Reduced Metabotropic Glutamate Receptor 5 Density in Major Depression Determined by [11C]ABP688 PET and Postmortem Study
Abstract
Objective:
Clinical and preclinical evidence suggests a hyperactive glutamatergic system in clinical depression. Recently, the metabotropic glutamate receptor 5 (mGluR5) has been proposed as an attractive target for novel therapeutic approaches to depression. The goal of this study was to compare mGluR5 binding (in a positron emission tomography [PET] study) and mGluR5 protein expression (in a postmortem study) between individuals with major depressive disorder and psychiatrically healthy comparison subjects.
Method:
Images of mGluR5 receptor binding were acquired using PET with [11C]ABP688, which binds to an allosteric site with high specificity, in 11 unmedicated individuals with major depression and 11 matched healthy comparison subjects. The amount of mGluR5 protein was investigated using Western blot in postmortem brain samples of 15 depressed individuals and 15 matched comparison subjects.
Results:
The PET study revealed lower levels of regional mGluR5 binding in the prefrontal cortex, the cingulate cortex, the insula, the thalamus, and the hippocampus in the depression group relative to the comparison group. Severity of depression was negatively correlated with mGluR5 binding in the hippocampus. The postmortem study showed lower levels of mGluR5 protein expression in the prefrontal cortex (Brodmann's area 10) in the depression group relative to the comparison group, while prefrontal mGluR1 protein expression did not differ between groups.
Conclusions:
The lower levels of mGluR5 binding observed in the depression group are consonant with the lower levels of protein expression in brain tissue in the postmortem depression group. Thus, both studies suggest that basal or compensatory changes in excitatory neurotransmission play roles in the pathophysiology of major depression.
Almost all established antidepressants target the monoamine systems (1). However, full and partial resistance to these drugs and their delayed onset of action suggest that dysfunctions of monoaminergic neurotransmitter systems found in major depressive disorder represent the downstream effects of other, more primary abnormalities. Several lines of evidence suggest a glutamatergic dysfunction as a primary abnormality in depression. A single dose of ketamine, a glutamate N-methyl-d-aspartate (NMDA) receptor antagonist, has been shown to produce rapid and large antidepressant effects in patients with treatment-resistant depression (2). Inhibitors of glutamate release (e.g., lamotrigine, riluzole) have antidepressant properties (3). Abnormal glutamate levels were found in depressed patients in MR spectroscopy (4). Finally, there is evidence for abnormal NMDA signaling in postmortem tissue preparations in major depression (5).
Metabotropic glutamate receptors are known to regulate glutamate neurotransmission and interact with monoamine neurotransmitters that are involved in the neurobiology of depression (6). The metabotropic glutamate receptor 5 (mGluR5) has been proposed as an attractive target for modulating glutamatergic neurotransmission (7) because it is present not only at postsynaptic neurons but also on glia cells, where it appears to modulate the stimulation of extrasynaptic NMDA receptors (8). In rats, prolonged blockade of mGluR5 exerts strong anxiolytic- and antidepressant-like effects (9), and mGluR5 knockout mice express an antidepressant-like phenotype (10).
This is the first study to assess mGluR5 binding and protein expression in depressed individuals. We used positron emission tomography (PET) with the radiolabeled mGluR5 antagonist [11C]ABP688 (3-(6-methyl-pyridin-2-ylethynyl)-cyclohex-2-enone-O-carbon-11-methyl-oxime) (11), which binds to an allosteric site of the mGluR5 with high selectivity. To support the results from the PET study, a second study was performed to investigate mGluR5 protein levels in the postmortem prefrontal cortex. Next, in order to evaluate the specificity of potential mGluR5 abnormalities in depression, postmortem mGluR1 protein quantities were measured in the same cohort of depressed individuals.
Method
PET Study
Participants.
