Aberrant Brain Activation During a Working Memory Task in Psychotic Major Depression
Abstract
Objective:
The authors sought to better understand the neural circuitry associated with working memory deficits in psychotic major depression by examining brain function during an N-back task.
Method:
Study subjects were 16 patients with psychotic major depression, 15 patients with nonpsychotic major depression, and 19 healthy comparison subjects. Functional MRI data were collected while participants responded to letter stimuli that were repeated from the previous trial (1-back) or the one before that (2-back).
Results:
Relative to the healthy comparison group, both the psychotic and nonpsychotic major depression groups had significantly greater activation in the right parahippocampal gyrus during the 2-back task, and the psychotic major depression group showed this overactivation during the 1-back task as well. The nonpsychotic major depression group showed significantly lower activation than other groups in the right dorsolateral prefrontal cortex and greater activation than the healthy comparison group in the superior occipital cortex. The psychotic major depression group was unique in showing greater activation than both other groups in the right temporoparietal junction, a cluster that also demonstrated connectivity with activation in the left prefrontal cortex.
Conclusions:
The psychotic major depression group showed aberrant parahippocampal activation at a lower demand level than observed in nonpsychotic major depression. While the nonpsychotic major depression group showed abnormalities in frontal executive regions, the psychotic major depression group showed abnormalities in temporoparietal regions associated with orienting to unexpected stimuli. Considering the functional connectivity of this cluster with left dorsolateral prefrontal cortex regions, these findings may reflect neural compensation for sensory gating deficits in psychotic major depression.
An estimated 5%–20% of patients with major depression experience psychotic symptoms, including hallucinations and delusions (1, 2). Psychotic major depression is associated with more severe psychomotor symptoms and guilt (3, 4), significantly greater impairment, longer illness duration, and greater likelihood of recurrence compared with nonpsychotic major depression (1, 5, 6). However, the severity of a depressive episode does not necessarily determine whether psychotic features will be present (7). Unfortunately, psychotic major depression is frequently misdiagnosed (8), which can deter effective treatment.
A better understanding of the brain pathophysiology underlying psychotic major depression can lead to earlier detection and more effective treatments. Neuropsychological data from our lab have shown that patients with psychotic major depression have greater cognitive impairment than do patients with nonpsychotic major depression or healthy comparison subjects on tests of working memory, verbal memory, and psychomotor speed but not on tests of simple verbal attention (9), which implies abnormal brain circuits associated with memory encoding and executive function (10). However, the relationship of these cognitive impairments to neural function is not clear. For example, impairments in the function of temporal lobe memory systems, prefrontal lobe executive regions, or visual and attention networks could underlie working memory deficits in psychotic major depression.
Working memory tasks require the temporary maintenance and manipulation of information as well as sustained attention. The neurofunctional system subserving verbal working memory has been studied extensively in healthy comparison subjects using functional MRI (fMRI). Studies consistently show that the dorsolateral prefrontal cortex, typically in Brodmann's area 46, sustains activation during the delay periods in which individuals hold information in working memory (11) and suppresses activation to nonrelevant stimuli (12). The sustained attention and target detection component of a working memory task often activates a right hemisphere attention system, including regions of the temporoparietal cortex and inferior and middle frontal gyri. This network is consistently activated during detection of task-relevant stimuli, especially when they are infrequent (13). In the left hemisphere, the inferior parietal region is believed to be part of a storage buffer for verbal information (14). The hippocampus is also active during the delay period of a working memory task, but the parahippocampal gyrus, in contrast, is activated during the encoding and recognition parts of the working memory task and predicts successful long-term memory encoding (15).
Because of the widespread brain circuitry involved, patients with various psychiatric disorders have been found to show abnormal brain activation during working memory tasks. Patients with nonpsychotic major depression show abnormal prefrontal function during a verbal working memory task, which emphasizes the importance of comparing patients with psychotic and nonpsychotic major depression (16–22). Because patients with schizophrenia also display deficits in working memory, several neuroimaging studies have found dorsolateral prefrontal cortex deficits in schizophrenia relative to comparison subjects (23–25) and to patients with nonpsychotic major depression (24, 26), which suggests a common substrate for working memory deficits across diagnoses but with greater severity in schizophrenia. Given that psychotic major depression involves symptoms of both nonpsychotic major depression and schizophrenia, these patients may share common deficits in executive function. However, because high cortisol levels in psychotic major depression (9) are likely to affect hippocampal and parahippocampal systems as well, deficits in medial temporal lobe regions may differentiate psychotic major depression from nonpsychotic major depression.
In this study, we investigated the neural correlates of a verbal working memory task in patients with psychotic major depression in order to better understand the brain circuitry underlying working memory deficits in psychotic major depression. Patients with nonpsychotic major depression and healthy comparison subjects were included to differentiate between depressive subtypes as well as between these subtypes and unaffected individuals. We predicted that the psychotic major depression group would show a unique pattern of activation that reflects abnormalities in medial temporal lobe and parietal regions and similarities to the nonpsychotic major depression group in prefrontal regions.
