Amygdala on the Lookout
Conditions of uncertainty, anxiety, or fear are typically associated with amygdala hyperactivity (1, 2). Accordingly, it has long been suspected that amygdala hyperactivity contributes to paranoia. Yet past blood-oxygen-level-dependent (BOLD) functional MRI (fMRI) studies have tended to show under-recruitment of the amygdala in paranoid compared with nonparanoid individuals with schizophrenia (3, 4). These results present an explanatory challenge: why would the amygdala be hypoactive in paranoid individuals who exhibit heightened threat perception and elevated physiological arousal? To explain this paradoxical finding, some investigators hypothesized that reduced frontal control over the amygdala due to dysconnectivity of frontolimbic circuitry could lead to hypervigilance and misattribution of fear to incoming signals (3). Another possibility is that methodological limitations of BOLD fMRI have obstructed a more accurate quantification of amygdala activity.
In this issue of the Journal, a new study conducted by Pinkham and colleagues (5) offers a methodological explanation for discrepant findings concerning aberrant amygdala activity in paranoid schizophrenia. The authors definitively demonstrate higher baseline amygdala activity in paranoid compared with nonparanoid patients with schizophrenia, by employing arterial spin labeling perfusion MRI. Arterial spin labeling allows for a noninvasive and quantitative measurement of regional cerebral blood flow (CBF) by using magnetically labeled arterial blood water as an endogenous tracer (as opposed to exogenous radioactive tracers used in positron emission tomography) (6).
There are both important methodological and conceptual advances here worthy of note. In addition to arterial spin labeling, the authors acquired BOLD fMRI data while participants rated photographs of faces for trustworthiness. By measuring resting state regional CBF and task-related BOLD activity in the same participants, the authors were able to examine group differences in baseline amygdala activity alongside differences in task-related fluctuations of amygdala activity. Such comparisons are particularly relevant to schizophrenia, as prior evidence of amygdala hypoactivation relied on BOLD contrasts blind to potential group differences in baseline activity.
Indeed, Pinkham and colleagues found that acutely paranoid patients exhibited higher resting CBF in the bilateral amygdala compared with nonparanoid patients and healthy comparison subjects, who did not differ from each other. On the other hand, the BOLD fMRI task analysis revealed a general trend of amygdala hypoactivation in paranoid compared with nonparanoid participants. Thus, observed task-related amygdala hypoactivity likely reflects higher baseline amygdala activity that mitigates the stimulus-driven signal change detected by the BOLD contrast. Additional whole-brain group comparisons found greater CBF in the internal capsule, the corpus callosum, and portions of the inferior temporal and fusiform gyri in paranoid compared with nonparanoid patients. Overall, these results suggest that paranoia is related to higher resting neuronal activity in the amygdala, as well as in broader sensory and frontal regions.
These findings provide an essential step toward integrating neurobiology with existing psychological accounts of paranoia. However, questions still remain regarding the functional implications of baseline amygdala hyperactivity. For example, how does irregular tonic amygdala activity affect the filtering, learning, and memory of environmental stimuli? And to what extent is amygdala hyperactivity a trait or state characteristic malleable to symptom change? Future research should focus on delineating these pathways between regional abnormality and behavior. Nonetheless, the work by Pinkham and colleagues highlights the importance of methodology and its constraints as we seek to answer these questions.
Scientific progress often emerges with breakthroughs in methodology. For example, optical inventions led to two of the greatest paradigm shifts in the history of science. Antonie van Leeuwenhoek’s significant improvement of the microscope allowed him to observe previously undetectable microorganisms that he called “animalcules,” paving the way for the rise of the germ theory (7). Furthermore, the invention of the optical telescope allowed increasingly accurate observations of the motions of the heavenly bodies and played a pivotal role in the Copernican revolution that placed the Earth as the third rock from the sun in the heliocentric universe (8). Similarly, our capacity to visualize the workings of brain structures in vivo is revolutionizing psychiatry, but as highlighted in the work by Pinkham and colleagues, we must choose our tools wisely. This work also demonstrates the power of combining complementary techniques to capture a more comprehensive picture of brain function in health and disease.
