Examining the Comorbidity Between Attention Deficit Hyperactivity Disorder and Bipolar I Disorder: A Meta-Analysis of Family Genetic Studies
Abstract
Objective
The existence of comorbidity between attention deficit hyperactivity disorder (ADHD) and bipolar I disorder has been documented in clinical and epidemiological studies, in studies of children and adults, and in diagnosed ADHD and bipolar I patient samples. Yet questions remain about the validity of diagnosing bipolar I disorder in ADHD youth. The authors aim to clarify these issues by reviewing family genetic studies of ADHD and bipolar I disorder.
Method
The authors applied random-effects meta-analysis to family genetic studies of ADHD and bipolar I disorder. Twenty bipolar proband studies provided 37 estimates of the prevalence of ADHD in 4,301 relatives of bipolar probands and 1,937 relatives of comparison probands. Seven ADHD proband studies provided 12 estimates of the prevalence of bipolar I disorder in 1,877 relatives of ADHD probands and 1,601 relatives of comparison probands.
Results
These studies found a significantly higher prevalence of ADHD among relatives of bipolar probands and a significantly higher prevalence of bipolar I disorder among relatives of ADHD probands. These results could not be accounted for by publication biases, unusual results from any one observation, sample characteristics, or study design features. The authors found no evidence of heterogeneity in the ADHD or bipolar family studies.
Conclusions
The results suggest that ADHD plus bipolar comorbidity cannot be accounted for by misdiagnoses, but additional research is needed to rule out artifactual sources of comorbidity. More research is also needed to determine whether comorbidity of ADHD and bipolar I disorder constitutes a familial subtype distinct from its constituent disorders, which if confirmed would have implications for diagnostic nosology and genetic studies.
The existence of comorbidity between attention deficit hyperactivity disorder (ADHD) and bipolar I disorder has long been noted in the scientific literature. In the 1950s, Sadler (1) described hyperactive children developing manic-depressive illness. In the 1970s and 1980s, additional cases of comorbidity were published (2–6), and in the 1990s, systematic studies found ADHD in 57%–98% of bipolar youths (7–9) and bipolar I disorder in 22% of ADHD inpatients (10). In 2005, a meta-analysis of extant studies estimated the prevalence of ADHD among bipolar youths to be 62% (11). Higher rates of ADHD were associated with better study quality, younger age, and use of parent reports.
Significantly elevated rates of bipolar I disorder have also been reported in studies of youths with ADHD (12–15), and this comorbidity has been confirmed in studies of adults with bipolar disorder and ADHD. Sachs et al. (16) found significant ADHD comorbidity in adults with an onset of bipolar I disorder before age 19, but not for those with older ages at onset. Likewise, several studies reported that rates of bipolar I disorder were significantly elevated in adults with ADHD (17, 18).
In youths, meta-analyses of epidemiological studies have reported a clinically significant prevalence of both ADHD and bipolar I disorder (19, 20). ADHD comorbid with bipolar I disorder is a particularly morbid and disabling condition. For example, Butler et al. (10) found high rates of bipolar I disorder (22%) in a hospitalized sample of ADHD patients. Wozniak et al. (7) reported that children with bipolar I disorder plus ADHD were at high risk for major depression, psychosis, psychiatric hospitalization, and severely impaired psychosocial functioning. Compared with other children with ADHD, these children also had significantly higher elevations on all of the clinical scales of the Child Behavior Checklist (21). Brent et al. (22) reported that adolescents who committed suicide had higher rates of bipolar I disorder and ADHD compared with those whose attempts were not successful. Likewise, Arnold et al. (23) found that youths with both disorders had poorer functioning, greater symptom severity, and more additional comorbidity than youths with only one of the disorders.
In summary, the comorbidity between ADHD and bipolar I disorder has been demonstrated in studies of children and in studies of adults regardless of whether samples were ascertained based on an ADHD or bipolar I diagnosis. Because this comorbidity has been repeatedly demonstrated across studies from different centers using different methodologies, we cannot attribute the documented co-occurrence to the diagnostic traditions or methods of a single research group. Nonetheless, these observations of comorbidity raise several questions: Do patients presenting with symptoms suggestive of bipolar I disorder and ADHD have ADHD, bipolar I disorder, or both? Is apparent comorbidity artifactual? Youngstrom et al. (24) reviewed five artifacts that could lead to ADHD and bipolar I comorbidity: 1) the two disorders could fall on a continuum of psychopathology, 2) overlapping clinical features could lead to misdiagnoses, 3) the artificial splitting of a single syndrome could lead to apparent comorbidity of subsyndromes, 4) one disorder could be a developmental precursor of the other, and 5) referral biases could exaggerate comorbidity because people with two disorders are more likely to be referred to treatment than those with one disorder. Because diagnosis drives treatment and the treatments for these two disorders are very different, determining whether ADHD and bipolar I comorbidity is valid or artifactual has considerable clinical, scientific, and public health significance.
To help address these issues, we applied meta-analysis to family genetic studies of ADHD and bipolar I probands. We reasoned that if ADHD and bipolar I disorder are associated by shared familial etiological factors, then family studies should find bipolar I disorder in the families of ADHD patients and find ADHD in the families of bipolar I patients. Furthermore, because the diagnosis of bipolar I disorder in adulthood is not controversial, studies assessing ADHD in the children of bipolar I adults or bipolar I disorder in the parents of children with ADHD provide a compelling cross-generational assessment of the validity of the comorbid condition. The application of family genetic methods to this issue is sensible given prior evidence that both disorders, considered separately, are known to be highly heritable (25–28). To the best of our knowledge, this report is the most comprehensive evaluation of the familial association between pediatric bipolar I disorder and ADHD.