Method
Participants
Participants were recruited from outpatient psychiatric clinics at Stanford University and through advertisements in the surrounding communities. Patients with psychotic and nonpsychotic major depression were diagnosed with the Structured Clinical Interview for DSM-IV (SCID) (27), and diagnoses were confirmed by the treating psychiatrist when available. Patients had to score at least 18 on the 21-item Hamilton Depression Rating Scale (HAM-D) (28) and at least 7 on the Thase Core Endogenomorphic Scale (29) to verify significant depressive and endogenous symptoms, respectively. Patients with psychotic major depression had to score at least 5 on the positive symptom subscale of the Brief Psychiatric Rating Scale (BPRS; 30). All patients met DSM-IV criteria for current unipolar major depressive episode. Patients with nonpsychotic major depression had no history of psychotic symptoms. Patients were excluded if they had active suicidality, obsessive-compulsive disorder, or bipolar disorder or had abused substances or received ECT within the past 6 months. All participants were allowed to continue their psychiatric medications but were required to maintain a stable medication regimen for at least 1 week before the study.
Healthy comparison subjects, recruited through advertisements, had to score less than 6 on the HAM-D, have no psychotic symptoms, and have no current or past axis I diagnoses according to SCID criteria.
Exclusion criteria for all participants included major medical illness, history of seizures or head injury, pregnancy or lactation, age <18 years, or use of estrogen supplements or birth control pills.
The study included 22 patients with psychotic major depression, 21 patients with nonpsychotic major depression, and 24 healthy comparison subjects. All participants gave written informed consent and received $250 for their participation. The study was approved by the Institutional Review Board of Stanford University.
The N-Back Task
Participants performed an N-back task during image acquisition. The task had three types of alternating blocks: four 1-back, four 2-back, and six control (“press for Z”) blocks. For all blocks, a succession of uniform-size single letters was presented, in both upper- and lowercase. Each letter was presented for 500 msec, with a 1,500 msec intertrial interval showing a fixation cross. Each block contained 12 letters and lasted for 24 seconds. Brief instructions were shown for 4 seconds at the beginning of the block (e.g., “Press for 1-back”). During the 1-back blocks, participants pressed the button when the current letter matched the one presented one trial back, e.g., a repeated letter such as A—A. During 2-back blocks, participants pressed the button when the current letter matched the one presented two trials back, e.g., in a “sandwich” pattern such as A—B—-A. During the “press for Z” blocks, participants pressed the button whenever the current letter was a Z. Participants pressed with the right index finger on a hand-held button box. Stimuli were projected onto a screen and viewed through a mirror attached to the head coil. All participants practiced the task before the scan. The scan lasted 7 minutes and 48 seconds, during which 232 frames were acquired. The E-Prime software program (www.pstnet.com) was used to present the task and collect responses.
fMRI Data Acquisition and Processing
Images were acquired on a 3-T General Electric (GE) Signa scanner using a standard GE whole-head coil (Lx platform, gradients 40 mT/m, 150 T/m/sec; GE Medical Systems, Milwaukee). A custom-built head restraint system and foam padding prevented head movement. A high-order shim (31) reduced blurring and signal loss from field inhomogeneities. Twenty-eight axial slices (4 mm thick, 0.5 mm skip) parallel to the anterior and posterior commissures and covering the whole brain were imaged using a T2*-weighted spiral pulse sequence (32) (repetition time=2,000 msec, echo time=30 msec, flip angle=80°, interleave=1, field of view=200 mm2, matrix=64×64, inplane spatial resolution=3.125 mm2).
fMRI data were processed using SPM5 (www.fil.ion.ucl.ac.uk/spm). Images were realigned to the third volume. Image distortion and spin history errors caused by abrupt motions were repaired by interpolation from the nearest unaffected volumes. These methods were implemented in the ArtRepair toolbox for SPM (http://cibsr.stanford.edu/tools/ArtRepair/ArtRepair.htm). Data were normalized to the Montreal Neurological Institute (MNI) echo-planar image template and resampled to a 2-mm3 matrix using sinc interpolation. Data were smoothed with a 4-mm full width at half maximum Gaussian filter and high-pass filtered at 120 seconds.
Individual statistics were computed using a fixed-effects model and a block design comparing 1-back with press for Z, and 2-back with press for Z. Group t tests used a random-effects model to examine activation within and between each group. A dual-significance corrected threshold of height at p=0.01 and cluster extent at p=0.01 was used within groups. To determine the location of significant clusters, coordinates were first converted to the Talairach template using the mni2tal function (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach), and then brain regions were localized using the Talairach Daemon software (http://www.talairach.org/client.html) and also visually inspected by an experienced neuroimager (A.G.).
