Antidepressants for Bipolar Depression: A Systematic Review of Randomized, Controlled Trials
Abstract
OBJECTIVE: This study reviewed the evidence from randomized, controlled trials on the efficacy and safety of antidepressants in the short-term treatment of bipolar depression. METHOD: The authors performed a systematic review and meta-analysis of randomized, controlled trials. They searched the Cochrane Collaboration Depression, Anxiety, and Neurosis Controlled Trials Register, incorporating results of searches of MEDLINE, EMBASE, CINAHL, PsycLIT, PSYNDEX, and LILACS. The main outcome measures were the proportion of patients who clinically responded to treatment and the rate of switching to mania. RESULTS: Twelve randomized trials were included, with a total of 1,088 randomly assigned patients. Five trials compared one or more antidepressants with placebo: 75% of these patients were receiving a concurrent mood stabilizer or an atypical antipsychotic. Antidepressants were more effective than placebo. Antidepressants did not induce more switching to mania (the event rate for antidepressants was 3.8% and for placebo, it was 4.7%). Six trials allowed comparison between two antidepressants. The rate of switching for tricyclic antidepressants was 10%, and for all other antidepressants combined, it was 3.2%. CONCLUSIONS: Antidepressants are effective in the short-term treatment of bipolar depression. The trial data do not suggest that switching is a common early complication of treatment with antidepressants. It may be prudent to use a selective serotonin reuptake inhibitor or a monoamine oxidase inhibitor rather than a tricyclic antidepressant as first-line treatment. Given the limited evidence, there is a compelling need for further studies with longer follow-up periods and careful definition and follow-up of emerging mania and partial remission.
The treatment of depressive episodes in patients with bipolar disorder presents a puzzling paradox. In perhaps most countries in the world where treatment is available, patients are offered antidepressants with little deliberation. By contrast, in all treatment guidelines, experts appear to agonize over whether antidepressants should be offered at all, at least as monotherapy. This division of opinion between the real world of treatment and the more refined world of evidence is one of the major current challenges in psychiatric management. It reflects genuine uncertainty about the benefit and harm of antidepressants in treating the 50 million people who may have bipolar disorder worldwide. This is a substantial therapeutic challenge because in bipolar disorder, depression—either recurrent or chronic—causes more disability than any other manifestation of the illness (1–3).
Clinically, the issue is an apparently straightforward balance between benefit and risk. Do antidepressants work in acute bipolar depression as well as they do in unipolar depression? If they do, is the potential cost—the risk of induction of a manic episode or mood instability—too high to justify their use? And should they be prescribed only in combination with mood stabilizers? These questions clearly concern both the short-term use of antidepressants and also the longer-term use for relapse prevention. Depression is often chronic and highly recurrent in bipolar patients, and it carries a significant risk of suicide (4, 5). In unipolar patients, there is strong evidence that long-term treatment with antidepressants prevents relapse of depression for at least 3 years and perhaps indefinitely (6). By contrast, the long-term use of antidepressants in bipolar patients may be associated with manic relapse (7, 8) and sometimes an increased frequency of bipolar episodes (rapid cycling) (9). Spontaneous switching from depression to mania is associated with poor long-term outcome (10).
The precautionary view is that to prescribe antidepressants puts bipolar patients at risk of iatrogenic episodes of mania, mixed states, or rapid cycling. Thus, antidepressants cannot stabilize mood because they may worsen the outcome of the opposite (elated) pole of the illness. All major reviews and guidelines for bipolar depression over the past 10 years have advised the use of a mood stabilizer (usually lithium or valproate) rather than an antidepressant as the first-line treatment for bipolar depression. However, the available evidence favoring lithium, for example, would not be sufficient to register it as an antidepressant in the current regulatory climate because there are no trials showing superiority versus placebo or an active comparator that meets current standards of trial design. Almost all were small crossover studies of very short duration (11). Antidepressants are recommended only as second-line treatment and then always with a concurrent mood stabilizer to prevent switching to mania. However, even in North America, where this policy is most strongly advocated, in clinical practice, bipolar patients are still frequently prescribed antidepressants as monotherapy (12).
The gap between practice and theory should make us all uncomfortable. There is a current need to make the best sense of the evidence, and we believe the key concerns are as follows:
| 1. | Previous reviews have all been essentially qualitative in approach. Accordingly, they have run the well-recognized risk of bias and overinterpretation of favored studies at the expense of more reliable average effects. Systematic reviews are less vulnerable to bias and more likely to highlight true areas of uncertainty (13, 14). | ||||
| 2. | Should we really be skeptical about the efficacy of antidepressants? How does the totality of the randomized evidence stack up? | ||||
| 3. | Can we be confident that the association between mood switches to mania and the use of antidepressants is usually causal? The natural course of bipolar disorder is to switch between the two poles of depression and mania. Can we quantify the increased risk from the existing data? | ||||
| 4. | Are some antidepressants either more effective than others or less likely to produce a manic switch? | ||||
| 5. | Conservative use of antidepressants complicates decision making in bipolar depression. When do we decide that a mood stabilizer alone has failed? It is easy to leave patients with mild but chronic depressive symptoms. | ||||
The objective of this study was systematically to assess the evidence from randomized, controlled trials of antidepressants in short-term treatment of bipolar depression using the rigorous methods developed by the Cochrane Collaboration (15). Our aim was to produce the best available quantitative estimates of the risks and benefits of antidepressant treatment in bipolar disorder.
