Increased Amygdala Responses to Sad But Not Fearful Faces in Major Depression: Relation to Mood State and Pharmacological Treatment
Abstract
Objective:
Increased amygdala response to negative emotions seen in functional MRI (fMRI) has been proposed as a biomarker for negative emotion processing bias underlying depressive symptoms and vulnerability to depressive relapse that are normalized by antidepressant drug treatment. The purpose of this study was to determine whether abnormal amygdala responses to face emotions in depression are related to specific emotions or change in response to antidepressant treatment and whether they are present as a stable trait in medication-free patients in remission.
Method:
Sixty-two medication-free unipolar depressed patients (38 were currently depressed, and 24 were in remission) and 54 healthy comparison subjects underwent an indirect face-emotion processing task during fMRI. Thirty-two currently depressed patients were treated with the antidepressant citalopram for 8 weeks. Adherence to treatment was evaluated by measuring citalopram plasma concentrations.
Results:
Patients with current depression had increased bilateral amygdala responses specific to sad faces relative to healthy comparison subjects and nonmedicated patients in stable remission. Treatment with citalopram abolished the abnormal amygdala responses to sad faces in currently depressed patients but did not alter responses to fearful faces.
Conclusions:
Aberrant amygdala activation in response to sad facial emotions is specific to the depressed state and is a potential biomarker for a negative affective bias during a depressive episode.
Cognitive theories of depression suggest that symptoms arise from mood-congruent emotion processing bias, whereby overly negative attention or interpretations tend to favor negative emotional stimuli (1, 2). Previous imaging studies have demonstrated that the amygdala plays a key role in face-emotion processing (3–5). Several studies have reported that depression is associated with greater amygdala responses to negative (sad and fearful) emotions in faces (6, 7). Furthermore, amygdala responses to negative face emotions have been reported to be attenuated by antidepressant treatment in individuals with depression (8); however, it remains uncertain whether these changes are related to remission or to drug treatment (9) and whether such changes apply to negative emotions in general or only to specific emotions. The amygdala is particularly sensitive to fearful faces, with greater responses to this emotion compared with responses to any other face emotion (10). It is unclear from the literature whether the negative bias present in depression relates to enhanced amygdala responses to fear or to sadness (or both) or to attenuated responses to happy faces. In a study of depressed patients, Sheline et al. (8) reported increased amygdala response to fearful (and happy) faces that decreased with antidepressant treatment. The finding of increased amygdala activation in depressed patients in response to fearful faces was replicated by the same group of investigators (11, 12), but other studies have failed to replicate this finding (13, 14). Most of the reports suggest that amygdala responses to sad faces are exaggerated in depression. In a recent article, Victor et al. (9) used a combined cross-sectional and longitudinal design and found greater amygdala responses to subliminal, but not overtly presented, sad faces in currently depressed individuals relative to healthy comparison subjects, and the inverse was observed in response to happy faces. This is only in partial agreement with the findings of several previous studies that reported enhanced amygdala activation in response to both masked and unmasked sad faces (7, 15–21). Victor et al. also reported that antidepressant treatment reversed the abnormal amygdala responses, which is consistent with findings from previous functional MRI (fMRI) and positron emission tomography (PET) pretreatment studies (8, 15, 22–32); however, the relevance of this finding to the mechanism of action of antidepressants is unclear, since the authors also reported exaggerated responses to sad faces in nonmedicated patients in remission. This may reflect biological differences in the targets of pharmacological action and clinical improvement. For example, Suslow et al. (7) reported that currently depressed patients receiving antidepressant treatment had enhanced amygdala responses to sad faces and blunted responses to happy faces, and Fu et al. (33) reported that increased amygdala responses to sad faces normalized in nonmedicated depressed patients after their symptoms improved following treatment with cognitive-behavioral therapy (CBT). In the present study, we used an indirect face-emotion processing task in a combined cross-sectional and longitudinal design to test the hypotheses that negative bias in depression involves enhanced processing of both sad and fearful emotions and that antidepressants induce remission by correcting these biases. We predicted that currently depressed patients would show greater amygdala responses to both sad and fearful faces relative to healthy comparison subjects and to untreated patients in stable remission and that the responses would normalize only in those patients achieving remission while receiving antidepressant treatment.