A multivariate analysis of variance was conducted in SPM5 using the independent factor group and the repeated factor task (1-back minus press for Z, and 2-back minus press for Z). Gender and response time were used as covariates (see below). A threshold of p=0.001, extent=10 defined significant activation that differed among the three groups across task conditions (main effect of group). Mean activation levels (t-scores) were extracted from significant clusters using MarsBar (http://marsbar.sourceforge.net/). Between-group t tests were then performed using SPSS (http://www.spss.com/) with a corrected significance threshold of p=0.0125 (or p=0.05/4 regions tested).
A connectivity analysis was conducted using the Psychophysiological Interaction module of SPM5. This post hoc analysis located brain regions functionally associated with the temporoparietal junction region in the psychotic major depression group. The temporoparietal junction cluster that was significantly increased in the psychotic major depression group was used as the seed region. For each participant, a 6-mm3 box was placed on the maximum voxel in the cluster. The average time course of the voxels that surpassed threshold (p=0.01) was extracted. A multiple regression identified voxels showing a significant interaction between that time course and the 2-back-press-for-Z contrast for each participant. A random-effects analysis combined individual results into a group result using a cluster-corrected threshold of dual height and extent at p=0.01.
Results
Data for five participants with psychotic major depression, two with nonpsychotic major depression, and three healthy comparison subjects were excluded because of response box failure resulting in N-back task accuracy below 50%. Scan data from one patient with psychotic major depression, four patients with nonpsychotic major depression, and two healthy comparison subjects were excluded because of movement artifacts during more than 20% of the scan. This left 16 participants in the psychotic major depression group, 15 in the nonpsychotic major depression group, and 19 in the healthy comparison group.
Table 1 summarizes participants' clinical and demographic characteristics. There were no group differences in age, handedness, or years of education, but the groups differed in gender distribution. The groups had similar IQ, as measured by full-scale estimates of premorbid intellectual functioning.
| Measure | Psychotic Major Depression Group (N=16) | Nonpsychotic Major Depression Group (N=15) | Healthy Comparison Group (N=19) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 34.13 | 10.68 | 39.81 | 12.74 | 34.85 | 12.54 |
| Education (years) | 16.25a | 3.72 | 14.40 | 1.40 | 15.95 | 2.15 |
| IQb | 111.56 | 9.40 | 104.00 | 10.63 | 111.28 | 9.35 |
| Hamilton Depression Rating Scale (21-item) scorec | 27.88 | 3.52 | 24.20 | 3.30 | 0.58 | 0.84 |
| Brief Psychiatric Rating Scale scored | 28.75 | 5.36 | 15.73 | 2.71 | 0.42 | 0.69 |
| Brief Psychiatric Rating Scale positive symptom subscoree | 8.38 | 3.70 | 0.13 | 0.35 | 0.00 | 0.00 |
| Length of current depressive episode (weeks) | 165.8f | 198.4 | 155.5g | 217.1 | ||
| Task performance | ||||||
| 1-Back | ||||||
| % Correct | 97.25 | 1.13 | 96.41 | 1.20 | 97.26 | 1.05 |
| Response time | 581.7 | 28.24 | 650.0 | 29.96 | 556.7 | 26.35 |
| 2-Back | ||||||
| % Correct | 98.47 | 2.84 | 99.03 | 0.38 | 99.77 | 0.33 |
| Response timeh | 629.6 | 30.28 | 683.9 | 32.13 | 559.7 | 28.26 |
| N | % | N | % | N | % | |
| Femalei | 7 | 43.8 | 10 | 66.7 | 6 | 31.6 |
| Left-handed | 2j | 15.4 | 2 | 13.3 | 2 | 10.5 |
| Current medication | ||||||
| None | 2 | 12.5 | 6 | 40.0 | ||
| Anxiolytics | 4 | 25.0 | 0 | 0.0 | ||
| Antidepressants | 8 | 50.0 | 9 | 60.0 | ||
| Antipsychotics | 10 | 62.5 | 0 | 0.0 | ||
| Mood stabilizer | 1 | 6.3 | 1 | 6.7 | ||
| Comorbid diagnoses | ||||||
| None | 5 | 31.3 | 5 | 33.3 | ||
| Anxiety disordersk | 3 | 18.8 | 8 | 53.5 | ||
| Bulimia nervosa | 1 | 6.3 | 0 | 0.0 | ||
TABLE 1. Clinical, Demographic, and Task Performance Measures, by Group, in a Study of the Neural Circuitry Associated With Working Memory Deficits in Psychotic Major Depression
The patients for whom medication data were available had established antidepressant medication regimens with no changes; the psychotic major depression group (N=6) had an average of 20.3 weeks of antidepressant use (range=3–56, SD=19.9) and 9.8 weeks of antipsychotic use (range=5–26, SD=9.2). The nonpsychotic major depression group (N=9) had an average of 42.6 weeks of antidepressant use (range=4–156, SD=56.4).