Method
Studies Included
We included randomized, controlled double-blind trials, published in any language, that compared antidepressants with placebo or alternative drug treatments. Antidepressants included all tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), noradrenergic reuptake inhibitors, reversible monoamine oxidase inhibitors (MAOIs), bupropion, St. John’s wort, and tryptophan but not mood stabilizers, anticonvulsants, lamotrigine, sulpiride, benzodiazepines, or ECT. Alternative comparator treatments included mood stabilizers, anticonvulsants, and other antidepressants. The inclusion criteria for the review were trials with patients with a current depressive or mixed depressive/manic episode with or without psychotic symptoms who had had at least one previous episode of mania or hypomania, including antidepressant-induced mania or hypomania. Trials that recruited nonbipolar patients as well as bipolar patients were included if they randomly assigned the bipolar patients separately or if the majority of the patients were bipolar. The primary outcome measures for the review were clinical response and remission rates (derived from observer-rated symptom reductions), induction of mania or hypomania, and the overall dropout rate as a proxy measure of the acceptability of treatment.
Search Strategy
We searched the Cochrane Collaboration Depression, Anxiety, and Neurosis Controlled Trials Register (December 2002), incorporating results of group searches of MEDLINE (1966 to the present), EMBASE (1980 to the present), CINAHL (1982 to the present), PsycLIT (1974 to the present), PSYNDEX (1977 to the present), and LILACS (1982–1999). These were searched by using the following terms for diagnosis: bipolar III disorder, unipolar mania, rapid-cycling disorder, affective disorders, affective psychosis, bipolar, bipolar disorder, bipolar I disorder, bipolar II disorder, cyclothymic disorder, depression, depressive psychosis, excited psychosis, hypomania, mania, manic depressive, manic disorder, manic episode, melancholia, mixed depression, mood disorders, bipolar affective disorder, bipolar not otherwise specified, dysphoric mania, manic episode, manic symptoms, schizoaffective disorder, psychoses, psychotic disorders, puerperal psychosis, and reactive depressive psychosis. The terms searched for intervention and antidepressive agents were monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, and tricyclic drugs. The following drug names were also searched: acetylcarnitine, alaproclate, amesergide, amiflamine, amineptine, amitriptyline, amoxapine, befloxatone, benactyzine, brofaromine, bupropion, butriptyline, caroxazone, chlorpoxiten, cilosamine, cimoxatone, citalopram, clomipramine, clorgyline, clorimipramine, clovoxamine, deanol, demexiptiline, deprenyl, desipramine, dibenzepin, diclofensine, dothiepin, doxepin, duloxetine, etoperidone, femoxetine, fluotracen, fluoxetine, fluparoxan, fluvoxamine, idazoxan, imipramine, iprindole, iproniazid, isocarboxazid, litoxetine, lofepramine, maprotiline, medifoxamine, melitracen, metapramine, mianserin, milnacipran, minaprine, mirtazapine, moclobemide, nefazodone, nialamide, nomifensine, nortriptyline, noxiptiline, opipramol, oxaflozane, oxaprotiline, pargyline, paroxetine, phenelzine, piribedil, pirlindole, pivagabine, prosulpride, protriptyline, quinupramine, reboxetine, rolipram, sertraline, setiptiline, teniloxine, tetrindole, thiazesim, thozalinone, tianeptine, toloxatone, tomoxetine, tranylcypromine, trazodone, trimipramine, venlafaxine, viloxazine, viqualine, and zimeldine.
The Cochrane Library was searched by using the same terms as the Cochrane Collaboration Depression, Anxiety, and Neurosis Controlled Trials Register excluding references that came from that database. The reference lists of selected studies were inspected for more published reports and citations of unpublished research. In addition, other relevant papers and major textbooks that cover affective disorder were checked.
Methods of the Review
Two of us (H.J.G., J.M.R.) independently checked studies generated by the search strategy to ensure that they met the inclusion criteria. Any disagreement was resolved by consensus discussion with another coauthor (J.R.G.). One reviewer (H.J.G.) assessed the methodological quality of the included studies according to the reporting of the randomization procedure, especially allocation concealment (15), blinding, and the reporting of withdrawals. Information about participant characteristics, intervention details, and outcome measures was extracted independently by two reviewers.
Data Analysis
Data were entered twice into Revman 4.2, a program developed by the Cochrane Collaboration for systematic reviews. For binary efficacy outcomes, a pooled relative risk (with 95% confidence intervals [CIs]) was calculated by using both fixed- and random-effects models to investigate the sensitivity of results to the choice of statistical method. Heterogeneity between studies was assessed by using the Q statistic (16). When significant heterogeneity was identified, sources were investigated by visual inspection of the forest plots for outlying trials. Two sensitivity analyses were conducted, one excluding two trials that included less than 100% bipolar patients and another including a trial with bipolar patients who were not randomized separately from unipolar patients. Treatment effects are also presented as the number needed to treat, which is the reciprocal of the absolute difference between the response rates of the compared treatments. The number needed to treat can assist clinical interpretation of the results of trials and expresses the number of patients that must be treated with the drug of interest to achieve one positive outcome more than would be achieved with a comparator or placebo (17).
Results
Description of Studies
Twelve trials were included in the primary analysis: five comparing one or more antidepressants with placebo (total participants, N=779), four studies comparing two different antidepressants only (N=236), and three trials comparing an antidepressant with another type of drug (N=73).
