Hippocampal Volume Development in Healthy Siblings of Childhood-Onset Schizophrenia Patients
Abstract
Objective:
Previous anatomic studies have established a reduction in hippocampal volume in schizophrenia, but few have investigated the progressive course of these changes and whether they are trait markers. In the present study, the authors examined hippocampal volumes in relation to age for patients with childhood-onset schizophrenia, their nonpsychotic healthy siblings, and healthy comparison subjects.
Method:
Anatomic brain magnetic resonance scans were obtained in childhood-onset schizophrenia probands (N=89, 198 scans), their nonpsychotic full siblings (N=78, 172 scans), and matched healthy comparison subjects (N=79, 198 scans) between the ages of 10 and 29 years. Total, left, and right hippocampal volumes were measured using FreeSurfer software and analyzed using a linear mixed-model regression covarying for sex and intracranial volume.
Results:
Childhood-onset schizophrenia probands had a fixed reduction in hippocampal volumes (total, left, and right) relative to both nonpsychotic siblings and healthy comparison subjects, whereas there were no significant volumetric or trajectory differences between nonpsychotic siblings and healthy comparison subjects.
Conclusions:
Fixed hippocampal volume loss seen in childhood-onset schizophrenia, which is not shared by healthy siblings, appears to be related to the illness. Decreased hippocampal volume is not strongly genetically related but represents an important intermediate disease phenotype.
Several lines of research support the hippocampus playing a role in the pathogenesis of schizophrenia. The hippocampus is intricately involved in declarative learning and memory (1–3), novelty detection (4, 5), and establishing semantic associations (6), such that functional deficits can resemble cognitive abnormalities seen in schizophrenia. Anatomic brain imaging studies have consistently shown patients with schizophrenia to have bilateral hippocampal volume reductions (7–9), and postmortem studies have confirmed these findings along with reduced neuronal size (10–12).
Adolescence appears to be a unique period of brain development in schizophrenia (13, 14), and changes during normal adolescence bring about hippocampal volume reduction (15). The mechanisms underlying hippocampal volume reduction in schizophrenia are unclear and could be related to genetic, environmental, antipsychotic medication, and/or illness-related factors (16, 17). To explore the contribution of a familial/genetic mechanism to the structural abnormalities, several studies have compared patients to their unaffected healthy siblings or other first-degree relatives (18, 19).
The results from these studies, and thus whether a reduction in hippocampal volume represents a familial/trait marker for schizophrenia, remain unclear. Table 1 in the present study provides an overview of studies examining hippocampal volumes in nonpsychotic relatives of adult-onset schizophrenia patients. Findings have been inconsistent, with six studies reporting smaller hippocampal volumes in first-degree relatives compared with healthy subjects, suggesting familial/genetic liability (16, 20–24). In contrast, five other studies failed to find any decrease in hippocampal volumes for unaffected siblings of adult patients (25–29) (Table 1). Studies in ultra-high-risk individuals also address the question of whether hippocampal volume reductions could be familial/trait markers and whether the changes could take place before the onset of psychosis. In the present analysis, the studies are also inconsistent, with four showing that volume deficits do not appear until after the onset of psychotic illness (30–33) and three finding ultra-high-risk patients to have decreased hippocampal volume (34–36). It is important to note that many subjects in these studies had various psychotic symptoms and were exposed to typical or atypical antipsychotics at clinical dosages lasting from a few days to approximately 1 month, and thus the effect of antipsychotics on hippocampal volume remains a confounding factor. As a whole, these studies suggest that hippocampal volumes may be differentially affected, depending on the stage and type of psychosis, but fail to provide convincing evidence about the use of hippocampal volume as a familial/trait marker.