Conceptually, this study raises questions concerning the nature of the amygdala. Given its central role in emotional processing, the amygdala merits its share of research on psychiatric conditions characterized by emotion dysfunction. Indeed, scientific interest in the amygdala has escalated, reflected by the exponential increase in animal and human studies on the amygdala since the 1960s (9). While the amygdala has been traditionally described as a threat detector, more recent work indicates the need for a broader conceptualization. Accumulating evidence of the amygdala’s role in associative learning and the rapid processing of positive, negative, and even unusual stimuli suggest that the amygdala acts as a “scout,” detecting information that is relevant, interesting, or salient to the organism. It then coordinates common autonomic responses to incoming stimuli deemed important (2). The amygdala is attentive—constantly monitoring subtle changes in the environment that demand ongoing adaptive responding.
Detecting change and salience depends on context, memory, and personal history (10). Salience may be partly driven by bottom-up fight-or-flight response (e.g., detecting a snake), but we adapt to our environment through personal, contextualized learning (e.g., becoming a snake charmer), such that salience becomes tailored to the individual and situation. Episodic memory and learning play a crucial role in modulating amygdala response to environmental changes. Here, we note the significance of corticolimbic connectivity, which involves the interaction between the frontal cortex and amygdala with contributions from the hippocampus (Figure 1). Prediction based on contextualized memory, necessary for detecting change, is dependent on these frontal and hippocampal circuits. This suggests that paranoid delusions must be understood within the context of a broader network that integrates memory and emotions dependent on the organism’s developmental and evolutionary history. A faulty calibration of the amygdala within this network could result in patterns of maladaptive behavior that become increasingly reinforced and reconsolidated. In summary, hyperactivity of the amygdala does not necessarily reflect a heightened response to threat-specific information, per se, but to any incoming information that signals a mismatch or divergence from predicted outcome (10). Within this framework, the amygdala embodies the border, or the limbus, of the brain, as the watchdog and the gatekeeper of the conscious self.
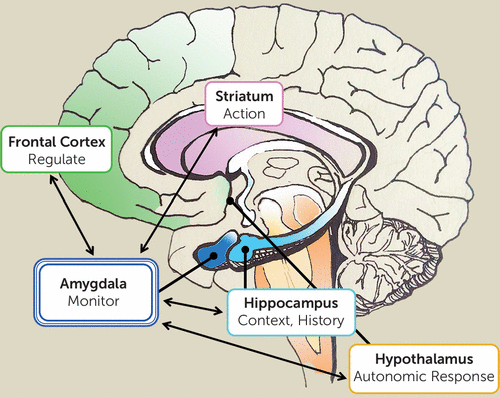
FIGURE 1. Corticolimbic Connectivity Involving the Frontal Cortex, Amygdala, and Hippocampus
1 : Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476–1488Link, Google Scholar
2 : The amygdala: inside and out. F1000 Biol Rep 2011; 3:2Crossref, Medline, Google Scholar
3 : Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am J Psychiatry 2004; 161:480–489Link, Google Scholar
4 : Amygdala recruitment in schizophrenia in response to aversive emotional material: a meta-analysis of neuroimaging studies. Schizophr Bull 2012; 38:608–621Crossref, Medline, Google Scholar
5 : Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am J Psychiatry 2015; 172:784–792Link, Google Scholar
6 : Perfusion imaging. Magn Reson Med 1992; 23:37–45Crossref, Medline, Google Scholar
7 : Antony van Leeuwenhoek and his “Little Animals”. New York, Dover Publications, 1960Google Scholar
8 : The Sleepwalkers: A History of Man’s Changing Vision of the Universe. London, Hutchinson, 1959Google Scholar
9 : From circuits to behaviour in the amygdala. Nature 2015; 517:284–292Crossref, Medline, Google Scholar
10 : Toward a neurobiology of delusions. Prog Neurobiol 2010; 92:345–369Crossref, Medline, Google Scholar