We had originally identified 23 trials in which bipolar depressed patients received an antidepressant, but we had to exclude 11 trials. Eight of these studies had included only a low proportion of bipolar patients, who were not randomized separately (18–25). Two excluded trials compared other treatments that were added to antidepressant treatment (26, 27). One trial compared two antidepressants but was not blinded (28).
Design Characteristics
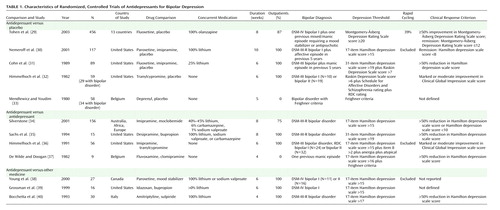
The characteristics of the included trials are presented in Table 1. All trials were described as randomized and used a parallel group design. Study duration varied between 4 and 10 weeks. All trials excluded patients with a diagnosis of serious physical illness or substance abuse. Participants were adult patients up to 70 years old. In most studies, the majority (approximately 60%–70%) of participants were women.
Outcomes
Dichotomous outcomes are presented here. With regard to continuous outcomes, nine studies reported Hamilton Depression Rating Scale or Montgomery-Åsberg Depression Rating Scale scores, but only some reported baseline scores of all randomly assigned patients. Thus, meta-analysis of continuous data was not feasible.
Antidepressants Versus Placebo
Five trials were available for this comparison. About 75% of the patients were taking a concurrent mood stabilizer or an atypical antipsychotic. One recent trial compared three groups: olanzapine plus fluoxetine (N=86), olanzapine alone (N=370), and placebo (N=377) (29). We only included the former two groups in this analysis as, respectively, the fluoxetine and placebo groups.
Clinical Response
One of these five trials only reported remission and was therefore excluded from this comparison (30). One other study did report responders but not how this was defined and was included in this analysis (33). As a result, four trials with a total of 662 randomly assigned patients were available for this comparison, 213 in the experimental group and 449 in the placebo group. Patients treated with an antidepressant were more likely to respond by the end of the trial (risk ratio=1.86, 95% CI=1.49–2.30) (Figure 1). The number needed to treat with antidepressants was 4.2 (95% CI=3.2–6.4). The treatment effect remained statistically significant after taking account of the heterogeneity by using a random-effect model (risk ratio=2.29, 95% CI=1.29–4.04). The direction of the effect favored antidepressants in all the trials, which implies that the heterogeneity was quantitative rather than qualitative. We could not distinguish response rates for patients taking and not taking concurrent medication within individual trials.
Clinical Remission
This was defined in only two studies, as a Hamilton depression scale score ≤7 (39) and a Montgomery-Åsberg Depression Rating Scale score ≤12 (29). However, these are the two largest studies, with a total of 160 patients in the experimental group and 413 patients in the comparison group. All of these patients were taking a concurrent mood stabilizer or an atypical antipsychotic. Patients treated with an antidepressant (paroxetine, imipramine, or fluoxetine) were more likely to reach remission than those who were not taking an antidepressant (risk ratio=1.41, 95% CI=1.11–1.80) (Figure 2). The number needed to treat was 8.4 (95% CI=4.8–33).
Switching to Mania
Two studies had predefined criteria for switching to mania, as DSM-III-R mania or hypomania (30) or a score ≥15 on the Young Mania Rating Scale (29). One study did not specifically describe how they monitored the emergence of mania (31). One study reported the absence of emergence of manic states (32). One small study did not address or report switches to mania but was included in the analysis on the inference that no mania did occur (33).
There was no evidence of an increased risk of switching to a manic episode in the trials (risk ratio=1.00, 95% CI=0.47–2.13) (Figure 3). However, there were very few manic events; hence, of course, this would have limited the power to detect a difference between antidepressant and placebo. The risk of switching for antidepressants was 3.8%, and for placebo, it was 4.7% (difference=0.9%, 95% CI=–2.0 to 3.8).
Overall Acceptability
The dropout rate was 32% in the antidepressant group, 49% in the placebo group, and 43% in total. There were significantly fewer study withdrawals while subjects were taking antidepressants (fixed-effect risk ratio=0.71, 95% CI=0.58–0.88; χ2=1.82, df=3, p=0.61, for heterogeneity).
Sensitivity Analysis
We performed a sensitivity analysis excluding the two studies with less than 100% bipolar patients (33, 36). This did not materially alter the estimate of treatment effect (fixed-effect risk ratio=1.61, 95% CI=1.27–2.04) and reduced heterogeneity (χ2=2.71, df=1, p=0.10). The risk of withdrawal also remained unchanged (fixed-effect risk ratio=0.74, 95% CI=0.60–0.92).
Tricyclic Antidepressants Versus Other Antidepressants
This comparison includes 134 patients from the antidepressant arms of two trials in which two antidepressants were compared with placebo (31, 30) and 236 patients from four trials with two antidepressant arms and no placebo group (34–37). In total, 201 of the 370 patients (54%) distributed throughout all of these studies were receiving co-therapy with mood stabilizers.
Clinical Response
A dichotomous measure of clinical response was reported in five trials. This was based on scores on the Hamilton depression scale in five trials and on the Clinical Global Impression scale in one trial. One trial did not report such a measure and was therefore excluded from this analysis (30). Tricyclic antidepressants may be less effective than other antidepressants, but this did not reach statistical significance (risk ratio=0.84, 95% CI=0.67–1.06) (Figure 4).