Method
A PubMed literature search identified family genetic studies that met the following criteria: 1) families were identified through probands diagnosed with bipolar I disorder or ADHD; 2) proband diagnoses were based on Research Diagnostic Criteria, DSM-II or subsequent version criteria, or DSM-equivalent ICD version criteria; 3) a group of families identified through nonaffected probands was available for comparison; and 4) the publication provided the numbers of ADHD and non-ADHD relatives (for family studies of bipolar I probands) or the numbers of bipolar I and non-bipolar I relatives (for family studies of ADHD probands). We used the following search algorithm: (family [Title/Abstract] OR genetic [Title/Abstract] OR twin [Title/Abstract]) AND ([ADHD OR “attention deficit” OR hyperactive] AND [mania OR manic OR bipolar OR mood OR affective]). Additional articles from the reference sections of these articles were also examined. Figure 1 depicts the number of articles identified and their disposition. We reanalyzed data from the Massachusetts General Hospital Pediatric Psychopharmacology Program to allow for stratifying relatives by parents, children, and siblings (29–33).
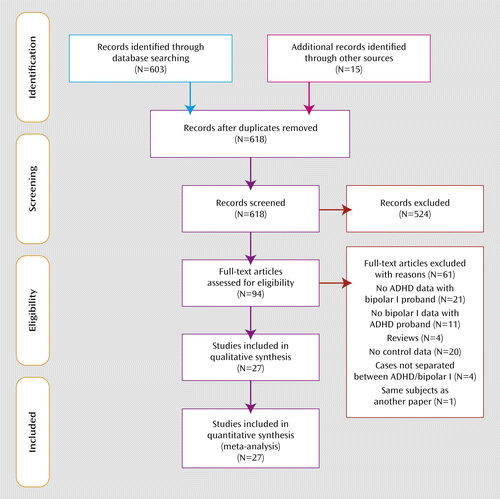
a PRISMA=Preferred Reporting Items for Systematic Reviews and Meta-Analyses (http://www.prisma-statement.org/); RDC=Research Diagnostic Criteria; MGH=reanalysis of child and adult proband studies from Massachusetts General Hospital's Pediatric Psychopharmacology Research Program.
In addition to the numbers of affected and nonaffected family members, we extracted the following methodological features of each article: mean age of relatives, mean age of probands, the percent of male probands, the percent of male relatives, the diagnostic system, the method of diagnosis (structured interview or rating scale), the source of probands (clinic, community, or both), whether diagnoses of relatives were made blind to diagnoses of probands, the fraction of the sample that was Caucasian, and information on the informant providing diagnostic data on children (interviewee, parent, teacher, or combinations of these).
We computed separate meta-analyses for the ADHD and bipolar I family studies. Our meta-analysis used the random-effects model of DerSimonian and Laird (34), which computes a pooled relative risk weighted by sample size. For one study (Massachusetts General Hospital adult ADHD), the relative risk could not be computed because the risks to the relatives of ADHD and comparison probands were both zero. To address that problem, we added the smallest number of affected relatives to both cells that maintained the lack of group difference. We used the I2 index to assess the heterogeneity of effect sizes (35). Its value lies between 0 and 100 and estimates the percentage of variation among effect sizes that can be attributed to heterogeneity. A significant I2 suggests that the effect sizes analyzed are not estimating the same population effect size. We used Egger’s method (36) to assess for publication biases and adjusted standardized mean differences for publication bias using Duval and Tweedie’s trim-and-fill method (37). To determine whether any one observation was skewing the results, we reran the meta-analysis deleting one observation at a time to determine whether the statistical significance of the pooled effect could be accounted for by any one observation.
We used meta-analytic regression to assess the degree to which the effect sizes varied with the methodological features of each study (38, 39). We estimated a separate model for each feature. The meta-analyses and meta-analytic regressions were weighted by the reciprocal of the variance of the effect size. Some research groups contributed more than one data set to the meta-analysis. Because measures reported from the same research group may not be statistically independent of one another, standard statistical procedures would produce inaccurate p values. To address this intrastudy clustering, variance estimates for the meta-analysis regression were adjusted using Huber’s formula (40) as implemented in Stata (41). This formula is a “theoretical bootstrap” that produces robust statistical tests. The method works by entering the cluster scores (i.e., the sum of scores within families) into the formula for the estimate of variance. The Huber estimate is also called the “sandwich” estimate because it is calculated as the product of three matrices: the matrix formed by taking the outer product of the observation-level likelihood score vectors is in the middle, and this matrix is pre- and postmultiplied by the usual model-based variance matrix. The resulting p values are valid even when observations are not statistically independent.
Results
Figure 1 presents the selection of studies in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) format. Twenty bipolar I studies provided 37 estimates of the prevalence of ADHD in 4,301 relatives of bipolar I probands and 1,937 relatives of comparison probands. Seven ADHD studies provided 12 estimates of the prevalence of bipolar I disorder in 1,877 relatives of ADHD probands and 1,601 relatives of comparison probands.