Method
Participants
Sixty-two nonmedicated patients meeting DSM-IV criteria for unipolar major depression (38 were currently depressed and 24 were in remission) and 54 healthy comparison subjects were recruited for participation in the study (Table 1) (for details regarding patients' medication history and medication-free period, see the data supplement accompanying the online edition of this article). Both patients and healthy comparison subjects were screened with the Structured Clinical Interview for DSM-IV Axis I Disorders (34). For patients, severity of illness was established using the Montgomery-Åsberg Depression Rating Scale (MADRS) (35) and the seven-item Clinical Anxiety Scale, adapted from the Hamilton Anxiety Rating Scale (36). Currently depressed patients were required to have a MADRS score ≥20, and patients in remission were required to have a score ≤10. Patients were excluded from analyses if they had a concurrent comorbid axis I psychiatric disorder or primary cluster A or B axis II disorder. Exclusion criteria for both patients and comparison subjects were as follows: any unstable medical condition, neurological disorders, history of significant head trauma, lifetime history of substance or alcohol abuse, and any contraindication to MRI scanning. For healthy comparison subjects, a positive family history of psychiatric disorders was an additional criterion for exclusion. IQ was assessed using the Quick Test (37). Recruitment was conducted at the University of Manchester (United Kingdom). Informed consent was obtained from all participants, and each individual was compensated for participation in the study. The study was approved by the local research ethics committee and conformed to the principles in the Declaration of Helsinki (38).
| Currently Depressed Patients (N=38) | Depressed Patients in Remission (N=24) | Healthy Comparison Subjects (N=54) | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD | Mean | SD | p |
| Age (years) | 36.1 | 8.8 | 33.8 | 10.7 | 32.4 | 9.6 | 0.19 |
| IQ | 98.3 | 12.0 | 100.8 | 9.9 | 99.9 | 14.1 | 0.72 |
| Age at onset (years) | 21.9 | 8.2 | 24.5 | 10.0 | 0.29 | ||
| Number of past episodes | 3.3 | 3.0 | 2.90 | 2.4 | 0.55 | ||
| Duration of episodes (weeks) | 21.7 | 19.1 | |||||
| Montgomery-Åsberg Depression Rating Scale score | 27.0 | 4.2 | 2.0 | 3.0 | 0.51 | 1.2 | <0.001 |
| Clinical Anxiety Scale score | 7.7 | 4.2 | 0.75 | 1.2 | 0.2 | 0.5 | <0.001 |
TABLE 1. Demographic and Clinical Characteristics of Nonmedicated Currently Depressed Patients, Depressed Patients in Remission, and Healthy Comparison Subjectsa
Longitudinal Analyses and Antidepressant Treatment
After initial assessment, 32 currently depressed patients were treated with citalopram following a baseline MRI scan (visit 1) and then returned for a second scan (visit 2) after 8 weeks. A cohort of 15 healthy comparison subjects also underwent two MRI scans within an 8-week interval to control for potential nonspecific order and test-retest effects. Citalopram hydrobromide (20 mg/day) was prescribed, and the dose could be increased to 40 mg after 4 weeks of treatment according to response. All currently depressed patients had received a stable dose of citalopram for at least 4 weeks prior to the second scan. Citalopram adherence was assessed by measurement of citalopram plasma collected at the time of the second scan. Participants for whom citalopram plasma could not be detected were excluded from the visit 2 analyses (see the online data supplement).
Indirect Emotion Processing Task
The fMRI task consisted of blocked presentation of faces with happy, sad, fearful, and neutral emotions and rest blocks, with each image presented on a back projection screen that was visible to the participant via two mirrors attached to the head coil. Six faces (three were female faces) from a standardized series of facial expressions were presented (39). Participants were unaware of the hypotheses tested in the experiment and were required to press a button to indicate whether the face presented was a male or female face (see the online data supplement).
fMRI Data Acquisition and Statistical Analysis
Data acquisition was performed at the Wellcome Trust Clinical Research Facility (Manchester, United Kingdom). T2*-weighted images were acquired using a Philips Intera 1.5-T MRI scanner (Philips Healthcare, Andover, Mass.), with a single-shot gradient echo planar sequence (TR=2.1 seconds, TE=40 msec). Functional imaging data were analyzed using statistical parametric mapping (http://www.fil.ion.ucl.ac.uk/spm). A block design was carried out using the following contrasts: sad versus neutral faces and fearful versus neutral faces, happy versus neutral faces and neutral faces versus rest blocks. Whole-brain exploratory analysis was initially performed with an uncorrected p value <0.001. As the key prehypothesized brain region of interest, the amygdala was explored using masks derived from the Wake Forest University School of Medicine PickAtlas (Winston-Salem, N.C.) (40–43), with a small-volume-corrected family-wise error threshold set at a p value <0.05. Potential effects of clinical variables were explored using statistical parametric mapping with correlation analyses set at a p value <0.05 (family-wise error corrected) (see the online data supplement).
fMRI Behavioral Data
fMRI behavioral data, including accuracy in recognition of emotional faces and reaction times in response to face emotions, were recorded using E-Prime software (Psychology Software Tools, Inc., Sharpsburg, Pa.) and analyzed using SPSS, version 15.0 (SPSS, Inc., Chicago). Normally distributed demographic and clinical data were analyzed using analyses of variance (ANOVAs) and t tests as appropriate, and categorical variables were analyzed using chi-square tests.