Clinical Remission
There was no difference in remission rates between imipramine and paroxetine based on one trial only (risk ratio=0.84, 95% CI=0.47–1.48) (30).
Switching to Mania
Three out of six trials had predefined criteria for switching to mania, as DSM-III-R mania or hypomania (30, 35) or a score higher than 5 on the Raskin Mania Scale (36). One trial used the Young Mania Rating Scale for follow-up but simply used “withdrawal from the study because of mania” as a clinical description (T. Silverstone, personal communication). One study did not specifically describe how the authors monitored the emergence of mania (31). Two studies reported the absence of emergence of manic states (32, 37).
On average, tricyclic antidepressants caused more switching to mania than other antidepressants (risk ratio=2.92, 95% CI=1.28–6.71) (Figure 5). The rate of switching for tricyclic antidepressants was 10%, and for other antidepressants combined, it was 3.2%. This gives an absolute risk difference of 6.8% (95% CI=1.7%–11.9%).
Overall Acceptability
The dropout risk in the tricyclic antidepressant group was 37%; in the other groups, it was 30%; and in total, it was 34%. The tricyclic antidepressants did not cause significantly more withdrawals than other antidepressants (risk ratio=1.23, 95% CI=0.92–1.64).
Subgroups
In the subgroups in Figure 4 and Figure 5, there are possible differences between treatments, although none was statistically significant. First, SSRIs may have greater efficacy than tricyclic antidepressants. Second, MAOIs may induce less switching to mania than tricyclic antidepressants.
Sensitivity Analysis
We performed a sensitivity analysis including 33 bipolar patients from a unipolar randomized, controlled trial of 381 patients comparing moclobemide and imipramine (25). This trial had been included in several previous reviews on bipolar depression. We excluded it from the primary analysis because, although the results for these patients were presented separately, they were not randomized separately. The sensitivity analysis did not alter the overall pattern of clinical response seen in our primary analysis.
Antidepressants Versus Other Medications
Only three small trials were identified comparing antidepressants with different types of agents, and therefore, no meta-analyses were performed. One study (N=30) compared an antidepressant with a second mood stabilizer but was unclear about which drugs were specifically used by the different groups of patients and did not report dichotomous response data (38). The second study (N=27) compared a low dose of amitriptyline (average 62 mg/day) with a low dose of sulpiride (average 55 mg/day) and reported response rates of 80% or higher in both groups (40). A third study (N=16) compared bupropion with idazoxan, a selective α2 antagonist, but only two of nine and three of seven patients in the respective groups responded (39).
Discussion
We raised five current concerns in the introduction, and we will address them as follows.
Comparison With Previous Reviews
This review demonstrates the advantages of a systematic search when reviewing the literature. Thus, we included five trials that were not included in previous reviews; two small studies compared antidepressants with other drugs not in wide use (39, 40). Another consisted of nine bipolar patients who were separately randomly assigned within a larger study (37). Finally, we included two placebo-controlled studies with fewer than 100% bipolar patients. One of these was included because the other 50% of patients suffered from anergic depression, which is very similar to typical bipolar depression (32). The other included 65% patients with bipolar depression, which is substantially more than any other trial with mixed inclusion (33).
We did not include several trials that have been cited in recent reviews on bipolar depression. One is a trial with no bipolar patients at all, since the patients are described as having manic-depressive psychosis, depressed type, the ICD-9 description for unipolar depression (21). This study was erroneously cited in several reviews on bipolar depression (41–43). In two other trials comparing bupropion with placebo in unipolar depression (22, 23), the majority of patients had a diagnosis of DSM-II manic-depressive, depressed, which is the DSM-II description for unipolar depression (“manic-depressive, circular” is used for bipolar patients). These two trials have been used to advocate the use of bupropion as a first antidepressant choice in bipolar depression (42, 44). Bupropion also takes a prominent place in recent expert guidelines (45). The actual evidence for the use of bupropion is very limited. Apart from some open studies, there is only one small study, which does not substantiate its advocated lower risk of switching to mania (35).
Antidepressant Efficacy
A quantitative review of the existing randomized data confirms that the numbers of bipolar depressed patients entered into clinical trials is around 1% of the figures for unipolar depression. However, in acceptance of that limitation, the data nevertheless strongly support an average positive efficacy for antidepressants versus placebo in trials up to 10 weeks. There is no coherent basis for doubting that conventional antidepressants have efficacy in bipolar depression on the basis of the existing evidence. The size of the antidepressant effect was comparable to that in unipolar depression (46), suggesting that antidepressants may be of comparable efficacy in unipolar and bipolar depression. Withdrawal rates also tended to be higher from placebo, compared with active treatment arms. This suggests a greater acceptability of active treatment, probably due to lower efficacy for placebo.
It is the advantage of a systematic review that individual negative trials are appropriately weighted. In a qualitative review, a trial that fails to achieve significant results may receive unwarranted attention at the expense of an overall pattern of positive findings (or vice versa).
Switching to Mania
The rates of switching to mania in the short time spans of 4 to 10 weeks covered by these studies were low and lent no support to the belief that switching is a common early complication of treatment with antidepressants. However, the numbers were small, and larger studies, especially with longer follow-up and systematic monitoring of manic symptoms, could change this conclusion. However, current guidelines for treatment of bipolar depression with antidepressants tend to stress use in the short term and early discontinuation. The present findings are relevant to that pattern of use.