Tables 1 and 2 summarize the characteristics of each study’s sample. Most studies of bipolar I probands (Table 1) ascertained parents with bipolar I disorder and reported the prevalence of ADHD in their offspring. The only exceptions were the Geller et al. (48) and Massachusetts General Hospital child studies, which ascertained pediatric bipolar I probands and assessed the prevalence of ADHD in parents and siblings. Most studies of ADHD probands (Table 2) ascertained child probands and reported the prevalence of bipolar I disorder in siblings or parents. The only exception was the Massachusetts General Hospital adult ADHD study, which ascertained adults with ADHD and evaluated bipolar I disorder in offspring, siblings, and parents. Tables 3 and 4 summarize the design features for each study. Most of the ADHD and bipolar I studies analyzed a mix of probands from clinical and population sources and used structured interviews and diagnoses of first-degree relatives by interviewers who were blind to proband diagnoses. There was more variability in the diagnostic systems used and in the informants used for child diagnoses.
| Probands | Relatives | Bipolar Probands | Comparison Subjects | ||||
|---|---|---|---|---|---|---|---|
| Study | Mean Age (Years) | % Male | Relation | Mean Age (Years) | % Male | N | N |
| Birmaher et al. (42) | 40 | 21 | Offspring | 12 | NA | 233 | 143 |
| Birmaher et al. (43) | 34 | 83 | Offspring | 4 | NA | 83 | 65 |
| Carlson and Weintraub (44) | 35 | NA | Offspring | NA | NA | NA | NA |
| Decina et al. (45) | NA | NA | Offspring | 11 | 45 | 18 | 14 |
| Duffy et al. (46) | NA | NA | Offspring | 19 | 45 | NA | NA |
| Duffy et al. (47) | NA | NA | Offspring | 16 | NA | 63 | 41 |
| Geller et al. (48) | 11 | 69 | Parent/sibling | 34 | 52 | 95 | 77 |
| Gershon et al. (49) | NA | NA | Offspring | NA | NA | NA | NA |
| Giles et al. (50) | NA | NA | Offspring | 14 | 49 | NA | NA |
| Grigoroiu-Serbănescu et al. (51) | 42 | 40 | Offspring | 13 | 47 | 47 | 110 |
| Hammen et al. (52) | 38 | 0 | Offspring | 12 | 49 | 13 | 17 |
| Henin et al. (53) | NA | NA | Offspring | 14 | 52 | 88 | NA |
| Kron et al. (54) | NA | NA | Offspring | 11 | NA | 18 | NA |
| MGH adult (33) | 5 | 56 | Offspring | 29 | 52 | 23 | 56 |
| MGH child (31) | 12 | NA | Sibling | 13 | 51 | 402 | 127 |
| MGH child (31) | 12 | NA | Parent | 43 | 47 | 402 | 127 |
| Neslihan Inal-Eiroglu et al. (55) | NA | NA | Offspring | 12 | NA | 29 | 29 |
| Nurnberger et al. (56) | NA | NA | Offspring | 16 | 50 | 88 | 58 |
| Petresco et al. (57) | 39 | 0 | Offspring | 12 | 51 | 43 | 53 |
| Simeonova et al. (58) | 43 | 19 | Offspring | 14 | 38 | 40 | 18 |
| Singh et al. (59) | NA | NA | Offspring | 10 | 41 | NA | NA |
| Zahn-Waxler et al. (60) | NA | NA | Offspring | NA | NA | 7 | NA |
| Probands | Relatives | ADHD Probands | Comparison Subjects | ||||
|---|---|---|---|---|---|---|---|
| Study | Mean Age (Years) | % Male | Relation | Mean Age (Years) | % Male | N | N |
| Bhatia et al. (61) | NA | NA | Parent/sibling | NA | NA | 112 | 112 |
| Cantwell (62) | NA | 100 | Parent | NA | 50 | 50 | 50 |
| Geller et al. (48) | 11 | 64 | Sibling | 15 | NA | 47 | 77 |
| Geller et al. (48) | 11 | 65 | Parent | 44 | NA | 47 | 77 |
| MGH adult (63) | 34 | NA | Offspring | 14 | 53 | 111 | 73 |
| MGH adult (63) | 34 | NA | Sibling | 31 | 42 | 111 | 73 |
| MGH adult (63) | 34 | NA | Parent | 58 | 32 | 111 | 73 |
| MGH child (64, 65) | 11 | NA | Sibling | 13 | 55 | 280 | 242 |
| MGH child (64, 65) | 11 | NA | Parent | 42 | 49 | 280 | 242 |
| Nigg and Hinshaw (66) | 41 | 100 | Parent | 9 | 47 | 80 | 62 |
| Stewart and Morrison (67) | NA | NA | Parent/sibling | NA | NA | 59 | 41 |
| Study | Ascertainment Source | Diagnostic System | Diagnostic Method | Blinded Study? | Informant for Child Diagnoses |
|---|---|---|---|---|---|
| Birmaher et al. (42) | Both | DSM-IV | SI | Yes | Self |
| Birmaher et al. (43) | Both | DSM-IV | SI | Yes | Self |
| Carlson and Weintraub (44) | Clinic | DSM-III | RS | Yes | Parent, self |
| Decina et al. (45) | Both | RDC | SI | Yes | Teacher |
| Duffy et al. (46) | Clinic | DSM-IV | SI | Yes | Self |
| Duffy et al. (47) | Both | DSM-IV | SI | Yes | Self |
| Geller et al. (48) | Both | DSM-IV | SI | Yes | Self |
| Gershon et al. (49) | Both | DSM-III | SI | No | Parent, self |
| Giles et al. (50) | Both | DSM-IV | RS | Yes | Parent |
| Grigoroiu-Serbănescu et al. (51) | Both | DSM-III | SI | Yes | Self |
| Hammen et al. (52) | Both | DSM-III | SI | Yes | Self |
| Henin et al. (53) | Clinic | DSM-IV | SI | Yes | Self |
| Kron et al. (54) | Clinic | RDC, DSM-III | SI | NA | Self |