N-Back Task Performance Results
All participants performed the task with a high degree of accuracy (Table 1). Response time for the 2-back task was different between groups, and further comparisons showed that this was due to significantly slower response time in the nonpsychotic major depression group compared to the healthy comparison group (p=0.002), even when controlling for group differences in gender (p=0.007). Therefore, all comparisons with the nonpsychotic major depression group during the 2-back task included response time as a covariate.
fMRI Results
Tables 2 and 3 list regions that were significantly activated during the 1-back and 2-back tasks within each group. All three groups activated regions typically associated with verbal working memory tasks, such as the left supramarginal gyrus, left inferior frontal gyrus, and left and right inferior parietal lobe.
| Region | Brodmann's Area | pa | Cluster Size (Voxels) | z Score | Talairach Coordinates | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Healthy comparison group (N=19) | |||||||
| Right inferior frontal gyrus | 46 | 0.0001 | 3,541 | 4.50 | 57 | 32 | 13 |
| Right middle frontal gyrus | 46 | 4.29 | 55 | 32 | 15 | ||
| 6 | 4.23 | 36 | 8 | 49 | |||
| Left inferior parietal lobe | 40 | 0.001 | 1,817 | 4.47 | −42 | −45 | 41 |
| Left supramarginal gyrus | 40 | 4.19 | −42 | −43 | 37 | ||
| Right inferior parietal lobe | 40 | 0.0001 | 2,200 | 4.19 | 51 | −36 | 52 |
| Right superior parietal lobe | 7 | 4.09 | 42 | −58 | 49 | ||
| Right postcentral gyrus | 40 | 3.94 | 50 | −32 | 51 | ||
| Left middle frontal gyrus | 6 | 0.003 | 1,398 | 3.91 | −30 | 14 | 55 |
| 9 | 3.41 | −57 | 17 | 30 | |||
| Left superior frontal gyrus | 6 | 3.54 | −32 | 14 | 51 | ||
| Left inferior frontal gyrus | 9 | 3.51 | −57 | 17 | 29 | ||
| Psychotic major depression group (N=16) | |||||||
| Right middle frontal gyrus | 6 | 0.0001 | 1,530 | 4.00 | 55 | 6 | 42 |
| 8 | 3.91 | 55 | 10 | 42 | |||
| Right inferior frontal gyrus | 44 | 3.86 | 53 | 9 | 20 | ||
| Left supramarginal gyrus | 40 | 0.0001 | 2,610 | 4.26 | −42 | −43 | 37 |
| Left inferior parietal lobe | 40 | 4.11 | −42 | −43 | 39 | ||
| Right precuneus | 19 | 0.0001 | 2,089 | 4.10 | 32 | −64 | 38 |
| Right inferior parietal lobe | 39 | 3.63 | 34 | −62 | 38 | ||
| 40 | 3.59 | 44 | −44 | 45 | |||
| Left inferior frontal gyrus | 9 | 0.0001 | 3,475 | 4.13 | −48 | 17 | 23 |
| Left middle frontal gyrus | 9 | 4.04 | −53 | 13 | 31 | ||
| 6 | 3.88 | −51 | 14 | 44 | |||
| Right medial frontal gyrus | 8 | 3.92 | 8 | 29 | 41 | ||
| Right medial frontal gyrus | 8 | 3.92 | 8 | 29 | 41 | ||
| Nonpsychotic major depression group (N=15) | |||||||
| Right inferior frontal gyrus | 46 | 0.0001 | 5,278 | 5.84 | 51 | 39 | 11 |
| Right middle frontal gyrus | 46 | 5.13 | 57 | 30 | 22 | ||
| 6 | 4.95 | 34 | 0 | 48 | |||
| Right inferior parietal lobe | 40 | 0.0001 | 4,347 | 5.74 | 40 | −50 | 45 |
| Left middle frontal gyrus | 6 | 0.0001 | 6,983 | 5.26 | −36 | 6 | 49 |
| Left middle frontal gyrus | 46 | 4.37 | −44 | 28 | 21 | ||
| Right medial frontal gyrus | 8 | 5.00 | 10 | 31 | 41 | ||
| Right superior frontal gyrus | 8 | 4.68 | 8 | 31 | 43 | ||
| Left inferior frontal gyrus | 45 | 4.23 | −46 | 26 | 21 | ||
| Left inferior parietal lobe | 40 | 0.0001 | 3,546 | 5.09 | −50 | −39 | 41 |
| Left supramarginal gyrus | 40 | 4.77 | −44 | −41 | 37 | ||
| Left lingual gyrus | 17 | 0.0001 | 1,748 | 3.82 | −20 | −97 | −7 |
| 18 | 3.71 | −20 | −99 | −5 | |||
| Left inferior occipital gyrus | 17 | 3.80 | −22 | −97 | −7 | ||
| Left inferior temporal gyrus | 20 | 3.50 | −57 | −49 | −14 | ||
| Left fusiform gyrus | 19 | 3.48 | −50 | −69 | −12 | ||
| 37 | 3.46 | −55 | −51 | −16 | |||
| Left middle occipital gyrus | 19 | 3.40 | −50 | −67 | −10 | ||
| Right inferior temporal gyrus | 20 | 0.0001 | 2,386 | 3.80 | 59 | −47 | −14 |
| Right fusiform gyrus | 37 | 3.64 | 55 | −49 | −16 | ||
| Right middle temporal gyrus | 37 | 3.50 | 59 | −49 | −9 | ||
TABLE 2. Significant Activation Within Each Group During the 1-Back Versus Press-for-Z Contrast
| Region | Brodmann's Area | pa | Cluster Size (Voxels) | z Score | Talairach Coordinates | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Healthy comparison group (N=19) | |||||||