Participants in the PET study were 11 patients who met DSM-IV criteria for a current major depressive episode and for major depressive disorder (five were female; the mean age was 40.8 years [SD=13.9, range=22–59]; mean Beck Depression Inventory [BDI] score, 25.3 [SD=8.0, range=13–35]; mean Beck Anxiety Inventory [BAI] score, 18.6 [SD=10.5, range=6–37]) and 11 healthy comparison subjects (five were female; the mean age was 40.6 years [SD=14.2, range=23–62 years]; mean BDI score, 1.0 [SD=1.4, range=0–4]; mean BAI score, 0.6 [SD=0.8, range=0–2]). Additional clinical characteristics of the depressed sample are provided in Table S1 in the data supplement that accompanies the online edition of this article. Participants were recruited through advertisements in local newspapers and posters at Zurich University Hospital and were evaluated during screening visits in the hospital's outpatient psychiatry clinic. Psychiatric diagnoses were established using both an unstructured clinical interview by a psychiatrist and the Structured Clinical Interview for DSM-IV (SCID; 12, 13). The clinical evaluation also included a physical examination (and in some cases electrocardiography) and laboratory tests, including liver and kidney function tests, hematology profile, thyroid function tests, urine analysis, and toxicology (drug screen). Exclusion criteria included current medical or neurological disorders; lifetime history of psychosis, manic episode, substance dependence, autism, posttraumatic stress disorder, or eating disorders; exposure to psychotropic medications within 4 weeks of scanning (8 weeks for fluoxetine); regular cigarette smoking; and pregnancy. MRI was performed on each participant, and scans were assessed by a radiologist to exclude any brain structural pathology. Patients were enrolled in the study after receiving a full explanation of the purpose of the study and the study procedures and after providing written consent, as approved by the local ethical committee (Kantonale Ethikkommission Zürich).
PET.
We used a bolus-infusion protocol (14), which we had previously evaluated for PET with [11C]ABP688 (15). The incentive to use the bolus-infusion protocol was that an equilibrium between tracer in tissue and in blood can be achieved (14, 16). Prior to scanning, catheters were placed in the right antecubital vein for tracer injection. Scanning was conducted in a single session for each participant, using PET with [11C]ABP688 in three-dimensional mode in a whole-body scanner (Discovery VCT, GE Healthcare, Milwaukee) with an axial field of view of 14.6 cm and an in-plane resolution of 7 mm. For attenuation correction, a low-dose CT was acquired before tracer injection. Using a previously evaluated equilibrium protocol (15, 17), a total of 500–650 MBq in a 50-ml solution was administered with an infusion pump. Half of the tracer was given as a bolus over 2 minutes, and the other half was then infused over 58 minutes. Simultaneously with the start of the bolus injection, we initiated a series of 20 scans. Ten frames of 60 seconds were followed by 10 frames of 300 seconds (total duration, 60 minutes per acquisition). Transaxial images were reconstructed to a 128×128 matrix with 35 slices of 2.34×2.34×3.27 mm voxel size using filtered back-projection. In each participant, we verified that equilibrium was reached by analyzing the tissue time-activity curve in a cingular (high receptor density) and a cerebellar (low receptor density) region. The activity reached an equilibrium at 30 minutes (see Figure S1 in the online data supplement). The ratio of tissue activity divided by the cerebellar activity at equilibrium (CT/Ccer at 45–60 minutes) was chosen as a measure for receptor density divided by affinity. At equilibrium, CT/Ccer is equal to the ratio of the distribution volumes of the tracer in target and reference tissue: (CT/Ccer)eq=VT/VND, where V ND is the distribution volume of the nondisplaceable compartment (18), in our case the cerebellum. VT/VND is referred to as the distribution volume ratio (DVR). One can furthermore demonstrate that DVR equals BPT+ 1, where BPT is the binding potential in the target tissue, which is often used as a measure for receptor density divided by affinity (14).
Statistical analysis.
All calculations were performed using the PMOD software package, version 3 (www.pmod.com), and SPM99 (Wellcome Department of Imaging Neuroscience, London). The DVR images were then transformed to a common space, the Montreal Neurological Institute template, using PMOD, and subsequently transferred to SPM99. The PET images were filtered using a 15-mm Gaussian smoothing kernel to compensate for misalignment error arising during spatial normalization and individual anatomical variation.
Based on the postmortem study, we had the a priori hypothesis that mGluR5 binding was lower in the right prefrontal cortex. To qualitatively compare mGluR5 binding between the PET study and the postmortem study, we extracted PET data from a predefined region of interest of 32.4 cm3 (4,051 voxels) corresponding to the location of the postmortem tissue sample (anatomical borders of the region in Talairach coordinates: x[+10 to + 30], y[+60 to + 70], z[0 to +20]) and calculated the mean value for this region for each study participant. In an additional exploratory analysis using SPM99, the [11C]ABP688 DVR whole brain images were compared between groups in a voxel-wise analysis using a two-sample t test model. In separate analyses in the depression group, we calculated correlations between DVR images and BDI and BAI scores. Volumes with significant differences in DVR (Table 1) were transferred to PMOD for data extraction and display. Extracted mean values of the volumes of interest were used to estimate the magnitude of group differences (in percent) and to correlate individual DVR values with symptom dimension scores on the BAI and BDI.