| Age (years) | |||
|---|---|---|---|
| Study and Sample | Mean/Range | SD | Main Findings |
| Ho and Magnotta (20)a | 1<3; 2<3 (left hippocampal volume, p=0.04). | ||
| 1. Adult-onset schizophrenia patients (N=46) | 13–28 | 4.5 | |
| 2. Nonpsychotic relatives (N=46) | 13–28 | 4.1 | |
| 3. Healthy comparison subjects (N=46) | 13–28 | 3.5 | |
| Honea et al. (24)b | 2<3 (only in right hippocampal volume [p=0.003]); 1 and 3: no difference. | ||
| 1. Patients with schizophrenia spectrum disorders (N=169) | 36.39 | 9.46 | |
| 2. Unaffected siblings (N=213) | 36.5 | 9.75 | |
| 3. Comparison subjects (N=212) | 33.31 | 9.86 | |
| Goldman et al. (26) | 2 did not differ from 1 or 3; 2<3 in post hoc analyses (left hippocampal volume, p=0.017, right hippocampal volume, p=0.006). | ||
| 1. Adult-onset schizophrenia patients (N=169) | 36.48 | 10.13 | |
| 2. Discordant siblings (N=183) | 36.38 | 9.60 | |
| 3. Comparison subjects (N=221) | 32.82 | 9.51 | |
| McDonald et al. (25) | 2 did not differ from 3. | ||
| 1. Adult-onset schizophrenia patients (N=24) | 37.9 | 10.3 | |
| 2. Relatives (N=32) | 47.1 | 13.1 | |
| 3. Comparison subjects (N=18) | 32.8 | 5.0 | |
| van Haren et al. (21) | 2<3 as main effect only (p=0.04); difference not significant after Bonferroni correction; no significant interaction reported. | ||
| 1. Monozygotic concordant twin pairs (N=14) | 34.36 | 8.48 | |
| 2. Monozygotic discordant twin pairs (N=10) | 36.70 | 13.81 | |
| 3. Monozygotic comparison twin pairs (N=17) | 38.06 | 10.38 | |
| Tepest et al. (22) | 3<4 (p=0.003). | ||
| 1. Adult-onset schizophrenia patients (N=12) | 29.8 | 5.5 | |
| 2. Affected siblings (N=13) | 31.1 | 8.4 | |
| 3. Unaffected siblings (N=13) | 30.5 | 5.6 | |
| 4. Comparison subjects (N=10) | 24.4 | 3.5 | |
| Seidman et al. (16) | 2<4 in left hippocampal volume (full sample); no significant differences reported for right hippocampal volume. | ||
| 1. Simplex relatives (N=28) | 41.9 | 12.7 | |
| 2. Multiplex relatives (N=17) | 38.9 | 10.6 | |
| 3. Adult-onset schizophrenia patients (N=18) | 43.2 | 8.3 | |
| 4. Comparison subjects (N=48) | 40.1 | 10.8 | |
| van Erp et al. (23) | 2<3 (p=0.001). | ||
| 1. Psychotic probands (N=72) | 40.2 | 5.4 | |
| 2. Nonpsychotic full siblings (N=58)c | 40.7 | 5.9 | |
| 3. Comparison subjects (N=53) | 40.9 | 3.1 | |
| Narr et al. (28) | 1<3 in left hippocampal volume (p=0.02); 2 did not differ from 4. | ||
| 1. Monozygotic discordant twin pairs (N=10) | 48.3 | 2.9 | |
| 2. Dizygotic discordant twin pairs (N=10) | 49.0 | 3.9 | |
| 3. Monozygotic comparison twin pairs (N=10) | 48.3 | 3.8 | |
| 4. Dizygotic comparison twin pairs (N=10) | 47.9 | 4.2 | |
| Baaré et al. (29) | 1 and 2<3 and 4 as main effect only (p<0.05); no significant interaction reported. | ||
| 1. Monozygotic discordant twin pairs (N=15) | 35.11 | 10.31 | |
| 2. Dizygotic discordant twin pairs (N=14) | 35.67 | 10.77 | |
| 3. Monozygotic comparison twin pairs (N=15) | 35.62 | 11.35 | |
| 4. Dizygotic comparison twin pairs (N=14) | 35.12 | 10.26 | |
| Staal et al. (27) | 2 did not differ from 3; no significant main effects or interactions reported. | ||
| 1. Adult-onset schizophrenia patients (N=32) | 40.6 | 8.2 | |
| 2. Unaffected siblings (N=32) | 40.9 | 8.6 | |
| 3. Comparison subjects (N=32) | 40.3 | 9.3 | |
TABLE 1. Cross-Sectional Studies of Hippocampal Volume in Schizophrenia Patients, Healthy Siblings, and Unaffected Relatives
Childhood-onset schizophrenia, defined as onset of psychotic symptoms before age 13 years and diagnosed using unmodified DSM-IV criteria, is a rare form of the illness that is continuous with its adult counterpart (13, 37) and shows a robust gray matter loss during adolescence that appears to be an exaggeration of the normal cortical gray matter developmental pattern (14, 38–41). We previously reported a moderate, nonprogressive reduction in hippocampal volume bilaterally (13, 42). Although there are salient volume reductions in the hippocampus associated with adult-onset schizophrenia, there have been no studies examining longitudinal hippocampal volume change in either the siblings of our patients or relatives of adult-onset patients.
In the present study, we investigated longitudinal development of hippocampal volumes in a large sample of childhood-onset schizophrenia probands, their unaffected healthy siblings, and healthy comparison subjects, all examined prospectively during childhood and adolescence using a fully automated, whole brain segmentation technique to determine hippocampal volume (43). Based on previous work, we hypothesized that hippocampal development in childhood-onset schizophrenia patients would show a nonprogressive bilateral hippocampal volume reduction. We further hypothesized that healthy siblings of childhood-onset schizophrenia probands would also demonstrate smaller and progressive reduction in hippocampal volume, and thus the trajectory of hippocampal development could be a familial/trait marker.