| Left inferior parietal lobe | 40 | 0.0001 | 12,750 | 7.06 | 44 | 43 | 41 |
| Left supramarginal gyrus | 40 | 6.02 | 42 | 41 | 37 | ||
| Right middle frontal gyrus | 6 | 0.0001 | 30,453 | 6.10 | 36 | 6 | 46 |
| Left middle frontal gyrus | 9 | 6.05 | 51 | 13 | 32 | ||
| Right superior frontal gyrus | 8 | 5.74 | 2 | 18 | 51 | ||
| Left superior frontal gyrus | 6 | 5.73 | 2 | 16 | 53 | ||
| Right cuneus | 17 | 0.005 | 509 | 3.84 | 14 | 79 | 11 |
| Right posterior cingulate | 30 | 3.26 | 26 | 64 | 7 | ||
| Left inferior temporal gyrus | 20 | 0.005 | 532 | 3.34 | 57 | 51 | 14 |
| Left fusiform gyrus | 37 | 3.23 | 53 | 51 | 16 | ||
| Left middle occipital gyrus | 37 | 3.08 | 55 | 65 | 10 | ||
| Psychotic major depression group (N=16) | |||||||
| Right inferior frontal gyrus | 44 | 0.0001 | 11,555 | 5.02 | 57 | 16 | 16 |
| 45 | 4.78 | 57 | 13 | 20 | |||
| Right middle frontal gyrus | 8 | 4.41 | 28 | 14 | 42 | ||
| 6 | 4.41 | 30 | 12 | 44 | |||
| Right insula | 13 | 4.33 | 30 | 17 | 4 | ||
| Right angular gyrus | 39 | 0.0001 | 10,413 | 4.59 | 34 | 60 | 36 |
| Right precuneus | 39 | 4.45 | 36 | 62 | 38 | ||
| Right inferior parietal lobe | 39 | 4.44 | 36 | 62 | 38 | ||
| Left inferior parietal lobe | 40 | 4.43 | 42 | 39 | 44 | ||
| Left precuneus | 19 | 4.32 | 28 | 74 | 41 | ||
| Right subgyral angular gyrus | 39 | 4.27 | 32 | 60 | 36 | ||
| Right medial frontal gyrus | 8 | 0.0001 | 6,339 | 4.58 | 6 | 29 | 43 |
| 6 | 4.28 | 6 | 35 | 37 | |||
| Right superior frontal gyrus | 8 | 4.42 | 6 | 31 | 43 | ||
| Left inferior frontal gyrus | 9 | 4.42 | 50 | 11 | 27 | ||
| Nonpsychotic major depression group (N=15) | |||||||
| Right inferior parietal lobe | 40 | 0.0001 | 10,087 | 5.81 | 48 | 44 | 43 |
| Right supramarginal gyrus | 40 | 5.26 | 44 | 45 | 37 | ||
| Left caudate body | NA | 0.0001 | 21,910 | 5.74 | 12 | 7 | 16 |
| Right caudate body | NA | 5.30 | 12 | 7 | 14 | ||
| Right caudate head | NA | 5.11 | 14 | 12 | 5 | ||
TABLE 3. Significant Activation Within Each Group During the 2-Back Versus Press-for-Z Contrast
Between-Group Analysis of Variance
Four clusters of activation were significant (Figure 1). Activation in the right parahippocampal gyrus was significantly greater during both the 1-back and 2-back tasks in the psychotic major depression group relative to the healthy comparison group. The nonpsychotic major depression group showed greater parahippocampal activation during the 2-back task relative to the comparison group. The nonpsychotic major depression group showed significantly less activation in the right dorsolateral prefrontal cortex during the 2-back task relative to both groups and greater occipital cortex activation relative to the healthy comparison group during both tasks. The psychotic major depression group had significantly greater activation in the right temporoparietal junction during the 2-back task relative to both the nonpsychotic major depression and healthy comparison groups and nonsignificantly greater activation during the 1-back task.

a Boxplots show the distribution of mean activation within each group, including the minimum, median, and maximum values. Error bars show the standard deviation, and data points outside the box are outliers. The y-axis represents the mean T value of extracted activation, statistically corrected for gender and task performance, ranging from 0 to 2.5. Four clusters were significant, including the right parahippocampal gyrus (Brodmann's area 20/36; peak z=3.68; extent=26 voxels, Talairach coordinates (x, y, z) of peak=30, −36, −15); the right dorsolateral prefrontal cortex (Brodmann's area 9; peak z=3.68; extent=24 voxels; Talairach coordinates of peak=22, 37, 31); the right temporoparietal junction (Brodmann's area 39; peak z=3.60; extent=20 voxels; Talairach coordinates of peak=44, −67, 24); and the right superior occipital gyrus (Brodmann's area 19; peak z=3.52; extent=27 voxels; Talairach coordinates of peak=32, −78, 37). Results of between-group t tests comparing extracted clusters are shown under each graph (corrected significance threshold, p=0.0125). PMD=psychotic major depression group; NPMD=nonpsychotic major depression group; HC=healthy comparison group.