Results
Details of the participants' clinical characteristics are summarized in Table 1. The groups did not differ significantly in gender distribution or with regard to mean age, IQ, number of episodes (for currently depressed and remitted patients), and age at illness onset. All participants were right-handed except four currently depressed patients and matched healthy comparison subjects.
fMRI Behavioral Performance
In all of the comparisons, the participants did not differ with regard to accuracy in recognition of the sex of the faces. Reaction times differed according to emotions (F=4.11, df=2.63, 113, p=0.01) and group (F=3.76, df=2, 113, p=0.03), but no significant emotion-by-group interaction was observed. Healthy comparison subjects were generally faster in face-emotion recognition than currently depressed patients (p=0.02) and depressed patients in remission (p=0.03). Pairwise comparisons indicated that currently depressed patients were generally slower than healthy comparison subjects in recognizing fearful faces than in recognizing neutral (p=0.02) and happy (p=0.03) faces.
fMRI Results
In an analysis of all participants across diagnostic groups, emotional faces, compared with neutral faces, elicited blood-oxygen-level-dependent (BOLD) responses bilaterally in the occipital areas, fusiform gyrus, amygdala, middle temporal gyrus, and frontal eye fields. There were no group differences in the neutral versus rest contrast, indicating that differences in responses to emotional faces were a result of altered response to the emotion rather than to the faces themselves (see the online data supplement).
Cross-Sectional Analyses
The full factorial ANOVA revealed an emotion-by-group interaction in the right amygdala (Montreal Neurological Institute [MNI] coordinates: x=21, y=0, z=–20; z=3.02; p=0.02, family-wise error corrected). Exploring each emotion separately revealed that this was a result of significant group differences in response to sad faces only in this region (MNI coordinates: x=32, y=0, z=–15; z=2.80; p=0.03, family-wise error corrected). We further explored each emotion versus neutral face-by-group interaction. Compared with depressed patients in remission and healthy comparison subjects, neural activity in currently depressed patients was greater in the amygdala in response to sad faces, whereas no statistical significance was observed for attenuated responses to happy faces (Figure 1). The ANOVA based on the sad-neutral contrast revealed a significant group interaction in the right and left amygdala (right: MNI coordinates: x=32, y=0, z=–15; z=2.99; p=0.02, family-wise error corrected; left: MNI coordinates: x=–28, y=0, z=–25; z=2.81; p=0.03, family-wise error corrected).
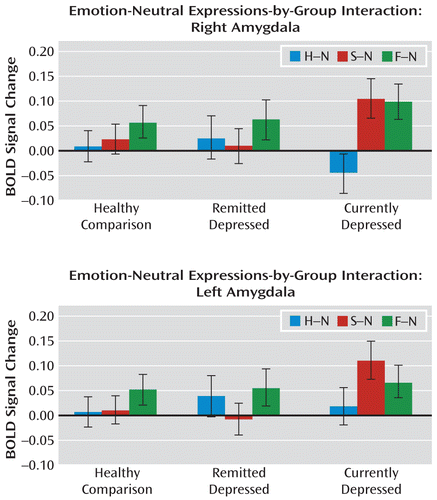
FIGURE 1. Emotional Versus Neutral Face-by-Group Interaction in Cross-Sectional Analyses of Currently Depressed Patients, Depressed Patients in Remission, and Healthy Comparison Subjectsa
a Analysis of variance revealed a group interaction for the sad-neutral contrast in the amygdala bilaterally (right: Montreal Neurological Institute [MNI] coordinates: x=32, y=0, z=–15; z=2.99; p=0.02, family-wise error corrected; left: MNI coordinates: x=–28, y=0, z=–25; z=2.81; p=0.03, family-wise error corrected); no significant differences in neural activity were measured for the happy or fear versus neutral contrasts. The three study groups are plotted against the averaged percentage of blood-oxygen-level-dependent (BOLD) signal change. Error bars indicate standard deviation. H–N=happy-neutral contrast; S–N=sad-neutral contrast; F–N=fear-neutral contrast.
Post hoc analyses.