| MGH adult (33) | Both | DSM-III-R | SI | Yes | Parent, self |
| MGH child (31) | Both | DSM-IV | SI | Yes | Parent, self |
| Neslihan Inal-Eiroglu et al. (55) | Both | DSM-IV | RS | No | Self |
| Nurnberger et al. (56) | Clinic | DSM-IV | SI | Yes | Self |
| Petresco et al. (57) | Clinic | DSM-IV | SI | Yes | Self |
| Simeonova et al. (58) | Both | DSM-IV | SI | NA | Teacher |
| Singh et al. (59) | Both | DSM-IV | SI | Yes | Teacher |
| Zahn-Waxler et al. (60) | Clinic | DSM-III | SI | NA | Parent |
| Study | Ascertainment Source | Diagnostic System | Diagnostic Method | Blinded Study? | Informant for Child Diagnoses |
|---|---|---|---|---|---|
| Bhatia et al. (61) | Clinic | DSM-III | Clinical | NA | Parent, self |
| Cantwell (62) | Clinic | DSM-II | SI | NA | Parent |
| Geller et al. (48) | Both | DSM-IV | SI | Yes | Self |
| Geller et al. (48) | Both | DSM-IV | SI | Yes | Self |
| MGH adult (63) | Both | DSM-IV | SI | Yes | Parent, self |
| MGH child (64, 65) | Both | DSM-III-R | SI | Yes | Parent, self |
| Nigg and Hinshaw (66) | Both | DSM-III-R | RS | NA | Self |
| Stewart and Morrison (67) | NA | DSM-II | Clinical | NA | Parent, teacher |
Across all bipolar I studies, our meta-analyses found a statistically significant pooled relative risk for ADHD in relatives (relative risk=2.6; 95% CI=2.1–3.2; z=8.8, p<0.0005). These analyses report the risk to relatives for ADHD, regardless of whether the relative also had bipolar I disorder. For bipolar I probands, the weighted prevalence of ADHD was 27.0% in offspring, 30.1% in siblings, and 16.5% in parents. For comparison probands, the weighted prevalence of ADHD was 9.6% in offspring, 11.6% in siblings, and 4.5% in parents. Figure 2 is a forest plot of these results. The pooled estimate remained significant after deleting each observation or contributing study in separate runs and then rerunning the meta-analysis. Thus, no single observation accounted for our results. We found no significant heterogeneity among studies, which suggests that these studies were estimating a common relative risk (I2=25, χ2=28, df=21, p=0.14). We found no significant effects of the study sample and design features listed in Tables 1–4 (p>0.10 in all cases). Egger’s test for publication bias was borderline significant (t=2.01, p=0.05). After adjusting for publication bias, the estimated relative risk became smaller (2.2) but was still statistically significant (z=10.7, p<0.0005). The relative risks for parents, offspring, and siblings were 3.7, 2.1, and 2.6, respectively.
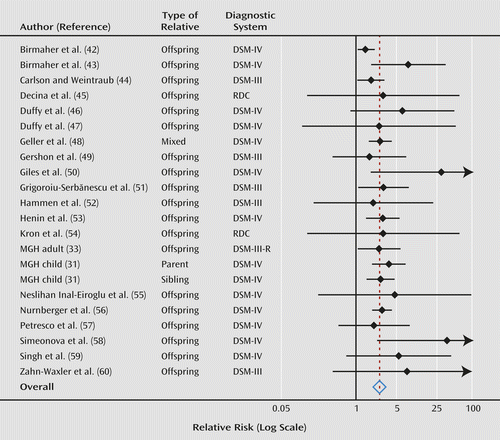
a For each comparison, the dot is the relative risk and the horizontal line is the 95% confidence interval (95% CI). The center of the diamond at the bottom is the weighted relative risk across all studies, and the width of the diamond is its 95% CI. MGH=reanalysis of child and adult proband studies from Massachusetts General Hospital Pediatric Psychopharmacology Program (see Method section).
Across all ADHD studies, our meta-analyses revealed a statistically significant pooled relative risk for bipolar I disorder in relatives (relative risk=1.8, 95% CI=1.3–2.6; z=3.3, p=0.001). These analyses report the risk to relatives for bipolar I disorder, regardless of whether the relative also had ADHD. For ADHD probands, the weighted prevalence of bipolar I disorder was 6.8% in offspring, 5.9% in siblings, and 5.1% in parents. For comparison probands, the weighted prevalence of bipolar I was 3.5% in offspring, 2.8% in siblings, and 3.1% in parents. Figure 3 is a forest plot of these results. The pooled estimate remained significant after deleting each observation or contributing study in separate runs and then rerunning the meta-analysis. We found no significant heterogeneity among studies, which suggests that these studies were estimating a common relative risk (I2=0, χ2=7.1, df=10, p=0.72). We found no significant effects of study sample and design features (p>0.06 in all cases) and found no evidence of publication bias (t=0.99, p=0.35). The relative risks for parents, offspring, and siblings were 1.7, 2.0, and 2.2, respectively.
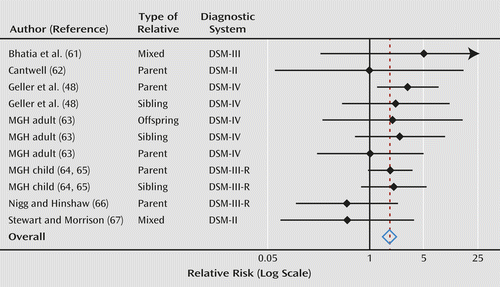
a For each comparison, the dot is the relative risk and the horizontal line is the 95% confidence interval (95% CI). The center of the diamond at the bottom is the weighted relative risk across all studies, and the width of the diamond is its 95% CI. MGH=reanalysis of child and adult proband studies from Massachusetts General Hospital Pediatric Psychopharmacology Program (see Method section).