An important question is whether differences between the psychotic and nonpsychotic major depression groups can be attributed solely to differences in severity of depressive symptoms. However, group differences in brain activation did not change when covaried for HAM-D score. Also, we attempted to better understand the influence of medication on our results by examining brain activation in medicated compared with unmedicated participants. Only the nonpsychotic major depression group contained a sufficient number of unmedicated participants to perform this analysis. With the group divided into unmedicated (N=6) and medicated (N=9) subgroups, none of the regions were significantly different between subgroups.
Connectivity Results
The psychophysiological interaction analysis was conducted to better understand functional circuits involving the temporoparietal junction during the 2-back task. Results showed that activation in a single large cluster in the left prefrontal cortex was significantly associated with activation in the right temporoparietal junction during the 2-back task (Figure 2). The left prefrontal cluster included three subpeaks: the dorsolateral prefrontal cortex, the middle frontal gyrus, and the inferior frontal gyrus.
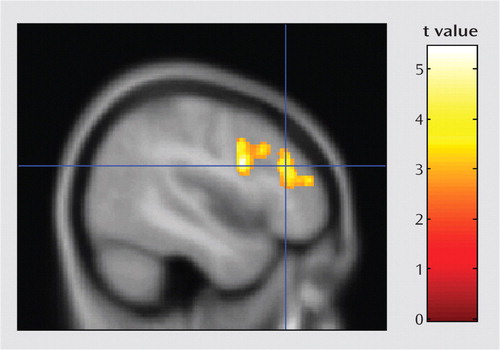
a A single significant cluster was found in the left prefrontal cortex (threshold: p=0.01 height and p=0.01 corrected cluster extent; cluster size=1,385 voxels; peak t=5.44; peak location, Talairach coordinates x, y, z=−46, 7, 24). The crosshairs shown in this figure arelocated at a subpeak in the dorsolateral prefrontal cortex (Brodmann'sarea 9/46). The cluster also includes the middle frontal gyrus (Brodmann's area 9/10) and the inferior frontal gyrus (Brodmann' area 44).
Discussion
We examined the neural correlates of working memory in patients with psychotic major depression compared to patients with nonpsychotic major depression and healthy comparison subjects. Given that several previous studies document working memory deficits in both psychotic and nonpsychotic major depression (9–10, 17, 20, 33), brain activation in these patient groups may reflect compensatory neural strategies as well as aberrant functional deficits. In the present study, both the psychotic and the nonpsychotic major depression groups showed increased parahippocampal activation relative to the healthy comparison group during the 2-back task, but the psychotic major depression group showed this difference during the less difficult 1-back task as well. These results suggest neural deficits common to both psychotic and nonpsychotic major depression in temporal lobe memory systems, albeit at a lower level of task demand in psychotic major depression. Only the nonpsychotic major depression group showed greater right occipital activation compared to the healthy comparison group, suggesting increased effort toward basic visual processing. The nonpsychotic major depression group also showed lower right dorsolateral prefrontal cortex activation compared to the psychotic major depression and healthy comparison groups during the 2-back task, although, contrary to predictions, no dorsolateral prefrontal cortex abnormalities were seen in the psychotic major depression group. The right temporoparietal junction was the only region where the psychotic major depression group showed significant overactivation compared to both the other groups during the 2-back task, with nonsignificantly greater activation during the 1-back task. In the psychotic major depression group, activation in the temporoparietal junction region showed psychophysiological connectivity with the left dorsolateral prefrontal cortex, which may reflect an executive function compensatory strategy. Controlling for group differences in severity of depressive symptoms did not eliminate observed differences between the psychotic and nonpsychotic major depression groups.
The temporoparietal junction region has been consistently shown in human and primate studies to be involved in reorienting attention to salient stimuli, especially when the stimuli are unexpected and important (13). The temporoparietal junction is part of the ventral frontoparietal system, as described by Corbetta and Shulman (13). Based on a large body of literature (34), these authors hypothesize that the ventral frontoparietal system functions as a “circuit breaker”; the system detects unexpected stimuli that both are behaviorally relevant and require a change in the current task set, such as reorienting attention to a fire alarm while working on your computer. This system is distinct from the dorsal attention system that prepares and applies goal-directed, top-down selection of task-relevant stimuli and responses. Also, the temporoparietal junction region is posterior and ventral to the inferior parietal lobe location (Brodmann's area 40/22) that is frequently activated bilaterally in neuroimaging studies of working memory, also called the “visuospatial scratch pad.”