Post hoc analyses were carried out to investigate the pattern of neural responses across groups for the sad-neutral contrast. Table 2 and Figure 2 demonstrate that neural activity in currently depressed patients was significantly increased in the amygdala bilaterally relative to both depressed patients in remission (left: MNI coordinates: x=–28, y=0, z=–25; z=3.36; p=0.006, family-wise error corrected; right: MNI coordinates: x=32, y=0, z=–15; z=3.60; p=0.002, family-wise error corrected) and healthy comparison subjects (left: MNI coordinates: x=–28, y=0, z=–25; z=2.73; p=0.03, family-wise error corrected; right: MNI coordinates: x=21, y=0, z=–20; z=2.83; p=0.03, family-wise error corrected).
| Comparison | Region | Voxelsa | pb | Montreal Neurological Institute Coordinates (x, y, z) | z Score |
|---|---|---|---|---|---|
| Cross-sectional | |||||
| Currently depressed > remitted depressed | |||||
| Right | 9 | 0.002 | 32, 0, –15 | 3.60 | |
| Left | 7 | 0.006 | –28, 0, –25 | 3.36 | |
| Currently depressed > healthy comparison | |||||
| Right | 1 | 0.03 | 21, 0, –20 | 2.83 | |
| Left | 1 | 0.03 | –28, 0, –25 | 2.73 | |
| Longitudinal (visit 1–visit 2)c | |||||
| Currently depressed | |||||
| Right | 1 | 0.03 | 32, –4, –20 | 2.87 | |
| Left | 0.05 | –28, 0, –25 | 2.61 | ||
| Currently depressed in full remission | |||||
| Right | 10 | 0.003 | 32, –4, –20 | 3.59 | |
| Left | 3 | 0.02 | –28, 0, –25 | 3.03 | |
TABLE 2. Suprathreshold Increased Neural Activity in the Amygdala in Response to the Sad-Neutral Contrast in Cross-Sectional and Longitudinal Comparisons in Currently Depressed Patients Relative to Nonmedicated Depressed Patients in Remission and to Healthy Comparison Subjects
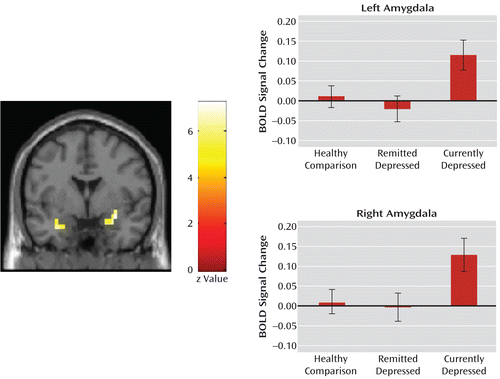
FIGURE 2. Neural Responses to the Sad-Neutral Contrast in the Left and Right Amygdala in Cross-Sectional Analyses of Currently Depressed Patients, Depressed Patients in Remission, and Healthy Comparison Subjectsa
a Post hoc analyses of the effect of group on the sad-neutral contrast revealed increased neural activity in the left and right amygdala in currently depressed patients relative to depressed patients in remission (right: Montreal Neurological Institute [MNI] coordinates: x=32, y=0, z=–15; z=3.60; p=0.002, family-wise error corrected; left: MNI coordinates: x=–28, y=0, z=–25; z=3.36; p=0.006, family-wise error corrected) and healthy comparison subjects (right: MNI coordinates: x=21, y=0, z=–20; z=2.83; p=0.03, family-wise error corrected; left: MNI coordinates: x=–28, y=0, z=–25; z=2.73; p=0.03, family-wise error corrected). The three study groups are plotted against the averaged percentage of blood-oxygen-level-dependent (BOLD) signal change (p=0.01). Error bars indicate standard deviation.
Longitudinal Analyses
Thirty-two currently depressed patients treated with citalopram returned for their second scan (mean MADRS score: 6.4 [SD=5.6]; mean Clinical Anxiety Scale score: 1.25 [SD=1.7]), and 25 had achieved full remission (mean MADRS score: 3.84 [SD=2.8]; mean Clinical Anxiety Scale score: 0.7 [SD=1.1]). The mean citalopram plasma concentration was 40.8 ng/ml (SD=21.1). Two remitted patients with undetectable citalopram were excluded from the visit 2 analyses, leaving 23 patients. Results for currently depressed patients and healthy comparison subjects are presented in Table 2 and Figure 3. For healthy comparison subjects, no significant difference was seen in amygdala responses to any emotion between visit 1 and visit 2. Amygdala responses to fearful and happy faces did not significantly change with treatment in currently depressed patients; however, there was an attenuated response to sad faces at visit 2 in the right and left amygdala in patients who achieved full remission (right: MNI coordinates: x=32, y=–4, z=–20; z=3.59; p=0.003, family-wise error corrected; left: MNI coordinates: x=–28, y=0, z=–25; z=3.03; p=0.02, family-wise error corrected). Including the two patients for whom citalopram could not be detected in their plasma at the second scan (N=25) and including patients who did and did not remit (N=30) did not alter these findings (see the online data supplement). In this sample, there was an insufficient number of depressed patients who did not achieve remission (those with poor response or lack of response) to compare those who did achieve remission with those who did not.