Method
Subjects
Childhood-onset schizophrenia patients were recruited nationwide and were diagnosed after inpatient observation that included a medication washout. Exclusionary criteria were medical or neurological illness, substance abuse, or an IQ <70 prior to the onset of psychotic symptoms. Further details are described elsewhere (44). All patients, along with their full siblings, were followed clinically with neurological rescan at 2-year intervals.
For this study, only childhood-onset schizophrenia patients (N=89 [198 scans]) and healthy full siblings of patients (N=78 [172 scans]) with two or more successive scans were examined. Siblings were interviewed using structured psychiatric interviews for axis I (using either the Schedule for Affective Disorders and Schizophrenia [SADS] [45] or the Schedule for Affective Disorders and Schizophrenia for School-Age Children [K-SADS] [46]) and axis II (using the Structured Interview for DSM-III Personality Disorders [47]) diagnoses. Siblings were considered healthy if they were free of any schizophrenia spectrum diagnoses, which included schizophrenia, schizoaffective disorder, or any psychotic illness on axis I or paranoid, schizotypal, schizoid, or avoidant personality disorders on axis II (48).
Seventy-nine healthy comparison subjects (172 scans) were selected from a larger prospective study of normal brain development and were matched for age, sex, and scan interval to the childhood-onset schizophrenia patients and healthy siblings. Only comparison subjects with two or more successive scans were included. As with siblings, comparison subjects were free of lifetime medical or psychiatric disorders as determined by means of clinical examination and standardized interview. Psychiatric illness in a first-degree relative was also exclusionary. Further details are described elsewhere (49).
Imaging Processing and Analysis
T1-weighted images with contiguous 1.5-mm slices in the axial plane were obtained using a three-dimensional spoiled gradient recalled echo sequence in the steady state. Imaging parameters were as follows: echo time=5 msec, repetition time=24 msec, flip angle=45°, acquisition matrix=256×192, number of excitations=1, and field of view=24 cm. Head placement was standardized as previously described (50).
The image files in DICOM (Digital Imaging and Communications in Medicine) format were transferred to a Linux workstation for analysis. Subcortical volumes were measured automatically with the FreeSurfer image analysis suite, which is documented and available online (http://surfer.nmr.mgh.harvard.edu/). A trained psychiatrist reviewed individual scans, and those with significant artifact or motion disturbance (childhood-onset schizophrenia group, N=7; healthy sibling group, N=4; healthy comparison group, N=4) were excluded from analysis. The automated procedures for subcortical volumetric measurements of different brain structures have been described previously (43, 51). This procedure automatically provides segments and labels for many brain structures and assigns a neuroanatomic label to each voxel in magnetic resonance imaging (MRI) volume on the basis or probabilistic information estimated automatically from a manually labeled training set. Briefly, this processing includes motion correction and averaging of multiple volumetric T1-weighted images (when more than one is available), removal of nonbrain tissue using a hybrid watershed/surface deformation procedure (52), automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (including the hippocampus, amygdala, caudate, putamen, and ventricles) (43, 51), intensity normalization, tessellation of the gray-white matter boundary, automated topology correction (53, 54), and surface deformation following intensity gradients to optimally place the gray-white matter and gray matter/CSF borders at the location where the greatest shift in intensity defines the transition to the other tissue class.
The segmentation uses the following data to disambiguate labels: 1) the prior probability of a given tissue class at a specific atlas location, 2) the likelihood of the image intensity given the tissue class, and 3) the probability of the local spatial configuration of labels given the tissue class. This technique has previously been shown to be comparable in accuracy to manual labeling (43) and has been demonstrated to show good test-retest reliability across scanner manufacturers and field strengths (55). However, all segmentations were visually inspected for accuracy prior to inclusion in the group analysis. Total hippocampal volume was calculated as the sum of the left and right hippocampal volumes for each study participant.
Statistical Analysis
Demographic differences between groups were tested using analysis of variance for continuous variables and chi-square tests of independence for categorical variables. To examine group differences between the developmental trajectories of total, left, and right hippocampal volume measures, we used mixed-effect regression models. The dependent measures were individual hippocampal volumes; fixed effects included age (centered at the sample average age of 17.58 years [SD=4.6]), group, group-by-age, intracranial volume, and sex. Random effects included an intercept per person (to account for within-person dependence) and an intercept for a person nested within a family (to account for within-family dependence). Hypotheses for model building were tested with F statistics to determine the order (cubic, quadratic, or linear) of the developmental growth curves. We graphed fitted regression lines for the middle 80% of the age range in our data set. Group differences in intercept (at the average age) and slope were tested using t tests.