| Talairach Coordinatesb | |||||
|---|---|---|---|---|---|
| Brain Region | x | y | z | T Values | Difference in DVR Between Groups (%) |
| Right inferior parietal lobe | 42 | –47 | 23 | 3.7 | –13.2 |
| Right mesencephalon/thalamus | 14 | –24 | –12 | 3.6c | –9.6 |
| Right middle/inferior temporal gyrus | 46 | –45 | –4 | 3.4 | –11.9 |
| Left inferior parietal lobe | –38 | –31 | 33 | 3.3 | –11.7 |
| Right anterior insula | 30 | 13 | 18 | 3.2 | –9.6 |
| Left middle/inferior temporal gyrus | –48 | –50 | 3 | 3.2 | –15.2 |
| Right precentral gyrus | 20 | –25 | 75 | 3.0 | –22.6 |
| Right anterior insula/inferior prefrontal gyrus | 34 | 33 | –2 | 2.9 | –9.6 |
| Right lateral prefrontal cortex (Brodmann's area 46) | 38 | 47 | 5 | 2.9 | –15.2 |
TABLE 1. Brain Regions With Lower [11C]ABP688 Distribution Volume Ratio (DVR) in Individuals With Major Depressive Disorder Relative to Comparison Subjects (N=22)a
Postmortem Study
Sample.
Postmortem brain samples were collected at autopsy from a total of 30 individuals at the Cuyahoga County Coroner's Office, Cleveland. Written informed consent was obtained from the legal next-of-kin, who were also interviewed. Retrospective psychiatric assessments were conducted in accordance with a protocol approved by the institutional review boards of the University Hospitals of Cleveland and the University of Mississippi Medical Center. There was no evidence of neurological disorders in any of the subjects. A trained interviewer administered the Schedule for Affective Disorders and Schizophrenia–Lifetime Version (SADS-L; 19) to knowledgeable next-of-kin of 10 of the depressed subjects and 10 of the comparison subjects. The SCID was administered to next-of-kin of the five remaining depressed and comparison subjects (13). Axis I psychopathology was assessed and consensus diagnosis was reached in conference using information from the interview and from medical records. Next-of-kin responses for the 10 depressed subjects evaluated with the SADS-L were also recorded in the SCID. All 15 of the depressed subjects were experiencing a depressive episode within the last 2 weeks of life. The mean duration of depression was 8.8 years (SD=10). Comparison subjects had no axis I diagnoses at the time of death other than nicotine dependence. Blood and urine samples from all subjects were examined by the coroner's office for psychotropic medications and substances of abuse, including alcohol. Fifteen depressed subjects and 15 comparison subjects were matched as closely as possible for age, gender, postmortem interval, brain pH, smoking history, and history of alcohol abuse (more details are presented in Tables S2 and S3 in the online data supplement).
Immunoblotting.
The study was carried out on blocks of tissue that were dissected from the frontal pole of the right hemisphere consisting of Brodmann's area 10. Tissue from the left hemisphere was not available. Frozen blocks were cut into sections 50 μm thick, and samples containing all six cortical layers of the gray matter of area 10 were collected and stored at –80°C until assayed. For the study of cerebellum tissue, several sections of the right cerebellar hemisphere were collected from psychiatrically healthy comparison subjects and stored until assayed. Western blotting was performed as previously described (5, 20) except that in the present study tissue samples were not heated before being subjected to gel electrophoresis. mGluR5 was labeled using anti-mGluR5 rabbit polyclonal antibody (1:1000; Millipore, Temecula, Calif.; no. AB5675), and mGluR1 was detected using anti-mGluR1 rabbit polyclonal antibody (1:500; Millipore; no. 07-617) and secondary anti-rabbit antibody (1:3000; Amersham Biosciences, Piscataway, N.J.; no. NA934). Actin was used as a control for transfer and loading and was detected on each blot using an anti-actin antibody (Millipore; no. MAB1501). To minimize interblot variability and to aid in quantifying blots, each gel was loaded with three concentrations of a cortical tissue standard (dissected from a psychiatrically healthy subject) consisting of 10, 20, and 40 μg of total protein. The same cortical tissue standard was used for all experimental gels.
Data analysis.