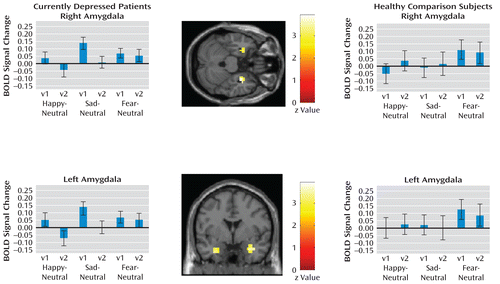
FIGURE 3. Longitudinal Analyses of Citalopram-Treated Currently Depressed Patients and Healthy Comparison Subjectsa
a Contrast plots depict change with antidepressant treatment in amygdala response to emotional relative to neutral faces in the whole group of currently depressed patients analyzed (those who did and did not achieve remission, N=30). There was attenuation with treatment in the sad-neutral condition in the whole group of currently depressed patients (right: Montreal Neurological Institute [MNI] coordinates: x=32, y=–4, z=–20; p=0.03, family-wise error corrected; left: MNI coordinates: x=–28, y=0, z=–25; p=0.05, family-wise error corrected) and in patients achieving remission (N=23), analyzed separately, in the same voxel bilaterally (right: MNI coordinates: x=32, y=–4, z=20; p=0.003, family-wise error corrected; left: MNI coordinates: x=–28, y=0, z=–25; p=0.02, family-wise error corrected). Happy or fearful face contrasts to neutral faces were not significantly different between groups in all cases. The overlays depict attenuated responses in the amygdala in the sad-neutral condition (N=23) (p=0.01). Contrast plots depict change with treatment in amygdala response to emotional relative to neutral faces in healthy comparison subjects (N=15) in the voxel that demonstrated a significant main effect of the fear-neutral condition across both healthy subjects and currently depressed patients in the amygdala bilaterally (p<0.05, family-wise error corrected) (right: MNI coordinates: x=21, y=–4, z=–20; left: x=21, y=–4, z=–20). Error bars indicate standard deviation. BOLD=blood oxygen level dependent; v1=visit 1; v2=visit 2.
Post hoc analyses
We compared neural activity in the amygdala in response to face emotions in medicated currently depressed patients who had achieved full remission at visit 2 (N=23) relative to nonmedicated depressed patients in remission (N=24). At visit 2, there was an effect of emotion in the right amygdala (MNI coordinates: x=32, y=0, z=–30; p=0.02, family-wise error corrected), which was driven by attenuated responses to faces in the happy-neutral contrast in medicated patients in remission relative to nonmedicated patients in remission (MNI coordinates: x=32, y=0, z=–30; p<0.05, family-wise error corrected). There were no differences between the groups in response to sad or fearful faces. Correlational analyses in currently depressed patients did not demonstrate a significant relationship between depression, anxiety severity, change in symptoms with treatment, or other clinical variables and amygdala response to happy, sad, or fearful faces or change in response with treatment. Exploratory correlational analyses revealed that bilateral parahippocampal activation in response to sad faces negatively correlated with change in depression score (see the online data supplement).
Discussion
The main finding of our study was that significantly increased activity in the amygdala in response to sad but not fearful faces was observed in currently depressed patients, and this aberrant activation was normalized with successful antidepressant treatment. No group differences were observed in response to happy faces or in response to simply viewing the faces themselves. Nonmedicated depressed patients in remission did not differ from healthy comparison subjects in any of their responses.
This study is the first, to our knowledge, to report amygdala responses to both fearful and sad faces in the same group of patients relative to healthy comparison subjects. Contrary to our prediction, responses to fearful faces did not differentiate depressed patients from healthy comparison subjects in any of the comparisons. Our findings support a selective role of the amygdala in the processing of sad faces in depression and in relation to improvement of symptoms. Major depression presents with a variable intensity of anxiety and frequently with comorbid axis I anxiety disorders (44); however, the contribution of anxiety symptoms to greater amygdala responses to fearful faces remains poorly investigated (8, 11, 12). We excluded patients with any comorbid anxiety disorder, and thus it is possible that comorbid anxiety disorders may have contributed to previous findings of increased amygdala response to fearful faces in depressed patients (6, 12). This is consistent with the findings of Fales et al. (12), who reported that controlling for anxiety disorders abolished some of the effects seen in the amygdala in depressed patients in response to fearful faces. Similarly, increased activation in this region in response to fearful faces was related to trait anxiety in healthy comparison subjects in another study (45) and to the severity of anxiety symptoms in a study of children with generalized anxiety disorder (46).