Results
Demographic characteristics are shown in Table 2. The three study groups were well matched with respect to age, sex, and handedness. Across the entire study sample, there were significant group differences in each hippocampal volume measure at the average age (Table 3). Childhood-onset schizophrenia patients had a significantly smaller (6%–7%) hippocampal volume (total, left, and right) relative to comparison subjects and healthy siblings. On the other hand, siblings and comparison subjects had comparable total, left, and right hippocampal volumes at the average age (Figure 1).
| Childhood-Onset Schizophrenia Patients (N=89) | Healthy Comparison Subjects (N=79) | Healthy Siblings (N=78) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Age (years) | Age (years) | Analysis | |||||||||
| Magnetic Resonance Imaging | N | Mean | SD | N | Mean | SD | N | Mean | SD | F | df | p |
| Scan 1 | 89 | 14.9 | 3.2 | 79 | 14.9 | 4.7 | 78 | 14.9 | 6.0 | 0.03 | 2, 243 | 0.99 |
| Scan 2 | 51 | 17.3 | 2.6 | 63 | 16.9 | 3.7 | 44 | 18.7 | 6.8 | 2.17 | 2, 155 | 0.12 |
| Scan 3 | 34 | 20.0 | 2.8 | 33 | 19.6 | 4.0 | 31 | 21.1 | 6.8 | 0.82 | 2, 95 | 0.44 |
| Scan 4 | 14 | 22.7 | 2.9 | 14 | 21.2 | 2.2 | 14 | 23.0 | 6.2 | 0.75 | 2, 39 | 0.47 |
| Scan 5 | 7 | 24.8 | 3.4 | 5 | 24.7 | 3.1 | 5 | 22.9 | 2.6 | 0.61 | 2, 14 | 0.55 |
| Scan 6 | 3 | 27.6 | 1.1 | 4 | 28.3 | 3.8 | 0.09 | 1, 5 | 0.77 | |||
| Total | 198 | 17.5 | 4.2 | 198 | 17.3 | 4.9 | 172 | 17.9 | 6.9 | 0.061 | 2, 565 | 0.54 |
TABLE 2. Demographic Characteristics Among Childhood-Onset Schizophrenia Probands, Healthy Comparison Subjects, and Healthy Siblingsa
| Childhood-Onset Schizophrenia Patients (N=89) | Healthy Comparison Subjects (N=79) | Healthy Siblings (N=78) | Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hippocampal Region | Mean | SD | Mean | SD | Mean | SD | F | df | p |
| Left | 4,221 | 419.63 | 4,477 | 470.33 | 4,435 | 396.89 | 14.52 | 2, 565 | <0.001 |
| Right | 4,197 | 425.14 | 4,480 | 498.10 | 4,475 | 418.01 | 25.32 | 2, 565 | <0.001 |
| Total | 8,411 | 807.35 | 8,953 | 944.34 | 8,902 | 790.54 | 20.81 | 2, 565 | <0.001 |
TABLE 3. Magnetic Resonance Imaging Hippocampal Volumes (mm3) Among Childhood-Onset Schizophrenia Probands, Healthy Comparison Subjects, and Healthy Siblingsa
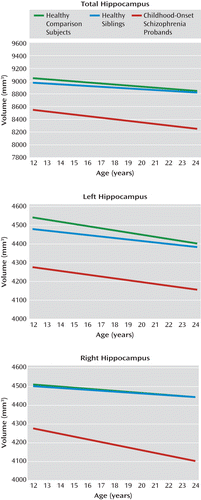
FIGURE 1. Longitudinal Trajectories (Slopes) of Total, Left, and Right Hippocampal Volumes in Childhood-Onset Schizophrenia Probands, Their Healthy Siblings, and Healthy Comparison Subjectsa
a The graphs depict group-by-age interaction effects. Images represent the progression of hippocampal volume for the middle 80% of the data range, from age 12 to 24 years. Mixed-model linear regression for the total, left, and right hippocampal volumes are shown. Pairwise group differences in total hippocampal volume (top) at the centered age (17.58 years [SD=4.6]) were statistically compared using t tests. Statistically significant differences in volume were found when comparing the childhood-onset schizophrenia group with healthy comparison subjects (t=5.53, df=229.90, p<0.001) and the childhood-onset schizophrenia group with healthy siblings (t=–4.96, df=236.40, p=0.004). There were no statistically significant group differences in slope for any of the volume measures. Pairwise group differences in left hippocampal volume (center) at the centered age (17.58 years [SD=4.6]) were statistically compared using t tests. Statistically significant differences in volume were found when comparing the childhood-onset schizophrenia group with healthy comparison subjects (t=5.18, df=227.37, p<0.001) and the childhood-onset schizophrenia group with healthy siblings (t=–4.27, df=234.51, p=0.006). There were no statistically significant group differences in slope for any of the volume measures. Pairwise group differences in right hippocampal volume (bottom) at the centered age (17.58 years [SD=4.6]) were statistically compared using t tests. Statistically significant differences in volume were found when comparing the childhood-onset schizophrenia group with healthy comparison subjects (t=5.41, df=232.08, p<0.001) and the childhood-onset schizophrenia group with healthy siblings (t=–5.25, df=239.18, p=0.001). There were no statistically significant group differences in slope for any of the volume measures.