Immunoreactive bands were analyzed using MCID Elite, version 7.0 (Imaging Research, St. Catherines, Ontario). Linear regression was used to plot a standard curve for each gel, from which relative optical density values of samples were converted to cortical standard protein units for each experimental sample for each gel. To control for accuracy of tissue loading and efficiency of transfer, data were normalized to actin detected on the same blots. The final data are expressed in cortical standard protein units and presented as ratio of mGluR to actin. The data were analyzed statistically using a two-tailed unpaired t test, and linear regression analysis was performed to test for potential associations among age, pH, postmortem interval, time in freezer, and duration of depression and mGluR protein level. The threshold for statistical significance was set at a p value of 0.05.
Results
PET Study
The major depression sample included four participants who were naive to psychotropic drugs and six who had been medication free for a mean of 15.3 months (SD=13.2, range=4–96). Three participants had a comorbid anxiety disorder. The mean [11C]ABP688 dose administered did not differ significantly between the depression and comparison groups (mean=600 MBq [SD=49] and mean=565 MBq [SD=186], respectively).
In a region of interest defined a priori in Brodmann's area 10 (prefrontal cortex) corresponding to the tissue sample of the postmortem study, mGluR5 DVR was 8.8% (p=0.018) lower in the depression group relative to the comparison group. Table 1 and Figure 1 display the results of the analysis comparing [11C]ABP688 DVR between the depression and comparison groups. The depression group had lower DVR than the comparison group in a region that included the right mesencephalon and the right thalamus (cluster-level corrected p=0.057) and in the right inferior parietal lobe, the right inferior temporal gyrus, the left inferior parietal lobe, the right anterior insula, the left middle temporal gyrus, the precentral gyrus bilaterally, the lateral prefrontal cortex bilaterally, the posterior cingulate cortex, the medial orbital cortex bilaterally, the left frontal polar cortex, and the left hippocampus. No region showed higher [11C]ABP688 DVR in the comparison group than in the depression group at an uncorrected p value <0.01.
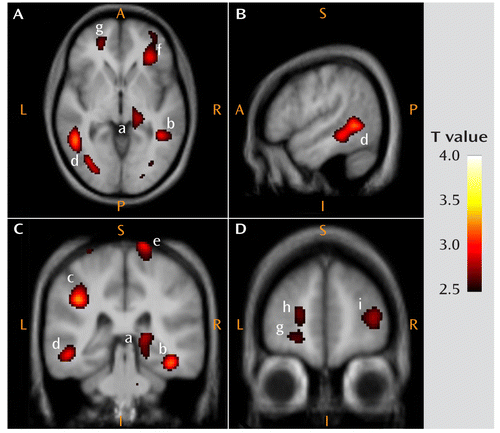
FIGURE 1. Brain Regions With Lower Metabotropic Glutamate Receptor Subtype 5 (mGluR5) Density in Patients With Major Depressive Disorder Relative to Comparison Subjectsa
a The voxel T values correspond to p<0.01 for the contrast of distribution volume ratio between groups and are displayed on axial (panel A [z=–2]), sagittal (panel B [x=–52]), and coronal (panel C [y=–33] and panel D [y=51]) sections of a spatially normalized and averaged MRI from the study sample. The regions are labeled with the following letters, ordered by decreasing T values (see Table 1): a=right mesencephalon/thalamus; b=right middle/inferior temporal gyrus; c=left inferior parietal lobe; d=left middle/inferior temporal gyrus; e=right precentral gyrus; f=right anterior insula/inferior prefrontal gyrus; g=left medial orbital cortex (Brodmann's area 11); h=left frontal polar cortex (Brodmann's area 10); i=right lateral prefrontal cortex (Brodmann's area 46). The color bar indicates the voxel T value. A=anterior; P=posterior; S=superior; I=inferior; L=left; R=right.
In the regions listed in Table 1 having lower mGluR5 binding in the depression group relative to the comparison group, we found significant negative correlations between depressive symptoms as assessed with the BDI and mGluR5 binding in the depression sample (N=11) in all regions (p values ranging from 0.04 to 0.0001). To identify correlations between illness severity and mGluR5 binding across the whole brain, we conducted a secondary voxelwise analysis on [11C]ABP688 DVR in the 11 participants with major depressive disorder. Depressive symptoms as assessed with the BDI were negatively correlated with mGluR5 binding in the hippocampus bilaterally (right side: cluster-level corrected p=0.029, see Figure 2; left side: uncorrected p<0.001). Anxiety symptoms as assessed with the BAI were negatively correlated with mGluR5 binding in the thalamus and orbital frontal cortex bilaterally, the right frontal polar cortex, and the left mid-cingulate cortex (all correlations at uncorrected p values <0.001). More details on these correlational analyses are presented in Table S4 in the online data supplement.