We could not show a difference between an antidepressant as monotherapy compared with when added to a mood stabilizer. To our knowledge, no trial has addressed this directly, and the scope for comparison between trials was limited by different definitions of switching and, probably, by different baseline rates of switching.
Interpretation of the data could have been improved by better definition and reporting of switching to mania, especially when it occurred in patients who had responded to treatment. Future studies also will have to pay more attention to the definition, follow-up, and reporting of manic symptoms.
Differences Between Antidepressants
At present, the data are inadequate to definitively favor one medicine over another within the several generic categories of the antidepressants. Our review, however, tends to support the findings of nonrandomized studies in bipolar patients and comparative studies in unipolar patients that have suggested a higher risk of switching to mania for tricyclic antidepressants (47, 48). Our results suggest that tricyclic antidepressants cause more switching to mania, and tricyclic antidepressants are not more, and may even be less, effective. This suggests that antidepressant response and mania may not be correlated, and indeed, the pharmacology of tricyclic antidepressants may make them less suitable for bipolar patients in general.
We did not include lamotrigine in this review since it is pharmacologically unlike existing antidepressants. However, data from the one published study in acute bipolar depression suggest that the effect size for the efficacy of antidepressants against placebo is comparable to that of lamotrigine (49). Lamotrigine, 200 mg/day, induced mania in 3% of patients (two of 63), which is no different from the risks we report for both nontricyclic antidepressants and placebo. Lamotrigine, 50 mg/day, was ineffective. Therefore, while there is no clear short-term benefit for lamotrigine over antidepressants for bipolar depression, it may be an alternative option for treatment.
The Antidepressant Effect of Mood Stabilizers
Only one small study in patients already taking a mood stabilizer compared the addition of an antidepressant with the addition of a second mood stabilizer. No firm conclusions can be drawn from that study because of its size and the absence of information on clinical response (38). Future large studies will need to address the efficacy of mood stabilizers alone in more detail. These studies should look at the long-term outcome of depression, quality of life, and the focus on duration of remission as well as response.
Conclusions
We will compare our current conclusions with the recent APA Practice Guideline for the Treatment of Patients With Bipolar Disorder(50) on two key points. First, there is no strong reason to avoid antidepressants for patients with bipolar depression. This is at odds with the recommendation to use lithium or lamotrigine as a first-line of treatment for bipolar depression. For patients already taking a mood stabilizer, we advise adding an antidepressant as a first-line treatment. For patients not taking a mood stabilizer but with a history of mania, the current consensus is to use antidepressants in combination with an antimanic agent or a mood stabilizer (51). Much of the data reviewed here reflect that practice.
Whether nontricyclic antidepressants can safely be used as monotherapy, especially in bipolar II depression, is not excluded by the existing data. Current APA guidelines suggest that the rate of switching is less of a worry in the acute treatment of bipolar II than in bipolar I depression and that, therefore, antidepressants can be added earlier in treatment. This is neither supported nor contradicted by the evidence from randomized, controlled trials. Consensus views are heavily influenced by clinical experience and formal audit, not randomized, controlled trials.
Second, it may be prudent to use an SSRI or an MAOI rather than a tricyclic antidepressant or bupropion as a first-line treatment. This also differs from the guidelines, which specifically recommend paroxetine and bupropion as antidepressants. We see only very limited evidence for bupropion and only limited evidence for a special place for paroxetine among the SSRIs. To prefer either is to move beyond the evidence.
Obviously, our conclusions are based on the results of randomized, controlled trials only, and we have not considered evidence from naturalistic and other nonrandomized studies. However, there is no strong signal that antidepressants (other than tricyclic antidepressants) cause mania or even rapid cycling from these studies either (52, 53). On the basis of current evidence, we believe that it is overcautious and potentially not in the best interest of patients to discourage the use of antidepressants for bipolar depression. We appreciate that the existing APA guidelines do recommend the use of specific antidepressants for severe depression. However, in practice, we have seen cases in which patients have not been treated with antidepressants and have been left chronically and significantly depressed for very long periods of time. This is almost certainly one consequence of an emphasis on the first-line use of mood stabilizers such as lithium and valproate for bipolar depression, despite the inadequate evidence that they actually work.
Finally, future research is required to address a number of key questions. Should we extrapolate from bipolar I trials to bipolar II disorder and the bipolar spectrum? Does the short-term use of antidepressants cause subsequent mood instability or cycling? Is there actually any disadvantage to the long-term use of nontricyclic antidepressants for the prevention of depressive relapse? Should we prefer an SSRI to lamotrigine? Will the long-term use of nontricyclic antidepressants reduce the risk of suicide in bipolar patients? Our capacity to answer such questions will depend upon the development of a trial-oriented culture in psychiatry and the active participation of patients and clinicians in appropriate clinical trials.
 |
Received Feb. 21, 2003; revision received Sept. 16, 2003; accepted Oct. 22, 2003. From the Department of Psychiatry, Warneford Hospital, Oxford, U.K.; the Department of Psychiatry, University Hospital, Groningen, the Netherlands. Address reprint requests to Dr. Gijsman, Scutari Clinic, St. Thomas’ Hospital, Lambeth Palace Rd., London, U.K.; [email protected] (e-mail). The authors thank the Cochrane Collaboration Depression, Anxiety, and Neurosis Group for methodological support and Dr. Mauricio Tohen for making available data from the fluoxetine/olanzapine trial. Dr. Goodwin, Dr. Geddes, and Ms. Rendell receive free medicines from Sanofi-Synthelabo to support the BALANCE trial. Dr. Nolen has been involved in a study within the Stanley Foundation Bipolar Network, which received free medicines from Wyeth, Pfizer, and GSK and currently receives research support from GSK. Dr. Goodwin and Dr. Nolen have acted as occasional advisers to several pharmaceutical companies and have spoken in industry-supported symposia in recent years.
