the cohort analysis, the number of prescriptions and the cumulated dose of benzodiazepines or Z-drugs at baseline were not associated with dementia, whereas in the nested case-control study, there were slightly higher odds of developing dementia among patients with the lowest rate of benzodiazepine or Z-drug use compared with patients with no lifetime use. However, patients with the highest rate of use appeared to have the lowest odds of developing dementia.
Comparison With Other Studies
The results from previous studies on the association between benzodiazepine use and dementia have been inconsistent. Some studies have demonstrated that benzodiazepine use is a risk factor for developing dementia (14, 17), whereas other studies, in line with ours, found no association (18, 19), including no protective association (20, 21). In a recent meta-analysis, the authors reported a 38% increased odds of dementia among users of benzodiazepines; however, the heterogeneity of the included studies was high (98%), and the 95% prediction interval of 0.58–3.25 indicated low certainty (7). The effect of elimination time has also been examined. Some investigators have found higher risk of dementia with use of long-acting compared with short-acting benzodiazepines (14, 22, 23), while others have found no difference in effect (17) or lack of effect (19). In terms of evidence regarding any association of Z-drugs specifically with dementia, findings are mainly restricted to subanalyses in benzodiazepine studies, which suggest a risk of dementia similar to that seen with benzodiazepines (4). However, few studies have accounted for simultaneous use of benzodiazepines (21). In the present study, we explored both the independent effect of each drug type (benzodiazepines, Z-drugs, and long-acting and medium- and short-acting drugs) on dementia risk and any interactions. This revealed that use of one drug type did not explain or modify the effect of other drug types. Studies on dose-response relationships with benzodiazepine use have been equally heterogeneous, with two case-control studies demonstrating a dose-response effect (14, 23), one study revealing an increased risk with a smaller cumulative dose only (24) (in line with our results), and three studies showing no trend (17, 19, 21). Our results are consistent with those from a Swiss case-control study (21) and a cohort study conducted in the United States using computerized pharmacy data (24). These two studies showed no association between benzodiazepines or Z-drugs and dementia. In line with our results, the Swiss study suggested a protective effect of the highest cumulative dose when adjusting for depression measured by antidepressant use (21). Most previous studies also accounted for reverse causality by introducing a latency period (lag time) before the outcomes were counted in order to account for the potential bias that may occur when benzodiazepines are used to treat prodromal symptoms of dementia. In our study, benzodiazepine use was associated with lower risk of dementia the first 2 years after study entry, which may indicate that physicians are less likely to make a diagnosis of dementia in patients taking benzodiazepines shortly after they are diagnosed with an affective disorder. However, the largest methodological limitation in previous studies was confounding by indication, which may explain the weak association between benzodiazepine use and dementia revealed in some studies. Most previous studies adjusted for depression, but less than half adjusted for anxiety, and none, to our knowledge, adjusted for depression severity or alcohol abuse.
Strengths and Limitations
One strength of this study is the sample size and use of nationwide population-based registers in a country with free access to health care, which provided a large, unselected patient population. The Danish personal identification numbers allowed individual patient data to be linked with different registers and complete follow-up information for dementia outcomes with positive predictive test values >70% to be obtained (11, 12). Analyses were conducted for patients with a first-time hospital admission for an affective disorder, a group assumed to be more homogeneous regarding psychiatric comorbidity, and this restriction was assumed to reduce confounding by indication. We also accounted for confounding by adjustment for several sociodemographic variables, comorbidity, and comedication. Because benzodiazepines and Z-drugs were frequently used in this patient population, it was not possible to account for confounding in a propensity-score-matched study, since there was not a sufficient number of nonexposed individuals for matching, and a simple adjustment including a propensity score would not have added to the multiple regression performed (25, 26). We intended additionally to use other anxiolytics as a negative control exposure because these drugs may have confounding similar to that for benzodiazepines but no assumed shared pathogenesis. Thus, if the association between other anxiolytics and dementia was due to confounding, we would expect to find a similar association between the negative control exposure and dementia. Unfortunately, this analysis was hampered by considerable contemporary use of benzodiazepines. Cohort entry was defined at hospital contact. This may have limited the risk of detection bias, which may hamper studies in which patients who are not exposed to benzodiazepines or Z-drugs may have less contact with health professionals and thus be at lower risk of being diagnosed with dementia. Information on the use of benzodiazepines, Z-drugs, and other anxiolytics was obtained from nationwide registers, which limits misclassification of exposure. Benzodiazepines may be used sporadically over longer periods, and we cannot exclude the possibility that some patients who received prescriptions for benzodiazepines before 1995 were misclassified as not exposed or as new users. However, any such misclassification would need to be substantial in order to move our results away from unity. Our benzodiazepine measure was based on prevalent users, and among these, patients who remained under treatment were those who tolerated the treatment well, but we also showed that the exclusion of patients with dementia before study entry did not appear to introduce any selection that may have influenced our estimates. However, we cannot exclude the possibility that the apparent positive effect of long-term benzodiazepine use was due to depletion of susceptibility (24). We accounted for competing mortality risk. This is especially relevant when examining outcomes in elderly patients or for conditions in which mortality is more commonly observed, because the competing risk event may substantially alter the probability of the occurrence of dementia, precluding its onset. However, to our surprise, our analyses showed that this did not appear to explain the results from our cohort study.