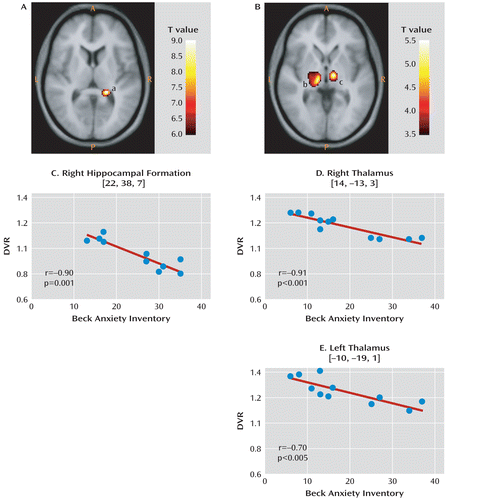
FIGURE 2. Correlations Between Metabotropic Glutamate Receptor 5 (mGluR5) Distribution Volume Ratio and Severity of Depressive and Anxiety Symptomsa
a Panels A and B show correlation maps on axial sections of a spatially normalized and averaged MRI from the study sample. In panel A (z=7), voxel T values correspond to p<0.0001; in panel B (z=–4), voxel T values correspond to p<0.003. In these panels, a=right hippocampal formation; b=left thalamus; c=right thalamus; A=anterior; P=posterior; L=left; R=right. In panels C, D, and E, symptom scores in the depressed sample (N=11) are plotted against the mGluR5 distribution volume ratio (DVR). Correlation coefficients and p values in the right hippocampal formation, the right thalamus, and the left thalamus were determined using Pearson's correlation. The regression lines were determined using linear regression.
Gender-based subsamples were too small for assessment of gender differences within diagnostic groups. Across both the depression and comparison groups, no significant gender difference in mGluR5 binding was evident in the regions of interest defined over the areas where the groups had differed most in the initial voxel-wise analysis. There was also no significant correlation between mGluR5 binding and age in these brain regions, either in the depression group or in the comparison group. In the depression group, there was no correlation between illness duration and mGluR5 binding in any of the regions that showed lower mGluR5 binding. Participants with a history of antidepressant use (N=7) had lower mGluR5 binding in the left precentral gyrus than those who were drug naive (N=4; p=0.003), and there was no difference in mGluR5 binding between these two subgroups in all other regions of interest. Figure S2 in the online data supplement displays mGluR5 DRV in the 11 participants classified by drug status and presence of comorbid anxiety disorders.
Postmortem Study
Western blot analyses of the prefrontal cortex from 15 depressed and 15 matched comparison subjects consistently revealed mGluR5 as a band corresponding to the molecular mass of 150 kDa. Figure 3 shows a representative immunoblot of mGluR5 and actin from three male depressed subjects and three male comparison subjects used in the analysis. The average mGluR5:actin ratio from depressed subjects (mean=0.85, SD=0.30) was significantly lower (–32%) than that of comparison subjects (mean=1.25, SD=0.52; t=2.61, df=28, p=0.014; Figure 4). As cerebellum radioactivity concentration was used to perform normalization of the PET data, we investigated the level of mGluR5 protein in the cerebellum. We did not detect mGluR5 immunoreactivity in the cerebellum from comparison subjects using Western blotting (see Figure S3 in the online data supplement).
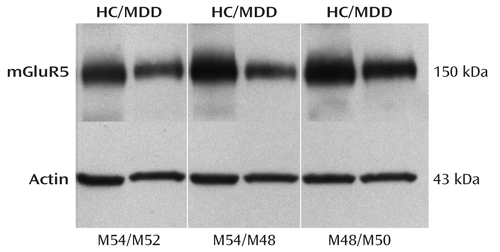
FIGURE 3. Metabotropic Glutamate Receptor 5 (mGluR5) Immunoreactivity in the Right Prefrontal Cortex (Brodmann's Area 10) From Six Male Subjects in the Postmortem Analysisa
a The bottom panel shows immunoreactive actin detected on the same blot. Each well was loaded with 15 μg of total protein. HC=psychiatrically healthy comparison subject; MDD=subject with major depressive disorder; M=male.