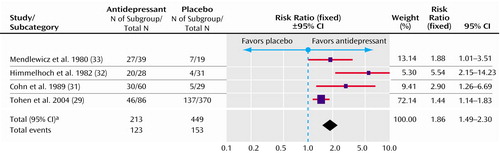
Figure 1. Fixed-Effect Model of Clinical Response in Randomized, Controlled Trials of Antidepressants Versus Placebo for the Treatment of Bipolar Depression
aSignificance test for heterogeneity (χ2=10.51, df=3, p=0.01; I2=71.4%). Significance test for overall effect (z=5.60, p<0.00001).
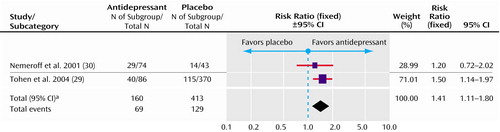
Figure 2. Fixed-Effect Model of Clinical Remission in Randomized, Controlled Trials of Antidepressants Versus Placebo for the Treatment of Bipolar Depression
aSignificance test for heterogeneity (χ2=0.54, df=1, p=0.46; I2=0%). Significance test for overall effect (z=2.79, p=0.005).
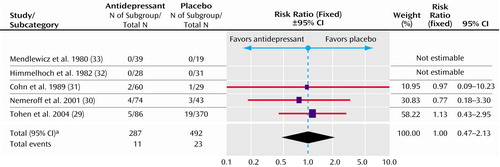
Figure 3. Fixed-Effect Model of Switching to Mania in Randomized, Controlled Trials of Antidepressants Versus Placebo for the Treatment of Bipolar Depression
aSignificance test for heterogeneity (χ2=0.18, df=2, p=0.91; I2=0%). Significance test for overall effect (z=0.01, p=0.99).
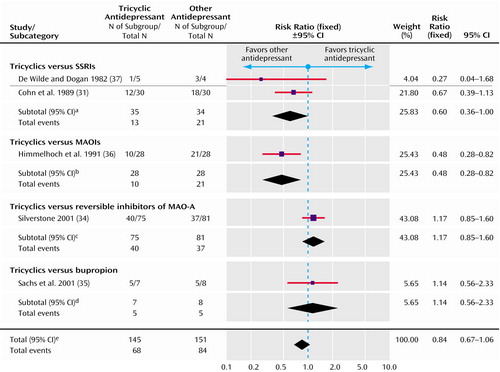
Figure 4. Fixed-Effect Model of Clinical Response in Randomized, Controlled Trials of Tricyclic Antidepressants Versus Other Antidepressants for the Treatment of Bipolar Depression
aSignificance test for overall effect (z=1.96, p=0.05). Significance test for heterogeneity (χ2=0.89, df=1, p=0.35; I2=0%).
bSignificance test for overall effect (z=2.69, p=0.007).
cSignificance test for overall effect (z=0.95, p=0.34).
dSignificance test for overall effect (z=0.37, p=0.71).
eSignificance test for overall effect (z=1.46, p=0.14). Significance test for heterogeneity (χ2=11.26, df=4, p=0.02; I2=64.5%).
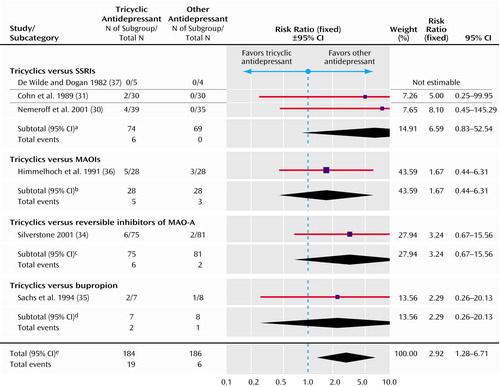
Figure 5. Fixed-Effect Model of Switching to Mania in Randomized, Controlled Trials of Tricyclic Antidepressants Versus Other Antidepressants for the Treatment of Bipolar Depression
aSignificance test for overall effect (z=1.78, p=0.08). Significance test for heterogeneity (χ2=0.05, df=1, p=0.82; I2=0%).
bSignificance test for overall effect (z=0.75, p=0.45).
cSignificance test for overall effect (z=1.47, p=0.14).
dSignificance test for overall effect (z=0.74, p=0.46).
eSignificance test for overall effect (z=2.53, p=0.01). Significance test for heterogeneity (χ2=1.35, df=4, p=0.85; I2=0%).