Explanations and Implications
If these results are not due to selection bias as discussed above, they suggest that benzodiazepines are not a risk factor for dementia, and in fact there may be a protective effect of long-term benzodiazepine use on dementia risk in patients with affective disorder. This may be, as suggested, attributed to an inhibitory effect of benzodiazepines on excitotoxic or other pathological lesions or symptoms (6). Anxiety and insomnia are frequent in these patients and are also associated with risk of dementia. Thus, benzodiazepines may also exert a beneficial effect through treatment of these comorbidities.
In summary, many older people and patients with severe anxiety and sleeping problems benefit from using benzodiazepines and Z-drugs as recommended for short-term treatment. The adverse effects of benzodiazepines on cognition and studies reporting elevated risk with any use of benzodiazepines have fueled the fear of dementia among patients and clinicians. This study demonstrated that notwithstanding other possible adverse short-term or long-term effects, there is not sufficient evidence that benzodiazepines or Z-drugs increase the risk of dementia.
1 : History of benzodiazepine dependence. J Subst Abuse Treat 1991; 8:53–59Crossref, Medline, Google Scholar
2 : The acute cognitive effects of zopiclone, zolpidem, zaleplon, and eszopiclone: a systematic review and meta-analysis. J Clin Exp Neuropsychol 2014; 36:691–700Crossref, Medline, Google Scholar
3 : Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J 2013; 13:214–223Medline, Google Scholar
4 : Benzodiazepines and Z-drugs: an updated review of major adverse outcomes reported on in epidemiologic research. Drugs R D 2017; 17:493–507Crossref, Medline, Google Scholar
5 : Benzodiazepines and human memory: a review. Anesthesiol 1990; 72:926–938Crossref, Medline, Google Scholar
6 : The benzodiazepine-dementia disorders link: current state of knowledge. CNS Drugs 2016; 30:1–7Crossref, Medline, Google Scholar
7 : Association between development of dementia and use of benzodiazepines: a systematic review and meta-analysis. Pharmacotherapy 2018; 38:1010–1020Crossref, Medline, Google Scholar
8 : A systematic review and meta-analysis of the risk of dementia associated with benzodiazepine use, after controlling for protopathic bias. CNS Drugs 2018; 32:485–497Crossref, Medline, Google Scholar
9 : Association between hypnotics use and increased mortality: causation or confounding? Eur J Clin Pharmacol 2015; 71:637–642Crossref, Medline, Google Scholar
10 : The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015; 7:449–490Crossref, Medline, Google Scholar
11 : Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord 2007; 24:220–228Crossref, Medline, Google Scholar
12 : Overdiagnosis of dementia in young patients: a nationwide register-based study. Dement Geriatr Cogn Disord 2012; 34:292–299Crossref, Medline, Google Scholar
13 : Data resource profile: the Danish National Prescription Registry. Int J Epidemiol 2017; 46:798–798fMedline, Google Scholar
14 : Regular benzodiazepine and Z-substance use and risk of dementia: an analysis of German claims data. J Alzheimers Dis 2016; 54:801–808Crossref, Medline, Google Scholar
15 : Use of benzodiazepines or benzodiazepine related drugs and the risk of cancer: a population-based case-control study. Br J Clin Pharmacol 2013; 75:1356–1364Crossref, Medline, Google Scholar
16 : Point: incident exposures, prevalent exposures, and causal inference: Does limiting studies to persons who are followed from first exposure onward damage epidemiology? Am J Epidemiol 2015; 182:826–833Crossref, Medline, Google Scholar
17 : The risk of Alzheimer’s disease associated with benzodiazepines and related drugs: a nested case-control study. Acta Psychiatr Scand 2018; 138:91–100Crossref, Medline, Google Scholar
18 : The risk of cognitive impairment in older community-dwelling women after benzodiazepine use. Age Ageing 2009; 38:226–228Crossref, Medline, Google Scholar
19 : Benzodiazepine use and risk of developing Alzheimer’s disease or vascular dementia: a case-control analysis. Drug Saf 2015; 38:909–919Crossref, Medline, Google Scholar
20 : Benzodiazepines may have protective effects against Alzheimer disease. Alzheimer Dis Assoc Disord 1998; 12:14–17Crossref, Medline, Google Scholar
21 : Benzodiazepine use and risk of developing Alzheimer’s disease: a case-control study based on Swiss claims data. CNS Drugs 2017; 31:245–251Crossref, Medline, Google Scholar
22 : Benzodiazepine, psychotropic medication, and dementia: a population-based cohort study. Alzheimers Dement 2016; 12:604–613Crossref, Medline, Google Scholar
23 : Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 2014; 349:g5205Crossref, Medline, Google Scholar
24 : Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. BMJ 2016; 352:i90Crossref, Medline, Google Scholar
25 : Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol 2006; 163:262–270Crossref, Medline, Google Scholar
26 : The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014; 33:1242–1258Crossref, Medline, Google Scholar