The above two meta-analyses demonstrate the transmission of ADHD in bipolar I families and of bipolar I disorder in ADHD families, but they do not address the potential for heterogeneity in the familial transmission of comorbid ADHD and bipolar I disorder. Because only the Massachusetts General Hospital studies addressed the issue of heterogeneity, we could not conduct a multistudy meta-analysis. Instead, we present a combined analysis of the Massachusetts General Hospital pediatric samples. In these samples, the prevalence of comorbid ADHD and bipolar I disorder was 1.6% among 61 relatives of 19 bipolar I probands, 2.3% among 511 relatives of 162 ADHD probands, 5% among 626 relatives of 220 comorbid ADHD and bipolar I probands, and 1% among 411 relatives of 136 comparison probands (χ2=35.1; p<0.0001). Pairwise comparisons revealed significant differences between the relatives of comorbid ADHD and bipolar I probands and the relatives of ADHD probands (odds ratio=2.21; 95% CI=1.11–4.44; p=0.03) and comparison probands (odds ratio=6.58; 95% CI=1.99–21.75; p=0.002). No significant differences were found 1) between relatives of comorbid ADHD and bipolar I probands and relatives of bipolar I probands; 2) between relatives of ADHD probands and comparison probands; 3) between relatives of bipolar I probands and comparison probands; and 4) between relatives of ADHD probands and relatives of bipolar I probands.
Discussion
From our two meta-analyses, we can draw several firm conclusions based on 6,238 relatives in pediatric bipolar I family studies and 3,478 relatives in ADHD family studies. These studies found a significantly higher prevalence of ADHD among relatives of bipolar I probands and a significantly higher prevalence of bipolar I disorder among relatives of ADHD probands. These results could not be accounted for by publication biases, unusual results from any one observation, sample characteristics, or study design feature. We also found no evidence of heterogeneity among the ADHD or bipolar I family studies.
The family studies of pediatric bipolar I disorder are especially compelling because there are many studies and all of them found a greater risk for ADHD among relatives. However, in contrast to the bipolar I family studies, four of the ADHD family studies reported relative risks less than or equal to 1.0, which suggests no familial link between ADHD and bipolar I disorder. Notably, two of these studies used DSM-II criteria, and all of the negative studies had wide confidence intervals that overlapped with the pooled relative risk. Thus, although those findings were nominally negative, they are consistent with the significant pooled relative risk that we report here, a conclusion that is also supported by the lack of heterogeneity among studies.
Our meta-analyses cannot completely address all the potential artifacts that might compromise the diagnosis of ADHD and bipolar I disorder in youths (24), but our findings do provide some insights. One potential artifact is overlap in clinical features. The mood criterion associated with mania in youths is frequently that of severe and pervasive irritability, which, although not a diagnostic criterion for ADHD, is observed (albeit to a much lesser degree) in people with ADHD because of deficits in emotional self-regulation (68–71). In addition, mania and ADHD share the symptoms of talkativeness, distractibility, and hyperactivity. Although the pressured speech and agitation of mania differ phenomenologically from the talkativeness and hyperactivity of ADHD, it is possible that these symptom classes could be confused in some cases.
Given the greater predominance of irritable mood in youths compared with adults, if misdiagnosis from overlapping symptoms causes ADHD and bipolar I comorbidity in pediatric bipolar disorder, we should have observed a stronger relative risk for studies diagnosing bipolar I disorder in children compared with those diagnosing it in adults. In contrast to this prediction, the relative risk for ADHD from the child studies (2.8, 95% CI=2.1–3.7) was similar to the relative risk from the adult proband studies (2.6, 95% CI=1.9–3.4).
The ADHD proband studies also provided some insights into the issue of misdiagnosis. Among the positive studies, three diagnosed bipolar I disorder in adult relatives of ADHD probands and two diagnosed bipolar I disorder among the child relatives of ADHD probands. The relative risk for bipolar I disorder was 2.2 in the studies of adult relatives (95% CI=1.4–3.6) and 2.1 in the studies of child relatives (95% CI=0.9–4.6). These nearly identical relative risks suggest that misdiagnosing emotionally dysregulated ADHD youths as pediatric bipolar I in relatives may not account for the positive results. These data suggest that the familial link between ADHD and bipolar I disorder cannot be attributed to the diagnostic controversies about pediatric bipolar disorder unless one posits that those same controversies apply to adults.
Regarding misdiagnosis, the samples diagnosed by the Massachusetts General Hospital group (data collected by the authors of this article) have been described as deviating from “classic” presentations of bipolar I disorder by allowing “severe, nonepisodic irritability” to qualify for the mood criterion (72). It is more accurate to describe the Massachusetts General Hospital pediatric bipolar I samples as including patients who meet DSM-IV criteria by having distinct episodes of severe irritable mood lasting 1 week or longer on a background of chronic psychopathology. However, despite these concerns, if we exclude the Massachusetts General Hospital studies (or any other research group) from our meta-analyses, the estimated relative risks do not change and remain significant. Moreover, as Figures 2 and 3 show, the estimates of relative risk and confidence intervals from the Massachusetts General Hospital child proband studies are clearly consistent with the work of Geller et al. (48), which is considered a more conservative approach to the diagnosis of pediatric bipolar I disorder.
The existence of two potential artifacts that could cause comorbidity suggests that ADHD and bipolar I are the same disorder but that the diagnostic nomenclature has made an error by splitting one categorical syndrome into two or by separating a developmental precursor or causal risk factor (ADHD) from its ultimate outcome (bipolar I disorder) (24). The idea that ADHD and bipolar I disorder are the same disorder predicts that the familial risk for comorbid ADHD and bipolar I disorder should be the same for relatives of ADHD, bipolar I, and comorbid ADHD and bipolar I probands. As our pooled analysis of the Massachusetts General Hospital data sets showed, this prediction was rejected. Moreover, if ADHD and bipolar I are a unitary disorder, then rates of comorbidity should be the same regardless of whether they are estimated from samples ascertained for ADHD or bipolar I disorder, yet the rates are much higher for the latter method of ascertainment (11–14, 29). Finally, the idea that the two disorders are the same suggests that the treatments of the two disorders would be the same, which is not the case (25, 73, 74).