1. Hlastala SA, Frank E, Mallinger AG, Thase ME, Ritenour AM, Kupfer DJ: Bipolar depression: an underestimated treatment challenge. Depress Anxiety 1997; 5:73–83Crossref, Medline, Google Scholar
2. Judd LL, Akiskal HS, Schettler PJ, Coryell W, Endicott J, Maser JD, Solomon DA, Leon AC, Keller MB: A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry 2003; 60:261–269Crossref, Medline, Google Scholar
3. Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser JD, Solomon DA, Leon AC, Rice JA, Keller MB: The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry 2002; 59:530–537Crossref, Medline, Google Scholar
4. Suppes T, Leverich GS, Keck PE, Nolen WA, Denicoff KD, Altshuler LI, McElroy SC, Rush AJ, Kupka R, Frye MA, Bickel M, Post RM (the Stanley Foundation Bipolar Treatment Outcome Network): Demographic and illness characteristics of the first 261 patients. J Affect Disord 2001; 67:45–59Crossref, Medline, Google Scholar
5. Lopez P, Mosquera F, de Leon J, Gutierrez M, Ezcurra J, Ramirez F, Gonzalez-Pinto A: Suicide attempts in bipolar patients. J Clin Psychiatry 2001; 62:963–966Crossref, Medline, Google Scholar
6. Geddes J, Carney SM, Davies C, Furukawa TA, Kupfer DJ, Frank E, Goodwin GM: Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet 2003; 361:653–661Crossref, Medline, Google Scholar
7. Prien RF, Klett CJ, Caffey EM: Lithium carbonate and imipramine in prevention of affective episodes. Arch Gen Psychiatry 1973; 29:420–425Crossref, Medline, Google Scholar
8. Lewis J, Winokur G: The induction of mania: a natural history study with controls. Arch Gen Psychiatry 1982; 39:303–306Crossref, Medline, Google Scholar
9. Wehr TA, Goodwin FK: Rapid cycling in manic-depressives induced by tricyclic antidepressants. Arch Gen Psychiatry 1979; 36:555–559Crossref, Medline, Google Scholar
10. Maj M, Pirozzi R, Magliano L, Bartoli L: The prognostic significance of “switching” in patients with bipolar disorder: a 10-year prospective follow-up study. Am J Psychiatry 2002; 159:1711–1717Link, Google Scholar
11. Bhagwagar Z, Goodwin GM: The role of lithium in the treatment of bipolar depression. Clin Neurosci Res 2002; 2:222–227Crossref, Google Scholar
12. Blanco C, Laje G, Olfson M, Marcus SC, Pincus HA: Trends in the treatment of bipolar disorder by outpatient psychiatrists. Am J Psychiatry 2002; 159:1005–1010Link, Google Scholar
13. Antman EM, Lau J, Kupelnick B, Mosteller F, Chalmers TC: A comparison of results of meta-analyses of randomized control trials and recommendations of clinical experts: treatments for myocardial infarction. JAMA 1992; 268:240–248Crossref, Medline, Google Scholar
14. Cipriani A, Geddes J: Comparison of systematic and narrative reviews: the example of the atypical antipsychotics. Epidemiol Psichiatr Soc 2003; 12:146–153Crossref, Medline, Google Scholar
15. Mulrow CD, Oxman AD: Cochrane Collaboration Handbook, 4th ed. Oxford, UK, Update Software, 1997Google Scholar
16. DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188Crossref, Medline, Google Scholar
17. Cook RJ, Sackett DL: The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995; 310:452–454Crossref, Medline, Google Scholar
18. Aberg-Wistedt A: A double-blind study of zimelidine, a serotonin uptake inhibitor, and desipramine, a noradrenaline uptake inhibitor, in endogenous depression. Acta Psychiatr Scand 1982; 66:50–65Crossref, Medline, Google Scholar
19. Kessell A, Holt NF: A controlled study of a tetracyclic antidepressant—maprotiline (Ludiomil). Med J Aust 1975; 1:773–776Medline, Google Scholar
20. Watanabe S, Yokoyama S, Kubo S, Iwai H, Kuyama C: A double-blind controlled study of clinical efficacy of maprotiline and amitriptyline in depression. Fol Psychiatr Neurol Jpn 1978; 32:1–31Medline, Google Scholar
21. Fieve RR, Platman SR, Plutchik RR: The use of lithium in affective disorders, I: acute endogenous depression. Am J Psychiatry 1968; 125:487–491Link, Google Scholar
22. Fabre LF, Brodie KH, Garver D, Zung WWK: A multicenter evaluation of bupropion versus placebo in hospitalized depressed patients. J Clin Psychiatry 1983; 44:88–94Medline, Google Scholar
23. Merideth CH, Feighner JP: The use of bupropion in hospitalized depressed patients. J Clin Psychiatry 1983; 44:85–87Medline, Google Scholar
24. Amsterdam JD: Efficacy and safety of venlafaxine in the treatment of bipolar II major depressive episode. J Clin Psychopharmacol 1998; 18:414–417Crossref, Medline, Google Scholar
25. Baumhackl U, Biziere K, Fischbach R, Geretsegger C, Hebenstreit G, Radmayr E, Stabl M: Efficacy and tolerability of moclobemide compared with imipramine in depressive disorder (DSM-III): an Austrian double-blind, multicentre study. Br J Psychiatry Suppl 1989; 6:78–83Medline, Google Scholar
26. Benedetti F, Barbini B, Lucca A, Campori E, Colombo C, Smeraldi E: Sleep deprivation hastens the antidepressant action of fluoxetine. Eur Arch Psychiatry Clin Neurosci 1997; 247:100–103Crossref, Medline, Google Scholar