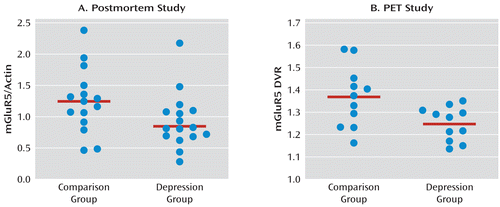
FIGURE 4. Metabotropic Glutamate Receptor 5 (mGluR5) Protein Levels and mGluR5 Binding Within the Right Frontal Polar Cortex (Brodmann's area 10)a
a In panel A, the mGluR5 protein levels were normalized to actin, and in panel B, to mGluR5 distribution volume ratio (DVR). Unpaired t tests were used to compare subjects with major depressive disorder with psychiatrically healthy comparison subjects. In panel A, the group means of the mGluR5/actin ratio were 1.25 (SD=0.52) in the comparison group and 0.85 (SD=0.30) in the depression group. In panel B, the group means of the mGluR5 DVR were 1.37 (SD=0.14) in the comparison group and 1.25 (SD=0.08) in the depression group.
Amounts of mGluR1 protein (150 kDa) were analyzed in the prefrontal cortex from 13 depression subjects and 13 matched comparison subjects. The same subjects (plus two additional comparison subjects and two additional depression subjects) were used in the mGluR5 analysis (see Table S3). The average mGluR1:actin ratio in depressed subjects (mean=0.98, SD=0.31) was not significantly different from that in comparison subjects (mean=1.07, SD=0.19).
Linear regression analysis showed no significant correlations between the amount of mGluR5 or mGluR1 immunoreactivity and age, postmortem interval, brain pH, time in freezer, or duration of depression.
Discussion
This is the first study to analyze mGluR5 binding and protein expression in individuals with major depressive disorder and comparison subjects using imaging and postmortem approaches. In the PET study, we found lower mGluR5 binding in depressed subjects relative to comparison subjects in multiple areas of the frontal, temporal, and parietal cortices and in the right thalamus, the insula bilaterally, the left hippocampus, the left posterior cingulate cortex, and the precentral gyrus. In the depression group, the severity of depressive symptoms was negatively correlated with mGluR5 binding in the hippocampus bilaterally, and anxiety symptoms were negatively correlated with mGluR5 binding in the thalamus and orbital frontal cortex bilaterally, the right frontal polar cortex, and the left mid-cingulate cortex. In line with the PET study results, the postmortem examination revealed lower mGluR5 protein levels in the right frontal polar cortex in depressed subjects relative to psychiatrically healthy comparison subjects. The postmortem study also revealed that the lower level of mGluR5 protein was specific given that we did not detect a difference in mGluR1 immunoreactivity between diagnostic groups.
Abnormalities in the glutamate receptor system have previously been observed in postmortem brain tissue from individuals with major depression and suicide victims (6). We have reported brain region-specific abnormalities in the NMDA receptor complex in depression (5, 20). These studies coincide with clinical reports demonstrating the potent antidepressant activity of ketamine, an NMDA receptor antagonist (2). In addition, several studies have demonstrated that selective mGluR5 antagonists have antidepressant-like effects in animals (21), and mGluR5 knockout mice showed decreased immobility in the forced swim test, which has been interpreted as an antidepressant-like phenotype (10). It has been postulated that the antidepressant properties of mGluR5 antagonists may involve inhibition of NMDA receptor-mediated neurotransmission and/or induction of brain-derived neurotrophic factor gene expression in the hippocampus (22).
In our PET study, the lower regional levels of DVR in major depression indicate reduced binding of [11C]ABP688, which may reflect reduced mGluR5 density, a change in the affinity of the binding site, or increased concentration of an unknown endogenous ligand. A reduction in mGluR5 density is consistent with our postmortem study showing lower mGluR5 protein levels in the prefrontal cortex in major depression. Thus, reduced binding to mGluR5 in depression most likely reflects reduced density of functional receptors because of decreased total protein concentration.
At present, we can only speculate about mechanisms that might explain the widespread lower levels of [11C-ABP688 binding to mGluR5 in depression in the parieto-temporo-frontal regions, including the insula and orbitofrontal cortex, in depression. Reduced mGluR5 binding may represent a biological trait associated with an elevated risk of depression, possibly due to genetic factors. Alternatively, the mGluR5 receptor binding may be reduced because receptor expression was down-regulated, possibly through the influence of repeated stress, increased glutamate activity, or hormonal changes (e.g., glucocorticoids) in depression. The negative correlation between mGluR5 in the hippocampus and depression severity and between mGluR5 in the ventrolateral thalamus and anxiety suggests that reduced mGluR5 binding reflects a primary pathogenic marker or an ineffective compensatory change.