As part of an automatic attention system, the temporoparietal junction could be important for sensory gating processes. Sensory gating has been suggested to be impaired in psychotic major depression, as data show that patients with this disorder have difficulties distinguishing relevant from irrelevant stimuli (35). In addition, we have observed abnormalities unique to patients with psychotic major depression during performance of the Stroop task (10), which requires sensory gating and filtering processes to resolve response conflict. A well-known measure of sensory gating is the prepulse inhibition test of the acoustic startle reflex (36). Although no studies of prepulse inhibition have focused on patients with psychotic major depression, it has been shown to be deficient in patients with schizophrenia but not in patients with nonpsychotic major depression (37–39). Furthermore, a study of transgenic mice that overexpress corticotropin-releasing factor (proposed to be a model of psychotic depression) also show startle reflex gating deficits that are reversed by antipsychotic drugs (40). Because of psychotic symptoms and problems with filtering, the psychotic major depression group studied here may be expending significantly more neural resources for sensory gating and filtering, and this may be reflected in the increased activation of the temporoparietal junction region. The temporoparietal junction region may function to continually reorient attention to the task, perhaps because of internal and external distractions, including noise from the scanner, or possibly the intrusion of psychotic symptoms. Notably, the psychotic major depression group showed insula activation during the 2-back task, and reduced insula volume has been linked to psychotic symptoms in patients with psychotic major depression (J.D. Cohen et al., unpublished 2009 data). The post hoc analysis showing functional connectivity of the temporoparietal junction with the left prefrontal cortex suggests that top-down executive control over temporoparietal junction resources may compensate for sensory gating deficits, allowing accurate task performance.
The nonpsychotic major depression group showed increased activation in the parahippocampal cortex during the 2-back task, as did the psychotic major depression group during both the 1- and 2-back tasks. Traditionally, medial temporal lobe regions and the adjacent parahippocampal cortex have not been considered to be necessary for working memory tasks but to have an essential role in memory encoding and retrieval. However, a growing body of literature suggests that parahippocampal activation during working memory tasks is associated with encoding of the stimuli and predicts subsequent recall (15). Similar to the present study, a previous investigation (18) showed increased parahippocampal activation in patients with nonpsychotic major depression relative to healthy comparison subjects during a verbal working memory task. The present study goes further to find a similar deficit in psychotic major depression but at a lower level of task demand than in nonpsychotic major depression.
The dorsolateral prefrontal cortex is strongly associated with working memory tasks in healthy volunteers (11). Our study replicates previous reports of abnormal dorsolateral prefrontal cortex activation in nonpsychotic major depression. However, other studies show increased, rather than decreased, dorsolateral prefrontal cortex activation in nonpsychotic major depression during accurate task performance, usually in the left hemisphere (20–22), but also in the right (19). The nonpsychotic major depression group also had lower right dorsolateral prefrontal cortex activation compared to the psychotic major depression group, and this difference remained after covarying for severity of depressive symptoms. This suggests that right hemisphere dorsolateral prefrontal cortex abnormalities contribute to working memory function in nonpsychotic major depression but not in psychotic major depression. However, the connectivity analysis suggests that the left dorsolateral prefrontal cortex may compensate for aberrant brain activation related to sensory gating in psychotic major depression.
These results support the hypothesis that psychotic major depression is distinct from nonpsychotic major depression on a neurofunctional basis. The study has several limitations, however, including the small sample size and medications taken by the clinical subjects, allowing us to detect only relatively large group differences. Although our analyses covaried for gender, imbalanced gender distributions across diagnoses may have contributed to our findings. The task was not difficult enough to demonstrate the previously shown deficits in working memory that have been documented for both patients with psychotic and nonpsychotic major depression. Greater group differences might have been detected by using a task that more rigorously challenged participants' working memory abilities, and an event-related analysis could separate trials on the basis of accuracy or response time for more detailed comparisons between groups.
It is possible that group differences in medication may have influenced brain activation. However, examining the distribution of medicated and unmedicated patients in each group did not reveal patterns suggesting a strong effect of medication. Moreover, we previously reported, using this sample of patients (9, 10), that medication status does not affect differences in cognitive test performance between symptomatic psychotic and nonpsychotic depressed groups. However, as psychiatric medications clearly affect brain function, we cannot rule out the possibility that medications influenced our results, which should thus be considered preliminary. Future studies would benefit from including only unmedicated subjects.
