Cognitive Abilities and 50- and 100-msec Paired-Click Processes in Schizophrenia
Abstract
Objective:
Abnormal 50- and 100-msec event-related brain activity derived from paired-click procedures are well established in schizophrenia. There is little agreement on whether group differences in the ratio score, i.e., the ratio of EEG amplitude after the second stimulus (S2) to the amplitude after the first stimulus (S1), refiect an encoding or gating abnormality. In addition, the functional implications remain unclear. In the present study, EEG and magnetoencephalography (MEG) were used to examine paired-click measures and cognitive correlates of paired-click activity.
Method:
EEG and whole-cortex MEG data were acquired during the standard paired-click paradigm in 73 comparison subjects and 79 schizophrenia patients. Paired-click ratio scores were obtained at 50 msec (P50 evoked potential at Cz, M50 at left and right superior temporal gyrus [STG]) and 100 msec (N100 at Cz, M100 at left and right STG). A cognitive battery assessing attention, working memory, and long-delay memory was administered. IQ was also estimated.
Results:
Groups differed on ratio score and amplitude of S1 response. Ratio scores at 50 msec and 100 msec and S1 amplitude predicted variance in attention (primarily S1 amplitude), working memory, and long-delay memory. The attention findings remained after removal of variance associated with IQ.
Conclusions:
Associations between paired-click measures and cognitive performance in patients support 50-msec and 100-msec ratio and amplitude scores as clinically significant biomarkers of schizophrenia. In general, cognitive performance was better predicted by the ability to encode auditory information than the ability to filter redundant information.
For more than 25 years the auditory paired-click paradigm has been used to study auditory processes in individuals with schizophrenia (1). Subjects are presented with two auditory stimuli (S1 and S2) separated by 500 msec, and the amplitude of evoked brain activity 50 msec (P50) and 100 msec (N100) after each click is typically measured with EEG at electrode Cz. A higher than normal ratio of the S2 amplitude to the S1 amplitude is frequently observed in schizophrenia (2). The functional implications of this ratio score deficit are currently in dispute. There is little evidence of a relationship between P50 ratio scores and positive or negative symptoms in schizophrenia (3). A few studies have examined the association between paired-click ratio scores and cognitive measures, with some evidence of a relationship to processing speed (4), attention (5 and 6, although see 7), and explicit and implicit memory (8).
There is also ongoing disagreement as to whether differences between the ratio scores of patients and comparison subjects refiect encoding or gating abnormalities. A fundamental question is whether interpretations of ratio score phenomena should focus on S1 or S2. The response to S1 is driven by encoding and attention processes, whereas the S2 response primarily refiects sensory gating and filtering. Although the paired-click ratio score deficit is often observed in the absence of group differences in S1, several studies have shown a lower S1 response in patients at 50 msec (e.g., references 9–12) and 100 msec (e.g., references 9, 13–15). De Wilde et al. (16) suggested that ratio scores and amplitude should both be investigated (see also 17).
There are at least two basic limitations to linking Cz activity and cognitive dysfunction. P50 recorded at the scalp is multidetermined, making it difficult to identify specific brain-performance relationships (18), and several studies have shown that the P50 Cz ratio score is not a reliable measure in traditional psychometric terms (e.g., 19–21, although see 22). Both limitations can be overcome by using source-localization methods. Studies using magnetoencephalography (MEG) to localize sources of brain activity during the paired-click paradigm in comparison subjects and schizophrenia patients focused on M50 and M100, the magnetic manifestations of the P50 and N100 neuroelectric components, and established that the bilateral superior temporal gyrus (STG) is the primary area active during the 50-msec period (18, 23). Whereas group differences in the ratio score may be specific to the left STG at 50 msec, bilateral STG deficits exist at 100 msec (24). In addition, source-localized M50 STG ratio scores are reliable (21). In contrast to paired-click EEG studies, paired-click MEG studies have shown relationships with clinical measures: negative symptoms correlated positively with the right hemisphere M50 ratio score (25).
Since our original study examining the relationship between EEG and MEG 50-msec ratio scores and cognitive abilities (26), the size of our study group has increased approximately fourfold. In addition, the Wisconsin Card Sorting Test, which is sensitive to prefrontal/executive function (27), was added to the cognitive battery. The present study examined the relationship between cognitive measures and EEG and MEG 50- and 100-msec ratio scores. Whereas the 50-msec component is thought to refiect bottom-up, preattentive processes (28, although see 29–32), 100-msec activity may refiect the onset of top-down modulation by the frontal cortex (33–35), a hypothesis supported by studies showing that N100 amplitude is influenced by attentional manipulations (e.g., references 33, 36, 37). Thus, 50- and 100-msec ratio scores may be differentially related to cognitive tests.