Another potential artifact suggests that ADHD and bipolar I comorbidity is due to a referral bias whereby people with two disorders are more likely to be clinically referred than people with one disorder. This bias is unlikely because ADHD and bipolar I comorbidity has been observed in epidemiological samples (17, 18). In family studies of ADHD and bipolar I probands, if comorbidity in the probands was due to referral biases, we would not expect to see comorbidity among the relatives because they were not selected based on clinical referral. In contrast to this prediction, comorbidity among relatives has been seen in ADHD (29, 30) or bipolar I (48, 75) family studies that addressed this issue.
More research is needed to fully assess the idea that ADHD and bipolar I comorbidity is an artifact. Such research should assess the mechanisms that explain the comorbidity of these disorders and their cotransmission in families. One possibility is that the condition we refer to as comorbid ADHD and bipolar I disorder is familially distinct from both ADHD and bipolar I disorder when these disorders are not comorbid with one another. As described by Pauls et al. (76), if two comorbid disorders form a familially distinct syndrome, the elevated risk to relatives for the comorbid condition should be limited to the relatives of comorbid patients. This idea was supported by our pooled analysis of the transmission of comorbid ADHD and bipolar I disorder in the Massachusetts General Hospital samples. Relative to comparison subjects, the risk for comorbid ADHD and bipolar I disorder was significantly higher among relatives of comorbid ADHD and bipolar I probands but not among relatives of probands with only one of these disorders. However, one ambiguity in these analyses is that although the risk in relatives for comorbid ADHD and bipolar I disorder was significantly higher in those of comorbid ADHD and bipolar I probands compared with those of ADHD probands, it did not differ from the risk to relatives of bipolar I probands, possibly because of the low number of bipolar I probands, which limited statistical power. Thus, these data provide fairly strong evidence that comorbid ADHD and bipolar I disorder is familially distinct from ADHD, but more research is needed to conclude that it is familially distinct from bipolar I disorder.
The validation of a distinct syndrome expressing both ADHD and bipolar I psychopathology requires more research. If confirmed, it could prove useful for researchers seeking to create homogeneous samples and to increase statistical power, as we have shown empirically for genetic studies (77). Considering the possibility that such a distinct syndrome exists might also provide a useful paradigmatic shift in how we view pediatric-onset bipolar I disorder. Rather than debating whether pediatric-onset bipolar I disorder is truly bipolar I disorder or whether it is misdiagnosed as ADHD, it might be more productive to study the empirical features of this syndrome to define it more accurately, assessing its concurrent and predictive validity and determining which ADHD youths are at highest risk for bipolar I comorbidity.
For clinicians, our findings establish that relatives of bipolar I patients have a greater risk for ADHD, and relatives of ADHD patients have a greater risk for bipolar I disorder. Although the risk for ADHD in bipolar I families is relatively high (27% for offspring and 30% for siblings), the risk for bipolar I in ADHD families is much lower (6.8% for offspring, 5.9% for siblings, and 5.1% for parents). Thus, although clinicians should be alert to the cotransmission of these disorders in families, the overall risk—especially for bipolar I disorder—should not be overemphasized.
The conclusions from our review of ADHD and bipolar I family studies are limited by some methodological issues. Like all meta-analyses, our analyses of covariates were limited by the information provided in the articles we reviewed. As is apparent in Tables 1–4, data were not uniformly available for some covariates, and other covariates did not have substantial variability among studies. Both of these problems limited our ability to detect significant covariate effects, but they would not have created spurious results. Although we found many relevant family studies of bipolar I disorder, we found fewer relevant family studies of ADHD, and two of these used DSM-II criteria. Nevertheless, because the power of meta-analysis derives from the total number of subjects across studies, we had sufficient power for our primary analyses.
Despite these limitations, our meta-analyses provide substantial evidence for a greater prevalence of ADHD among relatives of bipolar I probands and a significantly greater prevalence of bipolar I disorder among relatives of ADHD probands. These results could not be accounted for by publication biases, unusual results from any one observation, sample characteristics, or study design features. Our results suggest that ADHD and bipolar I comorbidity cannot be accounted for by misdiagnoses, but further work is needed to rule out artifactual sources of comorbidity.