Longitudinal trajectories (slopes) of hippocampal volume for the three groups did not differ significantly. Each group had a negative linear volumetric trajectory over time, which was not significantly different from zero. Furthermore, for the total and right hippocampal volumes, the childhood-onset schizophrenia group had qualitatively steeper volume loss over time compared with the other two groups, but the slopes for the trajectories between the groups did not differ significantly. When individual groups were divided by gender, no statistically significant differences in total, right, or left hippocampal volume emerged for either volume amount or slope of the trajectories.
Discussion
Healthy siblings of childhood-onset schizophrenia probands had no hippocampal volume deficits relative to healthy comparison subjects. However, childhood-onset schizophrenia patients showed bilateral fixed deficits in total hippocampus volumes. Similarly, the linear trajectories (slopes) across age for total, left, and right hippocampal volumes in siblings as well as childhood-onset schizophrenia probands did not differ from those of healthy comparison subjects.
These findings extend our previous reports of fixed total hippocampal volume deficits in childhood-onset schizophrenia patients (13, 42) in a much larger sample. The volume deficits, 6%–7% at the average age, are larger than those seen in cross-sectional studies of adult schizophrenia (4%–5%) (17, 31), which is consistent with the clinical evidence that childhood-onset schizophrenia represents a more severe phenotype of the illness. Since hippocampal volume deficits appear early in childhood-onset schizophrenia and are comparatively nonprogressive, these findings also support a static hippocampal lesion suggested by the animal models of schizophrenia (56–59).
Contrary to our a priori hypothesis, healthy siblings of childhood-onset schizophrenia probands showed no hippocampal volume loss. Previously, mostly cross-sectional MRI studies in healthy siblings as well as first-degree relatives have shown inconsistent findings (Table 1). Similarly, many studies of ultra high-risk individuals have also failed to show consistent hippocampal loss prior to the onset of psychosis (30, 32, 33, 60). Many of these studies, including those of high-risk populations, have included patients who have some schizophrenia spectrum symptoms or have been exposed to antipsychotic medications, which could have resulted in some of the inconsistencies. In a separate pilot analysis, we attempted to address this issue by comparing hippocampal (total, left, and right) volumes in medication-naive siblings of schizophrenia spectrum patients (N=15 [24 scans]) with volumes in healthy comparison subjects (N=15 [24 scans]). The siblings, which were probably comparable to an ultra high-risk group with schizotypal symptoms, also failed to show hippocampal volume reduction (p=0.7 [unpublished data available upon request from A. Mattai]). Overall, these findings strongly suggest the state-/disease-dependent nature of hippocampal volume loss. Strengths of the present study are the large sibling cohort, which enabled the selection of truly healthy comparison subjects, and the longitudinal nature of the study, which strengthened the stability and significance of the findings.
The effects of antipsychotic medication on hippocampal volume have been addressed by a few studies. The single longitudinal study (N=107) showed unchanged anterior hippocampal volume in patients regardless of cumulative antipsychotic dose (61). A cross-sectional study (N=56) showed that atypical antipsychotics rather than haloperidol were associated with larger hippocampal volumes after controlling for disease severity (62). On the other hand, studies of hippocampal volume in antipsychotic-naive and minimally medicated first-episode schizophrenia patients showed that hippocampal volume deficits were present at the onset of schizophrenia prior to any treatment (9, 63). All of our patients were exposed to antipsychotic medications, but the volume deficits remained fixed throughout the age range, suggesting minimal medication influence at least on the developmental trajectory, which we have also seen in the cortex (64). Although the effect of medications cannot be definitively ruled out with these observations, combined with the lack of volume loss in siblings, they support evidence that medications have minimal effect on hippocampal volume loss. A direct comparison of medicated and medication-naive childhood-onset schizophrenia patients may address this issue more definitively, but drug-naive childhood-onset schizophrenia patients are almost impossible to recruit.
Postmortem work investigating morphometric hippocampal changes in schizophrenia suggests that the illness reduces hippocampal neuronal size. Benes et al. (12) measured pyramidal neuron size in the posterior hippocampus and found reductions of 13%–18% in regions CA1–CA4 in schizophrenia patients relative to comparison subjects. Correction for the effects of age, fixation interval, and neuroleptic exposure did not alter the results. Similarly, Arnold (65) reported reductions in neuronal size in hippocampal subfields that mediate interactions with the cortex, thus possibly altering the neural circuits (66). Processes occurring during embryonic development and early childhood, such as neuronal migration, neuron enlargement and differentiation, and apoptosis in brain maturation, all have some bearing on hippocampal cytoarchitecture and neuronal arrangement (65). While it is not clear which of these factors may cause volume loss in the hippocampus, the nature of these neuropathologic changes suggests that at least part of the hippocampal disease process occurs during early development.