Our findings suggest that the increased amygdala responses to sad faces were state anxiety-related in the currently depressed group, since this activation was abolished after short-term citalopram treatment and was not observed in nonmedicated patients in remission. This observation is consistent with findings from previous treatment studies (8, 9, 15, 22–32). However, in a study by Victor et al. (9), nonmedicated depressed patients in remission were reported to have enhanced amygdala responses to masked sad faces that were similar to responses in nonmedicated patients for whom successful treatment had normalized the amygdala response. This contrasts with our findings and could be interpreted as a direct effect of antidepressant treatment masking a continuing abnormality that could confer vulnerability to depression. Consistent with this are results from studies of both acute (47) and repeated (48) administration of selective serotonin reuptake inhibitors (SSRIs) in healthy volunteers that have demonstrated attenuated amygdala responses to fearful faces, indicating that there are direct effects of SSRIs on amygdala responses to emotional faces. However, this cannot explain our finding of similar amygdala responses between nonmedicated patients in remission and healthy comparison subjects, and we previously reported the same finding in a larger cohort of both medicated and nonmedicated depressed patients in remission, which included the patients reported in the present study (49). However, there has been relatively little research of nonmedicated patients in remission. In a task using fearful faces, Norbury et al. (50) observed no alteration in amygdala responses in nonmedicated patients in remission, similar to our findings. Additionally, Fu et al. (33) reported that improvement in depression following CBT normalized pretreatment increased amygdala responses to sad faces. In contrast, in a study using PET imaging in combination with an indirect face-emotion processing task, Neumeister et al. (51) observed increased metabolism in the amygdala in response to sad faces in major depressive disorder patients in remission. Thus, it is not currently possible to fully reconcile the contradictory findings in the literature, but one possible explanation could be the face-emotion paradigm used and that a subliminal presentation is better able to uncover abnormal amygdala sensitivity in individuals with remitted depression than an indirect task in which the emotion can be recognized.
We did not observe significant differences between groups in amygdala responses to happy faces in our cross-sectional analyses. Other studies have not demonstrated a consistent pattern of amygdala activation in response to happy faces in depressed individuals, with results ranging from no significant differences (17, 52) to attenuated responses (8). Some studies have reported increased amygdala responses following treatment (9, 17), whereas we found no significant effect, and in fact the response appeared to be lower in our patients (Figure 1). Thus, it is not clear what role, if any, altered amygdala response to happy faces plays in depression and response to treatment.
Given previous evidence for the attenuation in amygdala responses to fear following repeated SSRI treatment in healthy volunteers (47, 48) and acute citalopram administration in comparison subjects and nonmedicated patients in remission (53), we expected to find attenuated responses to this emotion in currently depressed patients treated with citalopram compared with nonmedicated depressed patients in remission. A number of possible explanations exist with regard to our finding of no differences between the two groups. These possible explanations are that 1) there exists a difference in amygdala modulation between patients with a history of depression and healthy volunteers as a result of chronic SSRI treatment in depressed patients; 2) there is a disappearance of the effect with a longer duration of treatment (8 weeks in the present study compared with 1–2 weeks in studies of healthy volunteers); or 3) the stage of remission (early versus established) modulates the effect. The one significant difference we observed between the groups was a decreased right amygdala response to happy faces in patients receiving the SSRI. While this may have been a chance finding, a genuine difference could reflect the difference in the duration of remission, or more speculatively, this could contribute to the poorly understood description by some patients of a damping down of emotions with SSRI treatment (54).
Victor et al. (9) reported group differences, similar to those reported in our study, in a task using masked emotional faces but not when emotional faces were presented without masking. The authors inferred that the emotional biases in amygdala responses in depressed individuals are automatic, below conscious awareness, and strengthened by masking. This reasoning is in line with our results and the findings of others (15) of robust differences in amygdala response to sad faces in tasks using indirectly presented unmasked faces. The important factor may be that both masked and indirect unmasked tasks reduce conscious attention to the emotional information in faces, leaving automatic emotion processing to predominate. In keeping with this theory, a recent study of depression reported enhanced amygdala responses in depressed patients relative to comparison subjects only in response to unattended (but not attended) fearful faces (12), although, as discussed above, the enhanced responses to this emotion may relate to an anxiety component rather than to depression itself.