1. : Prevalence and clinical characteristics of psychotic versus nonpsychotic major depression in a general psychiatric outpatient clinic. Depress Anxiety 2009; 26:54–64Crossref, Medline, Google Scholar
2. : Prevalence of depressive episodes with psychotic features in the general population. Am J Psychiatry 2002; 159:1855–1861Link, Google Scholar
3. : Detecting psychotic major depression using psychiatric rating scales. J Psychiatr Res 2006; 40:22–29Crossref, Medline, Google Scholar
4. : Distinguishing psychotic and non-psychotic melancholia. J Affect Disord 1991; 22:135–148Crossref, Medline, Google Scholar
5. : Depressive symptom profiles and severity patterns in outpatients with psychotic vs nonpsychotic major depression. Compr Psychiatry 2008; 49:421–429Crossref, Medline, Google Scholar
6. : Current issues in the classification of psychotic major depression. Schizophr Bull 2007; 33:877–885Crossref, Medline, Google Scholar
7. : Is depression severity the sole cause of psychotic symp-toms during an episode of unipolar major depression? a study both between and within subjects. J Affect Disord 2009; 114:103–109Crossref, Medline, Google Scholar
8. ;
9. : The neuropsychological profile of psychotic major depression and its relation to cortisol. Biol Psychiatry 2006; 60:472–478Crossref, Medline, Google Scholar
10. : Neuropsychological deficits in psychotic versus nonpsy-chotic major depression and no mental illness. Am J Psychiatry 2000; 157:1095–1100Link, Google Scholar
11. : From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci 2007; 362:761–772Crossref, Medline, Google Scholar
12. : Differential effects of distraction during working memory on delay-period activity in the prefrontal cortex and the visual association cortex. Neuroimage 2006; 29:1117–1126Crossref, Medline, Google Scholar
13. : Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 2002; 3:201–215Crossref, Medline, Google Scholar
14. : Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage 2004; 22:562–573Crossref, Medline, Google Scholar
15. : Interaction of working memory and long-term memory in the medial temporal lobe. Cereb Cortex 2008; 18:2868–2878Crossref, Medline, Google Scholar
16. : Pattern of impaired working memory during major depression. J Affect Disord 2006; 90:149–161Crossref, Medline, Google Scholar
17. : Neuroanatomy of verbal working memory as a diagnostic biomarker for depression. Neuroreport 2008; 19:1507–1511Crossref, Medline, Google Scholar
18. : A longi-tudinal functional magnetic resonance imaging study of verbal working memory in depression after antidepressant therapy. Biol Psychiatry 2007; 62:1236–1243Crossref, Medline, Google Scholar
19. : An fMRI study of prefrontal brain activation during multi-ple tasks in patients with major depressive disorder. Hum Brain Mapp 2008; 29:490–501Crossref, Medline, Google Scholar
20. : Cognitive control and brain resources in major de-pression: an fMRI study using the n-back task. Neuroimage 2005; 26:860–869Crossref, Medline, Google Scholar
21. : Increased left prefrontal activation in patients with unipolar depression: an event-related, parametric, performance-controlled fMRI study. J Affect Disord 2007; 101:175–185Crossref, Medline, Google Scholar
22. : Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry 2007; 12:158–166Crossref, Medline, Google Scholar
23. ;
24. : Working memory and prefrontal cortex dysfunction: specificity to schizophrenia compared with major depression. Biol Psychiatry 2003; 53:376–384Crossref, Medline, Google Scholar
25. : Abnormal parietal cortex activation during working memory in schizophrenia: verbal phonological coding distur-bances versus domain-general executive dysfunction. Am J Psychiatry 2007; 164:1090–1098Link, Google Scholar
26. : Working memory dysfunction in schizophrenia compared to healthy controls and patients with depression: evidence from event-related fMRI. Neuroimage 2007; 35:1551–1561Crossref, Medline, Google Scholar
27. : Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1997 Google Scholar
28. : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
29. : Validation of a Hamilton subscale for endogenomorphic depression. J Affect Disord 1983; 5:267–278Crossref, Medline, Google Scholar
30. : The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
31. : SVD regularization algorithm for improved high-order shimming. Proceedings of the 8th Annual Meeting of the International Society for Magnetic Resonance in Medicine, Denver, 2000 Google Scholar
32. : Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med 1998; 39:361–368Crossref, Medline, Google Scholar
33. : Effects of major depression diagnosis and cortisol levels on indices of neurocognitive function. Psychoneuroendocrinology 2009; 34:1012–1018Crossref, Medline, Google Scholar
34. : Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 2000; 3:292–297Crossref, Medline, Google Scholar
35. : Cortisol activity and cognitive changes in psychotic major depression. Am J Psychiatry 2001; 158:1612–1616Link, Google Scholar
36. : Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001; 156:234–258Crossref, Medline, Google Scholar
37. : No prepulse inhibition deficits in patients with unipolar depression. Depress Anxiety 2003; 17:224–225Crossref, Medline, Google Scholar
38. : Prepulse inhibition in patients with non-psychotic major depressive disorder. J Affect Disord 2004; 81:179–184Crossref, Medline, Google Scholar
39. : Normal prepulse inhibition and habituation of acoustic star-tle response in suicidal depressive patients without psychotic symptoms. J Affect Disord 2006; 92:299–303Crossref, Medline, Google Scholar
40. : Reversal of startle gating deficits in transgenic mice overexpressing corticotropin-releasing factor by antipsychotic drugs. Neuropsychopharmacology 2003; 28:1790–1798Crossref, Medline, Google Scholar