Given that the primary development of the brain occurs during fetal life, adverse environmental variables in early life could affect hippocampal development. Across environmental variables, obstetrical complications are one of the strongest predictors of risk for schizophrenia (67–70). There is persuasive evidence to suggest that obstetrical complications, particularly perinatal hypoxia and prenatal infections, are related to smaller hippocampal volumes in schizophrenia (71, 72). Furthermore, in animal models, prenatal- and birth-related hypoxic insults have been demonstrated to result in hippocampal neuron damage and a reduction in hippocampal cell number (73, 74).
Studies of hippocampal volume in monozygotic and dizygotic twins discordant for schizophrenia have also bolstered support for the idea that hippocampal volumes are differentially modulated by environmental factors to a greater degree when compared with healthy individuals (75, 76). However, a small retrospective chart study found no evidence for increased obstetrical risk in childhood-onset schizophrenia versus sibling comparison subjects (77). Collectively, such work highlights that unique environmental events can significantly influence hippocampal volume in patients with schizophrenia.
A model of the developmental pathology of the hippocampus in childhood-onset schizophrenia could consist of an early environmental risk factor, such as perinatal hypoxia or prenatal infections, imparting a constitutional vulnerability to the hippocampus. Studies have shown that the hippocampus is particularly susceptible to damage as a result of stress (78, 79). During early adolescence, the hippocampus regulates the hypothalamic-pituitary-adrenal axis that releases cortisol and consequently augments dopamine activity in certain brain regions, including the mesolimbic system (80, 81). Increased stress could lead to an increased demand placed on the hippocampus, eventually leading to an exaggerated response to stress and more hippocampal damage in a positive feedback system. Given the pronounced prefrontal cortical deficits seen in childhood-onset schizophrenia (38), the prefrontal cortex may have limited ability to take over functions, such as working memory, from the hippocampus, further increasing functional demands and leading to hippocampal damage. This framework could explain a hippocampal diathesis-stress model in which normal maturational processes and exaggerated responses to early stress lead to abnormal hippocampal development that could be static with continued illness burden.
Our findings have several broad-ranging implications in terms of prevention and treatment. First, given the potential significant environmental contribution to hippocampal volume in schizophrenia, measures to decrease exposure to the environmental influence could result in a reduction in the incidence of illness in the population. For example, Suvisaari et al. (82) found that a decline in bacterial illnesses and initiation of immunization programs may have led to a decline in the incidence of schizophrenia in Finland since the 1950s. In addition, prenatal and perinatal monitoring may also decrease the risk of schizophrenia in some genetically at-risk individuals (83). As hippocampal pathology likely begins and progresses in early brain development, measures to attenuate or reverse volume loss should be initiated early. The hippocampus is one of few brain structures with the ability to generate new neurons throughout its life (84). Although there is limited work linking schizophrenia to decreased hippocampal neurogenesis or on whether normalization of neurogenesis would improve atrophy (85), recent investigations suggest that exercise may promote hippocampal plasticity and improve memory (86, 87). While many obstacles need to be overcome, future translational studies should focus on early interventions, possibly in the fetal period, as a way to improve hippocampal development and potentially prevent or delay onset of illness.
A major limitation to this study is that we did not investigate hippocampal shape abnormalities that may have reflected on heterogeneous changes within the hippocampus. Shape and subregional analyses of the hippocampus within this population are ongoing. Another limitation of the study is the unknown bias of national recruitment for this very rare patient population that may favor healthy families and thus a population with lower genetic risk. Nonetheless, this study highlights that hippocampal deficits are not strongly related to genetic factors and may represent an important intermediate disease phenotype in childhood-onset schizophrenia.