27. Ebert D, Jaspert A, Murata H, Kaschka WP: Initial lithium augmentation improves the antidepressant effects of standard TCA treatment in non-resistant depressed patients. Psychopharmacology (Berl) 1995; 118:223–225Crossref, Medline, Google Scholar
28. Vieta E, Martinez-Aran A, Goikolea JM, Torrent C, Colom F, Benabarre A, Reinares M: A randomized trial comparing paroxetine and venlafaxine in the treatment of bipolar depressed patients taking mood stabilizers. J Clin Psychiatry 2002; 63:508–512Crossref, Medline, Google Scholar
29. Tohen M, Vieta E, Calabrese J, Ketter TA, Sachs G, Bowden C, Mitchell PB, Centorrino F, Risser R, Baker RW, Evans AR, Beymer K, Dube S, Tollefson GD, Breier A: Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003; 60:1079–1088; correction, 2004; 61:176Google Scholar
30. Nemeroff CB, Evans DL, Gyulai L, Sachs GS, Bowden CL, Gergel IP, Oakes R, Pitts CD: Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry 2001; 158:906–912Link, Google Scholar
31. Cohn JB, Collins G, Ashbrook E, Wernicke JF: A comparison of fluoxetine imipramine and placebo in patients with bipolar depressive disorder. Int Clin Psychopharmacol 1989; 4:313–322Crossref, Medline, Google Scholar
32. Himmelhoch JM, Fuchs CZ, Symons BJ: A double-blind study of tranylcypromine treatment of major anergic depression. J Nerv Ment Dis 1982; 170:628–634Crossref, Medline, Google Scholar
33. Mendlewicz J, Youdim MB: Antidepressant potentiation of 5-hydroxytryptophan by L-deprenyl in affective illness. J Affect Disord 1980; 2:137–146Crossref, Medline, Google Scholar
34. Silverstone T: Moclobemide vs imipramine in bipolar depression: a multicentre double-blind clinical trial. Acta Psychiatr Scand 2001; 104:104–109Crossref, Medline, Google Scholar
35. Sachs GS, Lafer B, Stoll AL, Banov M, Thibault AB, Tohen M, Rosenbaum JF: A double-blind trial of bupropion versus desipramine for bipolar depression. J Clin Psychiatry 1994; 55:391–393Medline, Google Scholar
36. Himmelhoch JM, Thase ME, Mallinger AG, Houck P: Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry 1991; 148:910–916Link, Google Scholar
37. De Wilde JE, Doogan DP: Fluvoxamine and chlorimipramine in endogenous depression. J Affect Disord 1982; 4:249–259Crossref, Medline, Google Scholar
38. Young LT, Joffe RT, Robb JC, MacQueen GM, Marriott M, Patelis-Siotis I: Double-blind comparison of addition of a second mood stabilizer versus an antidepressant to an initial mood stabilizer for treatment of patients with bipolar depression. Am J Psychiatry 2000; 157:124–126Link, Google Scholar
39. Grossman F, Potter WZ, Brown EA, Maislin G: A double-blind study comparing idazoxan and bupropion in bipolar depressed patients. J Affect Disord 1999; 56:237–243Crossref, Medline, Google Scholar
40. Bocchetta A, Bernardi F, Burrai C, Pedditzi M, Del Zompo M: A double-blind study of L-sulpiride versus amitriptyline in lithium-maintained bipolar depressives. Acta Psychiatr Scand 1993; 88:434–439Crossref, Medline, Google Scholar
41. Leibenluft E: Issues in the treatment of women with bipolar illness. J Clin Psychiatry 1997; 58(suppl 15):5–11Google Scholar
42. Sachs GS: Bipolar mood disorder: practical strategies for acute and maintenance phase treatment. J Clin Psychopharmacol 1996; 16(2 suppl 1):32S-47SGoogle Scholar
43. Zornberg GL, Pope HG: Treatment of depression in bipolar disorder: new directions for research. J Clin Psychopharmacol 1993; 13:397–408Crossref, Medline, Google Scholar
44. Compton MT, Nemeroff CB: The treatment of bipolar depression. J Clin Psychiatry 2000; 61(suppl 9):57–67Google Scholar
45. Sachs GS, Printz DJ, Kahn DA, Carpenter D, Docherty JP: The Expert Consensus Guideline Series: Medication Treatment of Bipolar Disorder 2000. Postgrad Med 2000(April Special Number)Google Scholar
46. Geddes J, Butler R, Hatcher S: Depressive disorders. Clin Evid 2003; 9:1034–1057Medline, Google Scholar
47. Peet M: Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants. Br J Psychiatry 1994; 164:549–550Crossref, Medline, Google Scholar
48. Bottlender R, Rudolf D, Strauss A, Moller HJ: Antidepressant-associated maniform states in acute treatment of patients with bipolar-I depression. Eur Arch Psychiatr Clin Neurosci 1998; 248:296–300Crossref, Medline, Google Scholar
49. Calabrese JR, Bowden CL, Sachs GS, Ascher JA, Monaghan E, Rudd GD: A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry 1999; 60:79–88Crossref, Medline, Google Scholar
50. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Bipolar Disorder (Revision). Am J Psychiatry 2002; 159(April suppl)Google Scholar
51. Goodwin GM: Evidence-based guidelines for treating bipolar disorder: recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2003; 17:149–173Crossref, Medline, Google Scholar
52. Moller H-J, Grunze H: Have some guidelines for the treatment of acute bipolar depression gone too far in the restriction of antidepressants? Eur Arch Psychiatry Clin Neurosci 2000; 250:57–68Crossref, Medline, Google Scholar
53. Post RM, Denicoff KD, Leverich GS, Frye MA: Drug-induced switching in bipolar disorder: epidemiology and therapeutic implications. CNS Drugs 1997; 8:352–365Crossref, Google Scholar