Neuroimaging, neuropathological, and lesion studies have provided consistent evidence that neuronal networks involving the medial and orbital prefrontal cortex and related mesiotemporal and striato-pallido-thalamic structures, as well as cortical areas, play a major role in the pathogenesis of depression (23). Neuroanatomical experiments in monkeys have shown that the orbital cortex is associated with sensory association areas in the inferior temporal cortex and somatosensory areas associated with the insula (24). Lower mGluR5 binding in this orbital prefrontal network may be related to impairments in the coding of affective characteristics of stimuli in depression (23). The other extended cortical network associated with depression is connected with the medial prefrontal cortex and includes regions where we also found lower mGluR5 binding: the frontal polar cortex, the posterior cingulate cortex, the hippocampal formation, and mesencephalic structures (25, 26). Since this “visceromotor” network is involved in visceral reactions to emotional stimuli (23), reduced mGluR5 binding in this network may be related to emotional dysregulation and vegetative symptoms in depression.
Several limitations of our study merit comment. Our cross-sectional design could not distinguish whether abnormalities in mGluR5 receptor binding reflected a biological vulnerability to depression or were a consequence of the illness (27). Another possible limitation is the confounding effect of medication. In the PET study, participants had been medication free for at least 4 weeks before scanning; nevertheless, we found an association between a history of antidepressant use and lower mGluR5 binding in the left precentral gyrus, which suggests that drug effects cannot be fully excluded. In the postmortem study, although depressed subjects were medication free at the time of death (based on postmortem toxicology screening), this does not exclude the possibility that antidepressants had a long-term effect on mGluR5 protein expression.
To avoid potentially painful arterial cannulation in participants in the PET study, we used the bolus-infusion technique and normalized the PET images using the cerebellar radioactivity concentration. The use of the cerebellum as a reference region was based on convincing in vivo and in vitro evidence showing that mGluR5 level is extremely low in the cerebellum relative to the brain regions that are thought to be involved in depression (28). However, some studies using the mGluR5 radioligand [18F]FPEB have questioned the use of the cerebellum as a reference region because of the relatively high specific cerebellar binding, particularly in rhesus brain tissue (29, 30) but also, to a weaker extent, in human brain tissue as measured in a single subject using a relatively nonspecific in vitro screen (“no-wash” assay) (30). In contrast, a recent study demonstrated that quantification of mGluR5 receptor with [18F]FPEB with noninvasive modeling using the cerebellum as reference region may be feasible (31). In support of this, in vitro and in vivo studies have suggested negligible binding in the cerebellum when using ABP688 and validated the use of the cerebellum as a reference region (28, 32). In addition, the postmortem study did not identify mGluR5 protein expression (see Figure S3 in the online data supplement), and we are not aware of any other study showing detectable expression of mGluR5 protein in the cerebellum using Western blotting. Previous studies of cerebellar mRNA expression have demonstrated the presence (33) and absence (34) of mGluR5 mRNA or weak mRNA expression exclusively in Bergmann glia (35). Taken together, all studies on cerebellar mGluR5 concentration using more specific measures than that used in one contradictory single-subject study (29) found negligible mGluR5 expression in the cerebellum, which suggests that the cerebellum can be used as a reference region.
In conclusion, the data we report here demonstrate lower levels of binding and protein expression of mGluR5 in depressed individuals relative to nondepressed comparison subjects. The findings suggest that neurotransmission at mGluR5 is reduced in depression, possibly as a result of basal or compensatory changes in glutamate system activity. Implications of these findings are that mGluR5 receptor expression might be well suited for use as a biomarker for depression and as a target for novel antidepressant medications. These findings in living subjects, corroborated in postmortem tissue, should encourage studies designed to investigate genetic and environmental influences on mGluR5 and interactions among mGluR5, ionotropic glutamate receptors, and monoaminergic receptor systems in mood and anxiety disorders.