Although given the literature, our study was focused a priori on the amygdala and its central role in emotion processing, it is important to recognize that the amygdala is part of a wider network involved in affective processing. The amygdala is not a unitary structure, and topographically lateral nuclei receive sensory inputs with projections to basolateral and central complexes involved in amygdala efferents and emotional memory (4). Taking into account that our study did not have the spatial resolution to explore regional activation within the amygdala, it is of interest that we observed the greatest signal change in lateral areas, similar to those reported by Victor et al. (9) and consistent with an abnormality at the early stages of information processing when sensory information is being integrated in emotion centers of the brain. Our whole-brain analysis demonstrates, similar to other studies (9, 22), that the processing of sad facial expressions involves a network that includes the amygdala, hippocampus/parahippocampal gyrus, and insula, and it is known that the anatomical pathways between the amygdala and hippocampus play a role in encoding, retrieval, memory consolidation, and storage of emotional information (55, 56). Additionally, decreased hippocampal volume has been consistently reported in depression (57). While we did not find that depression severity correlated with amygdala activation in response to sad or fearful faces, our exploratory analysis did reveal significant correlations between anxiety and depression severity scores and BOLD signal change in the parahippocampal gyrus (see the online data supplement).
Strengths of this study include the large sample of currently depressed patients and depressed patients in remission, measures repeated in the same patients before and after treatment, and control for nonspecific repetition effects by testing healthy comparison subjects over the same interval. However, our study does have several limitations. Currently depressed patients experienced symptoms in the moderate range, and we did not include more severely ill patients who may have shown a different pattern of abnormality. We also cannot be certain that the same results would have been obtained using face-emotion tasks of a different design. Further studies including masked expressions of sadness and fear could help clarify the role of these emotions in relation to pharmacological treatment and clinical improvement. The implications of the findings with regard to vulnerability to depression and to the effect of treatment itself are uncertain without studies of relatives of depressed patients or studies of medicated and nonmedicated depressed patients in remission at the same distance in time from a depressive episode. We aimed to compare patients who achieved remission with those who did not, but our study had insufficient power to test this hypothesis. Inspection of the data did not suggest a difference between patients who achieved remission and those who improved. A larger sample of patients who did not remit with treatment is needed to separate the role of drug effects from the effects of remission on face-emotion processing. Finally, a placebo-treated group of depressed patients would allow changes as a result of remission itself to be identified without the confounding variable of drug treatment.
In conclusion, we have demonstrated that abnormal amygdala responsivity to indirect processing of sad faces in depression appears specific to this particular emotion and that it is bilateral and appears to be a state, rather than trait, abnormality. Further work is needed to determine how specific this is to unipolar depression compared with other psychiatric disorders, such as bipolar depression. Our findings also raise a question about the validity of the use of fearful face-emotion processing with the parameters we used as a biomarker for antidepressant action in healthy volunteers, given that amygdala activation was not related to state or trait depression or altered by antidepressant treatment in our sample.
1. : The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry 2008; 165:969–977Link, Google Scholar
2. : Cognitive reactivity and vulnerability: empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clin Psychol Rev 2005; 25:487–510Crossref, Medline, Google Scholar
3. : The amygdala and its place in the cerebral hemisphere. Ann N Y Acad Sci 2003; 985:174–184Crossref, Medline, Google Scholar
4. : The amygdaloid complex: anatomy and physiology. Physiol Rev 2003; 83:803–834Crossref, Medline, Google Scholar
5. : The amygdala: vigilance and emotion. Mol Psychiatry 2001; 6:13–34Crossref, Medline, Google Scholar
6. : Amygdala hyperactivation in untreated depressed individuals. Psychiatry Res 2009; 173:158–161Crossref, Medline, Google Scholar
7. : Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry 2010; 67:155–160Crossref, Medline, Google Scholar
8. : Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry 2001; 50:651–658Crossref, Medline, Google Scholar
9. : Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry 2010; 67:1128–1138Crossref, Medline, Google Scholar
10. : Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 2009; 34:418–432Medline, Google Scholar
11. : Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord 2009; 112:206–211Crossref, Medline, Google Scholar
12. : Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry 2008; 63:377–384Crossref, Medline, Google Scholar
13. : Neural correlates of perception of emotional facial expressions in out-patients with mild-to-moderate depression and anxiety. A multicenter fMRI study. Psychol Med 2011; 41:2253–2264Google Scholar