1. : Regional dissociations within the hippocampus: memory and anxiety. Neurosci Biobehav Rev 2004; 28:273–283Crossref, Medline, Google Scholar
2. : Differential effects of early hippocampal pathology on episodic and semantic memory. Science 1997; 277:376–380Crossref, Medline, Google Scholar
3. : Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol 1995; 5:169–177Crossref, Medline, Google Scholar
4. : Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex 1996; 6:71–79Crossref, Medline, Google Scholar
5. : Novelty detection and encoding for declarative memory within the human hippocampus. Clin EEG Neurosci 2006; 37:309–314Crossref, Medline, Google Scholar
6. : Human hippocampus associates information in memory. Proc Natl Acad Sci U S A 1999; 96:5884–5889Crossref, Medline, Google Scholar
7. : Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry 2005; 162:2233–2245Link, Google Scholar
8. : Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry 2006; 188:510–518Crossref, Medline, Google Scholar
9. : Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr Res 2006; 82:75–88Crossref, Medline, Google Scholar
10. : The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain 1999; 122(
11. : The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004; 174:151–162Crossref, Medline, Google Scholar
12. : Reduced neuronal size in posterior hippocampus of schizophrenic patients. Schizophr Bull 1991; 17:597–608Crossref, Medline, Google Scholar
13. : Childhood-onset schizophrenia: progressive brain changes during adolescence. Biol Psychiatry 1999; 46:892–898Crossref, Medline, Google Scholar
14. : Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry 2006; 47:1003–1012Crossref, Medline, Google Scholar
15. : Dynamic mapping of normal human hippocampal development. Hippocampus 2006; 16:664–672Crossref, Medline, Google Scholar
16. : Left hippocampal volume as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Arch Gen Psychiatry 2002; 5:839–849Crossref, Google Scholar
17. : Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry 1998; 55:433–440Crossref, Medline, Google Scholar
18. : Are brain structural abnormalities useful as endophenotypes in schizophrenia? Int Rev Psychiatry 2007; 19:399–408Crossref, Google Scholar
19. : Can biological markers identify endophenotypes predisposing to schizophrenia? Int Rev Psychiatry 1997; 9:355–364Crossref, Google Scholar
20. : Hippocampal volume deficits and shape deformities in young biological relatives of schizophrenia probands. Neuroimage 2009; 49:3385–3393Crossref, Medline, Google Scholar
21. : A controlled study of brain structure in monozygotic twins concordant and discordant for schizophrenia. Biol Psychiatry 2004; 56:454–461Crossref, Medline, Google Scholar
22. : Hippocampal deformities in the unaffected siblings of schizophrenia subjects. Biol Psychiatry 2003; 54:1234–1240Crossref, Medline, Google Scholar
23. : Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry 2002; 159:1514–1520Link, Google Scholar
24. : Is gray matter volume an intermediate phenotype for schizophrenia?: a voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry 2008; 63:465–474Crossref, Medline, Google Scholar
25. : Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry 2006; 163:478–487Link, Google Scholar
26. : Heritability of brain morphology related to schizophrenia: a large-scale automated magnetic resonance imaging segmentation study. Biol Psychiatry 2008; 63:475–483Crossref, Medline, Google Scholar
27. : Structural brain abnormalities in patients with schizophrenia and their healthy siblings. Am J Psychiatry 2000; 157:416–421Link, Google Scholar
28. : A twin study of genetic contributions to hippocampal morphology in schizophrenia. Neurobiol Dis 2002; 11:83–95Crossref, Medline, Google Scholar
29. : Volumes of brain structures in twins discordant for schizophrenia. Arch Gen Psychiatry 2001; 58:33–40Crossref, Medline, Google Scholar
30. : Hippocampus abnormalities in at risk mental states for psychosis? a cross-sectional high resolution region of interest magnetic resonance imaging study. J Psychiatr Res 2010; 44:447–453Crossref, Medline, Google Scholar
31. : Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry 2006; 63:139–149Crossref, Medline, Google Scholar
32. : Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry 1999; 56:133–141Crossref, Medline, Google Scholar
33. : Non-reduction in hippocampal volume is associated with higher risk of psychosis. Schizophr Res 2002; 58:145–158Crossref, Medline, Google Scholar
34. : Gray matter abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls. Psychiatry Res 2009; 173:163–169Crossref, Medline, Google Scholar
35. : Hippocampal subdivision and amygdalar volumes in patients in an at-risk mental state for schizophrenia. J Psychiatry Neurosci 2010; 35:33–40Crossref, Medline, Google Scholar
36. : The volumetric differences of the fronto-temporal region in young offspring of schizophrenic patients. Eur Child Adolesc Psychiatry 2009; 19:151–157Crossref, Medline, Google Scholar
37. : Childhood-onset schizophrenia: rare but worth studying. Biol Psychiatry 1999; 46:1418–1428Crossref, Medline, Google Scholar
38. : Comparison of progressive cortical gray matter loss in childhood-onset schizophrenia with that in childhood-onset atypical psychoses. Arch Gen Psychiatry 2004; 61:17–22Crossref, Medline, Google Scholar
39. : Progressive brain volume loss during adolescence in childhood-onset schizophrenia. Am J Psychiatry 2003; 160:2181–2189Link, Google Scholar
40. : Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr Bull 2008; 34:30–36Crossref, Medline, Google Scholar
41. : Longitudinal brain changes in early-onset psychosis. Schizophr Bull 2008; 34:341–353Crossref, Medline, Google Scholar
42. : Dynamic mapping of hippocampal development in childhood onset schizophrenia. Schizophr Res 2007; 90:62–70Crossref, Medline, Google Scholar
43. : Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33:341–355Crossref, Medline, Google Scholar
44. : Looking for childhood-onset schizophrenia: the first 71 cases screened. J Am Acad Child Adolesc Psychiatry 1994; 33:636–644Crossref, Medline, Google Scholar
45. : Schedule for Affective Disorders and Schizophrenia (SADS). Acta Psychiatr Belg 1987; 87:361–516Medline, Google Scholar
46. : Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Crossref, Medline, Google Scholar
47. : A structured interview for the DSM-III personality disorders: a preliminary report. Arch Gen Psychiatry 1985; 42:591–596Crossref, Medline, Google Scholar
48. : Schizophrenia and schizophrenia-spectrum personality disorders in the first-degree relatives of children with schizophrenia: the UCLA Family Study. Arch Gen Psychiatry 2001; 58:581–588Crossref, Medline, Google Scholar
49. : Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 1999; 2:861–863Crossref, Medline, Google Scholar
50. : Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2001; 58:289–295Crossref, Medline, Google Scholar
51. : Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004; 23(suppl 1):S69–S84Crossref, Medline, Google Scholar
52. : A hybrid approach to the skull stripping problem in MRI. Neuroimage 2004; 22:1060–1075Crossref, Medline, Google Scholar
53. : Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 2001; 20:70–80Crossref, Medline, Google Scholar
54. : Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging 2007; 26:518–529Crossref, Medline, Google Scholar
55. : Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 2006; 32:180–194Crossref, Medline, Google Scholar
56. : Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
57. : The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry 2005; 10:434–449Crossref, Medline, Google Scholar
58. : A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci 2008; 28:12691–12699Crossref, Medline, Google Scholar
59. : Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology 1993; 9:67–75Crossref, Medline, Google Scholar
60. : Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry 2006; 63:139–149Crossref, Medline, Google Scholar
61. : Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry 2001; 49:487–499Crossref, Medline, Google Scholar
62. : Duration of illness and treatment effects on hippocampal volume in male patients with schizophrenia. Br J Psychiatry 2005; 186:26–31Crossref, Medline, Google Scholar
63. : Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J Psychiatry Neurosci 2010; 35:95–104Crossref, Medline, Google Scholar
64. : Effects of clozapine and olanzapine on cortical thickness in childhood-onset schizophrenia. Schizophr Res 2010; 116:44–48Crossref, Medline, Google Scholar
65. : Cellular and molecular neuropathology of the parahippocampal region in schizophrenia. Ann N Y Acad Sci 2000; 911:275–292Crossref, Medline, Google Scholar
66. : The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology 2004; 174:151–162Crossref, Medline, Google Scholar
67. : In utero infection and adult schizophrenia. Ment Retard Dev Disabil Res Rev 2002; 8:51–57Crossref, Medline, Google Scholar
68. : Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry 2002; 159:1080–1092Link, Google Scholar
69. : Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 2010; 167:261–280Link, Google Scholar
70. : Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr Bull 2008; 34:1083–1094Crossref, Medline, Google Scholar
71. : Perinatal complications and reduced size of brain limbic structures in familial schizophrenia. Schizophr Bull 1988; 14:185–191Crossref, Medline, Google Scholar
72. : Apical temporal lobe resection: “tailored” hippocampus-sparing resection based on presurgical evaluation data. Acta Neurochir(Epub ahead of print, July 17, 2010)Medline, Google Scholar
73. : Fetal and neonatal origins of altered brain development. Early Hum Dev 2005; 81:753–761Crossref, Medline, Google Scholar
74. : Animal models of obstetric complications in relation to schizophrenia. Brain Res Brain Res Rev 2004; 45:1–17Crossref, Medline, Google Scholar
75. : Relationship of obstetric complications and differences in size of brain structures in monozygotic twin pairs discordant for schizophrenia. Am J Psychiatry 2000; 157:203–212Link, Google Scholar
76. : Hippocampal volumes in schizophrenic twins. Arch Gen Psychiatry 2004; 61:346–353Crossref, Medline, Google Scholar
77. : Obstetrical complications and childhood-onset schizophrenia. Am J Psychiatry 1999; 156:1650–1652Link, Google Scholar
78. : Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci 2001; 933:265–277Crossref, Medline, Google Scholar
79. : Stress and brain atrophy. CNS Neurol Disord Drug Targets 2006; 5:503–512Crossref, Medline, Google Scholar
80. : Role of glucocorticoids in the regulation of dopaminergic neurotransmission. Pol J Pharmacol 2003; 55:667–674Medline, Google Scholar
81. : Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry 2002; 51:775–787Crossref, Medline, Google Scholar
82. : Decline in the incidence of schizophrenia in Finnish cohorts born from 1954 to 1965. Arch Gen Psychiatry 1999; 56:733–740Crossref, Medline, Google Scholar
83. : Time trends in schizophrenia: changes in obstetric risk factors with industrialization. Schizophr Bull 1995; 21:483–500Crossref, Medline, Google Scholar
84. : Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology 2010; 58:884–893Crossref, Medline, Google Scholar
85. : Neurogenesis and schizophrenia: dividing neurons in a divided mind? Eur Arch Psychiatry Clin Neurosci 2007; 257:290–299Crossref, Medline, Google Scholar
86. : Exercise and time-dependent benefits to learning and memory. Neuroscience 2010; 167:588–597Crossref, Medline, Google Scholar
87. : Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry 2010; 67:133–143Crossref, Medline, Google Scholar