1. : The medical management of depression. N Engl J Med 2005; 353:1819–1834Crossref, Medline, Google Scholar
2. : A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63:856–864Crossref, Medline, Google Scholar
3. : GABA and glutamate systems as therapeutic targets in depression and mood disorders. Expert Opin Ther Targets 2005; 9:153–168Crossref, Medline, Google Scholar
4. : Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 2007; 64:193–200Crossref, Medline, Google Scholar
5. : Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33:70–75Crossref, Medline, Google Scholar
6. : Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev 2009; 61:105–123Crossref, Medline, Google Scholar
7. : Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov 2008; 7:426–437Crossref, Medline, Google Scholar
8. : mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci USA 2007; 104:1995–2000Crossref, Medline, Google Scholar
9. : Multiple MPEP administrations evoke anxiolytic- and antidepressant-like effects in rats. Neuropharmacology 2002; 43:181–187Crossref, Medline, Google Scholar
10. : Metabotropic glutamate 5 receptor antagonism is associated with antidepressant-like effects in mice. J Pharmacol Exp Ther 2006; 319:254–259Crossref, Medline, Google Scholar
11. : Radiosynthesis and preclinical evaluation of 11C-ABP688 as a probe for imaging the metabotropic glutamate receptor subtype 5. J Nucl Med 2006; 47:698–705Medline, Google Scholar
12. : Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York, New York State Psychiatric Institute, Biometrics Research, 2001Google Scholar
13. : Structured Clinical Interview for DSM-IV Axis I Disorders: Non-Patient Edition (SCID-I/NP). New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
14. : Comparison of bolus and infusion methods for receptor quantitation: application to [18F]cyclofoxy and positron emission tomography. J Cereb Blood Flow Metab 1993; 13:24–42Crossref, Medline, Google Scholar
15. : Validation of a bolus/infusion protocol for 11C-ABP688, a PET tracer for mGluR5. Nucl Med Biol 2010; 37:845–851Crossref, Medline, Google Scholar
16. : Strategies for the study of the opiate receptor in brain: application to the opiate antagonist cyclofoxy. J Cereb Blood Flow Metab 1989; 9:S732Google Scholar
17. : Evaluation of the metabotropic glutamate receptor subtype 5 using PET and 11C-ABP688: assessment of methods. J Nucl Med 2007; 48:1207–1215Crossref, Medline, Google Scholar
18. : Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007; 27:1533–1539Crossref, Medline, Google Scholar
19. : A diagnostic interview: the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry 1978; 35:837–844Crossref, Medline, Google Scholar
20. : Elevated levels of NR2A and PSD-95 in the lateral amygdala in depression. Int J Neuropsychopharmacol 2009; 12:143–153Crossref, Medline, Google Scholar
21. : Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther 2007; 115:116–147Crossref, Medline, Google Scholar
22. : Effect of MPEP treatment on brain-derived neurotrophic factor gene expression. Pharmacol Rep 2006; 58:427–430Medline, Google Scholar
23. : Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 2008; 213:93–118Crossref, Medline, Google Scholar
24. : Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol 2008; 506:659–693Crossref, Medline, Google Scholar
25. : Orbitofrontal cortex function and structure in depression. Ann NY Acad Sci 2007; 1121:499–527Crossref, Medline, Google Scholar
26. : Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 2003; 460:425–449Crossref, Medline, Google Scholar
27. : Discovering endophenotypes for major depression. Neuropsychopharmacology 2004; 29:1765–1781Crossref, Medline, Google Scholar
28. : Synthesis, characterization, and first successful monkey imaging studies of metabotropic glutamate receptor subtype 5 (mGluR5) PET radiotracers. Synapse 2005; 56:205–216Crossref, Medline, Google Scholar
29. : Human PET studies of metabotropic glutamate receptor subtype 5 with 11C-ABP688. J Nucl Med 2007; 48:247–252Medline, Google Scholar
30. : Species differences in mGluR5 binding sites in mammalian central nervous system determined using in vitro binding with [18F]F-PEB. Nucl Med Biol 2007; 34:1009–1017Crossref, Medline, Google Scholar
31. : Quantitation of glutamate mGluR5 receptor with 18F-FPEB PET in humans. J Nucl Med 2010; 51(suppl 2):215Google Scholar
32. : In vivo and in vitro validation of reference tissue models for the mGluR(5) ligand [(11)C]ABP688. J Cereb Blood Flow Metab 2010; 30:1538–1549Crossref, Medline, Google Scholar
33. : Identification and characterization of a novel splice variant of the metabotropic glutamate receptor 5 gene in human hippocampus and cerebellum. Brain Res Mol Brain Res 2002; 109:168–178Crossref, Medline, Google Scholar
34. : Molecular and functional characterization of recombinant human metabotropic glutamate receptor subtype 5. Neuropharmacology 1995; 34:871–886Crossref, Medline, Google Scholar
35. : Expression of metabotropic glutamate receptor subtype mRNA (mGluR1–8) in human cerebellum. Neuroreport 1999; 10:3861–3867Crossref, Medline, Google Scholar