1 : Juvenile manic activity. Childs Nerv Syst 1952; 9:363–368Medline, Google Scholar
2 : Mania in childhood and adolescence, in Mania: An Evolving Concept. Edited by Belmaker RHvan Praag HM. Jamaica, Spectrum Publications, 1980, pp 111–117Google Scholar
3 : Manic-depressive variant syndrome of childhood: diagnostic and therapeutic considerations. Clin Pediatr (Phila) 1983; 22:495–499Crossref, Medline, Google Scholar
4 : Hypomania in a four-year-old. J Am Acad Child Psychiatry 1984; 23:105–110Crossref, Medline, Google Scholar
5 : Developmental manifestations in a boy with prepubertal bipolar disorder. J Clin Psychiatry 1985; 46:441–443Medline, Google Scholar
6 : Embryonic mania. Child Psychiatry Hum Dev 1976; 6:149–154Crossref, Medline, Google Scholar
7 : Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995; 34:867–876Crossref, Medline, Google Scholar
8 : Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry 1995; 152:271–273Link, Google Scholar
9 : Complex and rapid-cycling in bipolar children and adolescents: a preliminary study. J Affect Disord 1995; 34:259–268Crossref, Medline, Google Scholar
10 : Affective comorbidity in children and adolescents with attention deficit hyperactivity disorder. Ann Clin Psychiatry 1995; 7:51–55Crossref, Medline, Google Scholar
11 : Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar Disord 2005; 7:483–496Crossref, Medline, Google Scholar
12 : ADHD and bipolar disorder: common causes common cure? J Atten Disord 2011; 15:99–100Crossref, Medline, Google Scholar
13 : Bipolar disorder comorbidity in children with attention deficit hyperactivity disorder. Psychiatry Res 2011; 186:333–337Crossref, Medline, Google Scholar
14 : Comorbidity of bipolar disorder in children and adolescents with attention deficit/hyperactivity disorder (ADHD) in an outpatient Turkish sample. World J Biol Psychiatry 2009; 10:488–494Crossref, Medline, Google Scholar
15 : Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry 1997; 36:1046–1055Crossref, Medline, Google Scholar
16 : Comorbidity of attention deficit hyperactivity disorder with early- and late-onset bipolar disorder. Am J Psychiatry 2000; 157:466–468Link, Google Scholar
17 : The lifetime impact of attention deficit hyperactivity disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Psychol Med 2011; 42:1–13Medline, Google Scholar
18 : Adult ADHD and its comorbidities, with a focus on bipolar disorder. J Affect Disord 2010; 124:1–8Crossref, Medline, Google Scholar
19 : The worldwide prevalence of ADHD: a systematic review and meta-regression analysis. Am J Psychiatry 2007; 164:942–948Link, Google Scholar
20 : Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry 2011; 72:1250–1256Crossref, Medline, Google Scholar
21 : CBCL clinical scales discriminate prepubertal children with structured interview-derived diagnosis of mania from those with ADHD. J Am Acad Child Adolesc Psychiatry 1995; 34:464–471Crossref, Medline, Google Scholar
22 : Risk factors for adolescent suicide: a comparison of adolescent suicide victims with suicidal inpatients. Arch Gen Psychiatry 1988; 45:581–588Crossref, Medline, Google Scholar
23 : Pediatric bipolar spectrum disorder and ADHD: comparison and comorbidity in the LAMS clinical sample. Bipolar Disord 2011; 13:509–521Crossref, Medline, Google Scholar
24 : Bipolar and ADHD comorbidity: both artifact and outgrowth of shared mechanisms. Clin Psychol (New York) 2010; 17:350–359Crossref, Medline, Google Scholar
25 : Molecular genetics of attention deficit hyperactivity disorder. Psychiatr Clin North Am 2010; 33:159–180Crossref, Medline, Google Scholar
26 : Latent class analysis shows strong heritability of the Child Behavior Checklist: juvenile bipolar phenotype. Biol Psychiatry 2006; 60:903–911Crossref, Medline, Google Scholar
27 : Prevalence and genetic architecture of Child Behavior Checklist: juvenile bipolar disorder. Biol Psychiatry 2005; 58:562–568Crossref, Medline, Google Scholar
28 : Family, twin, adoption, and molecular genetic studies of juvenile bipolar disorder. Bipolar Disord 2005; 7:598–609Crossref, Medline, Google Scholar
29 : Attention-deficit hyperactivity disorder with bipolar disorder: a familial subtype? J Am Acad Child Adolesc Psychiatry 1997; 36:1378–1387, discussion 1387–1390Crossref, Medline, Google Scholar
30 : Attention deficit hyperactivity disorder with bipolar disorder in girls: further evidence for a familial subtype? J Affect Disord 2001; 64:19–26Crossref, Medline, Google Scholar
31 : A controlled family study of children with DSM-IV bipolar I disorder and psychiatric comorbidity. Psychol Med 2010; 40:1079–1088Crossref, Medline, Google Scholar
32 : A pilot family study of childhood-onset mania. J Am Acad Child Adolesc Psychiatry 1995; 34:1577–1583Crossref, Medline, Google Scholar
33 : Patterns of psychopathology and dysfunction in high-risk children of parents with panic disorder and major depression. Am J Psychiatry 2001; 158:49–57Link, Google Scholar
34 : Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188Crossref, Medline, Google Scholar
35 : Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560Crossref, Medline, Google Scholar
36 : Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634Crossref, Medline, Google Scholar
37 : A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 2000; 95:89–98Google Scholar
38 : Statistical Methods for Meta-Analysis. Orlando, Fla, Academic Press, 1985Google Scholar
39 : Methods of Meta-Analysis: Correcting Error and Bias in Research Findings. Newbury Park, Calif, Sage Publications, 1990Google Scholar
40 : The behavior of maximum likelihood estimates under non-standard conditions, in Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. Berkeley, Calif, University of California Press, 1967, pp 221–233Google Scholar
41 : Stata User’s Guide: Release 9. College Station, Tex, Stata Corp LP, 2005Google Scholar
42 : Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry 2009; 66:287–296Crossref, Medline, Google Scholar
43 : Psychiatric disorders in preschool offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study (BIOS). Am J Psychiatry 2010; 167:321–330Link, Google Scholar