14. : fMRI activation in the amygdala and the orbitofrontal cortex in unmedicated subjects with major depressive disorder. Psychiatry Res 2010; 183:209–217Crossref, Medline, Google Scholar
15. : Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry 2004; 61:877–889Crossref, Medline, Google Scholar
16. : Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. Neuroimage 2004; 21:1215–1223Crossref, Medline, Google Scholar
17. : Neural responses to happy facial expressions in major depression following antidepressant treatment. Am J Psychiatry 2007; 164:599–607Link, Google Scholar
18. : A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol Psychiatry 2005; 58:495–503Crossref, Medline, Google Scholar
19. : Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 2004; 55:578–587Crossref, Medline, Google Scholar
20. : Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry 2008; 165:90–98Link, Google Scholar
21. : Amygdalar activation associated with happy facial expressions in adolescents: a 3-T functional MRI study. J Am Acad Child Adolesc Psychiatry 2003; 42:979–985Crossref, Medline, Google Scholar
22. : Amygdala reactivity predicts automatic negative evaluations for facial emotions. Psychiatry Res 2007; 154:13–20Crossref, Medline, Google Scholar
23. : The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry 2003; 160:64–75Link, Google Scholar
24. : Neural markers of symptomatic improvement during antidepressant therapy in severe depression: subgenual cingulate and visual cortical responses to sad, but not happy, facial stimuli are correlated with changes in symptom score. J Psychopharmacol 2009; 23:775–788Crossref, Medline, Google Scholar
25. : Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychol Med 1993; 23:579–590Crossref, Medline, Google Scholar
26. : Changes in regional cerebral blood flow on recovery from depression. Psychol Med 1995; 25:247–261Crossref, Medline, Google Scholar
27. : Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biol Psychiatry 2001; 50:171–178Crossref, Medline, Google Scholar
28. : Brain blood flow changes in depressed patients treated with interpersonal psychotherapy or venlafaxine hydrochloride: preliminary findings. Arch Gen Psychiatry 2001; 58:641–648Crossref, Medline, Google Scholar
29. : Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry 2001; 158:899–905Link, Google Scholar
30. : Paralimbic hypoperfusion in unipolar depression. J Nucl Med 1994; 35:929–934Medline, Google Scholar
31. : Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999; 156:675–682Abstract, Google Scholar
32. : The functional neuroanatomy of the placebo effect. Am J Psychiatry 2002; 159:728–737Link, Google Scholar
33. : Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol Psychiatry 2008; 64:505–512Crossref, Medline, Google Scholar
34. : Structural Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP). New York, New York State Psychiatric Institute, Biometrics Research, 2002Google Scholar
35. : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
36. : The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Crossref, Medline, Google Scholar
37. : The Quick Test (QT): provisional manual. Psychol Reports 1960; 11:111–161Crossref, Google Scholar
38.
39. : Pictures of Facial Affect. Palo Alto, Calif, Consulting Psychologists Press, 1976Google Scholar
40. : Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15:273–289Crossref, Medline, Google Scholar
41. : An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19:1233–1239Crossref, Medline, Google Scholar
42. : Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 2004; 21:450–455Crossref, Medline, Google Scholar
43. : Clinical implementation of spin-tag perfusion magnetic resonance imaging. J Comput Assist Tomogr 2008; 32:403–406Crossref, Medline, Google Scholar
44. : Major depressive disorder and axis I diagnostic comorbidity. J Clin Psychiatry 2002; 63:187–193Crossref, Medline, Google Scholar
45. : Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron 2004; 44:1043–1055Crossref, Medline, Google Scholar
46. : Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry 2001; 58:1057–1063Crossref, Medline, Google Scholar
47. : Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry 2009; 194:535–540Crossref, Medline, Google Scholar
48. : Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 2006; 59:816–820Crossref, Medline, Google Scholar
49. : Interaction between a history of depression and rumination on neural response to emotional faces. Psychol Med 2011; 41:1845-1855Crossref, Medline, Google Scholar
50. : Increased neural response to fear in patients recovered from depression: a 3T functional magnetic resonance imaging study. Psychol Med 2010; 40:425–432Crossref, Medline, Google Scholar
51. : Effects of a alpha 2C-adrenoreceptor gene polymorphism on neural responses to facial expressions in depression. Neuropsychopharmacology 2006; 31:1750–1756Crossref, Medline, Google Scholar
52. : A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry 2005; 57:201–209Crossref, Medline, Google Scholar
53. : The effect of acute citalopram on face emotion processing in remitted depression: a pharmacoMRI study. Eur Neuropsychopharmacol 2011; 21:140–148Crossref, Medline, Google Scholar
54. : Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry 2009; 195:211–217Crossref, Medline, Google Scholar
55. : The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 2004; 27:1–28Crossref, Medline, Google Scholar
56. : The basolateral amygdala modulates specific sensory memory representations in the cerebral cortex. Neurobiol Learn Mem 2009; 91:382–392Crossref, Medline, Google Scholar
57. : Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol 2012; 22:1–16Crossref, Medline, Google Scholar