44 : Childhood behavior problems and bipolar disorder: relationship or coincidence? J Affect Disord 1993; 28:143–153Crossref, Medline, Google Scholar
45 : Clinical and psychological assessment of children of bipolar probands. Am J Psychiatry 1983; 140:548–553Link, Google Scholar
46 : The early manifestations of bipolar disorder: a longitudinal prospective study of the offspring of bipolar parents. Bipolar Disord 2007; 9:828–838Crossref, Medline, Google Scholar
47 : Temperament, life events, and psychopathology among the offspring of bipolar parents. Eur Child Adolesc Psychiatry 2007; 16:222–228Crossref, Medline, Google Scholar
48 : Controlled, blindly rated, direct-interview family study of a prepubertal and early-adolescent bipolar I disorder phenotype: morbid risk, age at onset, and comorbidity. Arch Gen Psychiatry 2006; 63:1130–1138Crossref, Medline, Google Scholar
49 : Diagnoses in school-age children of bipolar affective disorder patients and normal controls. J Affect Disord 1985; 8:283–291Crossref, Medline, Google Scholar
50 : Child Behavior Checklist profiles of children and adolescents with and at high risk for developing bipolar disorder. Child Psychiatry Hum Dev 2007; 38:47–55Crossref, Medline, Google Scholar
51 : Psychopathology in children aged 10–17 of bipolar parents: psychopathology rate and correlates of the severity of the psychopathology. J Affect Disord 1989; 16:167–179Crossref, Medline, Google Scholar
52 : Longitudinal study of diagnoses in children of women with unipolar and bipolar affective disorder. Arch Gen Psychiatry 1990; 47:1112–1117Crossref, Medline, Google Scholar
53 : Psychopathology in the offspring of parents with bipolar disorder: a controlled study. Biol Psychiatry 2005; 58:554–561Crossref, Medline, Google Scholar
54 : The offspring of bipolar manic-depressives: clinical features, in Adolescent Psychiatry: Developmental and Clinical Studies. Edited by Feinstein SLooney JSchwartzberg ASorosky A. Chicago, London, University of Chicago Press, 1982, pp 273–291Google Scholar
55 : Mood and disruptive behavior disorders and symptoms in the offspring of patients with bipolar I disorder. World Psychiatry 2008; 7:110–112Crossref, Medline, Google Scholar
56 : A high-risk study of bipolar disorder: childhood clinical phenotypes as precursors of major mood disorders. Arch Gen Psychiatry 2011; 68:1012–1020Crossref, Medline, Google Scholar
57 : The prevalence of psychopathology in offspring of bipolar women from a Brazilian tertiary center. Rev Bras Psiquiatr 2009; 31:240–246Crossref, Medline, Google Scholar
58 : Creativity in familial bipolar disorder. J Psychiatr Res 2005; 39:623–631Crossref, Medline, Google Scholar
59 : Temperament in child offspring of parents with bipolar disorder. J Child Adolesc Psychopharmacol 2008; 18:589–593Crossref, Medline, Google Scholar
60 : A follow-up investigation of offspring of parents with bipolar disorder. Am J Psychiatry 1988; 145:506–509Link, Google Scholar
61 : Attention deficit disorder with hyperactivity among pediatric outpatients. J Child Psychol Psychiatry 1991; 32:297–306Crossref, Medline, Google Scholar
62 : Psychiatric illness in the families of hyperactive children. Arch Gen Psychiatry 1972; 27:414–417Crossref, Medline, Google Scholar
63 : Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry 2006; 163:1720–1729Link, Google Scholar
64 : Family study of girls with attention deficit hyperactivity disorder. Am J Psychiatry 2000; 157:1077–1083Link, Google Scholar
65 : Further evidence for family-genetic risk factors in attention deficit hyperactivity disorder: patterns of comorbidity in probands and relatives psychiatrically and pediatrically referred samples. Arch Gen Psychiatry 1992; 49:728–738Crossref, Medline, Google Scholar
66 : Parent personality traits and psychopathology associated with antisocial behaviors in childhood attention-deficit hyperactivity disorder. J Child Psychol Psychiatry 1998; 39:145–159Crossref, Medline, Google Scholar
67 : Affective disorder among the relatives of hyperactive children. J Child Psychol Psychiatry 1973; 14:209–212Crossref, Medline, Google Scholar
68 : Deficient emotional self-regulation and adult attention deficit hyperactivity disorder: a family risk analysis. Am J Psychiatry 2011; 168:617–623Link, Google Scholar
69 : Deficient emotional self-regulation and pediatric attention deficit hyperactivity disorder: a family risk analysis. Psychol Med 2012; 42:639–646Crossref, Medline, Google Scholar
70 : Severity of the aggression/anxiety-depression/attention Child Behavior Checklist profile discriminates between different levels of deficits in emotional regulation in youth with attention-deficit hyperactivity disorder. J Dev Behav Pediatr 2012; 33:236–243Crossref, Medline, Google Scholar
71 : Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): clinical correlates and familial prevalence. J Child Psychol Psychiatry 2010; 51:915–923Crossref, Medline, Google Scholar
72 : Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry 2011; 168:129–142Link, Google Scholar
73 : Methylphenidate in the treatment of children and adolescents with bipolar disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2007; 46:1445–1453Crossref, Medline, Google Scholar
74 : How informative are open-label studies for youth with bipolar disorder? a meta-analysis comparing open-label versus randomized, placebo-controlled clinical trials. J Clin Psychiatry 2012; 73:358–365Crossref, Medline, Google Scholar
75 : Further evidence that pediatric-onset bipolar disorder comorbid with ADHD represents a distinct subtype: results from a large controlled family study. J Psychiatr Res (Epub ahead of print, Sep 11, 2012)Google Scholar
76 : Gilles de la Tourette’s syndrome and obsessive-compulsive disorder: evidence supporting a genetic relationship. Arch Gen Psychiatry 1986; 43:1180–1182Crossref, Medline, Google Scholar
77 : Familial links between attention deficit hyperactivity disorder, conduct disorder, and bipolar disorder. Curr Psychiatry Rep 2002; 4:146–152Crossref, Medline, Google Scholar