In the present study, MEG and EEG data were simultaneously collected during the paired-click paradigm, and Cz and MEG-derived STG activity was examined to uncover clinical relationships in a relatively large group of comparison subjects and patients. The following hypotheses were pursued: 1) in a replication of previous findings, patients with schizophrenia would show abnormally large P50, N100, left M50, and bilateral M100 ratio scores and 2) in keeping with 100-msec paired-click findings reported in previous studies (e.g., references 9, 38, 39), 100-msec ratio score group differences would refiect an encoding deficit. Given variable 50-msec amplitude findings, no predictions were made concerning group differences in the 50-msec S1 and S2 amplitudes (3). Given that inadequate inhibition of redundant sensory information is thought to underlie attention dysfunction in patients with schizophrenia and that some studies have shown relationships between P50 and M50 ratio scores and attention, higher 50-msec and perhaps also higher 100-msec ratio scores and S2 amplitudes would be associated with impaired performance on attention measures (4). As Cz ratio scores are unreliable, P50 and N100 ratio scores would be less (if at all) related to cognitive performance. In contrast, although EEG S1 and S2 amplitudes are determined by multiple sources, their reliability (20, 21) means that these EEG amplitude measures might show associations with cognitive performance.
Method
Subjects
The participants were 79 patients with chronic schizophrenia (17 female) and 73 comparison subjects (20 female) (Table 1). Recruitment procedures and information on inclusion and exclusion have been detailed previously (24, 38); see the online data supplement for additional demographic information. In the patient group, 62 were treated with therapeutic doses of second-generation antipsychotics (clozapine, N=11; olanzapine, N=15; aripiprazole, N=8; risperidone, N=12; quetiapine, N=14; ziprasi-done, N=2), and 17 were receiving therapeutic doses of first-generation antipsychotics (fluphenazine, N=4; haloperidol, N=12; perphenazine, N=1). Fifty-four patients were diagnosed with the paranoid subtype, 23 with the undifferentiated subtype, and two with the disorganized subtype.
| Characteristic | Comparison Subjects (N=73a) | Patients (N=79a) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Ageb | 41.48 | 10.44 | 42.94 | 10.30 |
| Education (years)c | 14.66 | 2.02 | 13.10 | 2.37 |
| Socioeconomic statusd | ||||
| Patientc | 37.97 | 14.20 | 58.12 | 13.16 |
| Parentb | 37.01 | 18.30 | 39.91 | 20.28 |
TABLE 1. Demographic Information on Comparison Subjects and Patients With Schizophrenia
Paired-Click Paradigm
The procedure followed the protocol of Adler et al. (40), in which 3-msec binaural clicks were presented in pairs (S1 and S2) with a 500-msec interstimulus interval and a variable intertrial interval of 7–11 seconds, averaging 9 seconds. The clicks were delivered through earphones placed in each ear canal. The peak intensity of the click was presented 30–40 dB above hearing threshold. If necessary, intensity was decreased to avoid elicitation of a startle refiex. Click pairs were presented until 150 trials were obtained without MEG or EEG artifact. The time to collect 150 paired-click trials did not differ between groups; for the comparison subjects the mean time was 32.43 minutes (SD=6.1), and for the patients it was 33.49 minutes (SD=6.8).
EEG Data Collection
EEG data were collected with Ag/AgCl electrodes. Impedances were below 10 kΩ. EEG was recorded by using two SynAmps amplifiers and SCAN software (Neuroscan, Herndon, Va.) with a bandpass filter (0.03–150 Hz) and a 60-Hz notch filter. The left mastoid and Cz were referenced to the right mastoid during recording and were re-referenced offiine to linked mastoids (41). An electro-oculogram (EOG) (bipolar oblique: upper right and lower left sites) and electrocardiogram (ECG) (at the collarbone) were also obtained.
MEG Data Collection
MEG data were collected by means of a 122-channel biomagnetometer (NeuroMag, Helsinki) (A. Ahonen et al., unpublished article, 1992). EEG and MEG data were collected simultaneously with NeuroMag acquisition software and hardware (Elekta, Stockholm). After a bandpass filter (0.03–150 Hz) and a 60-Hz notch filter, EEG and MEG signals were digitized at 300, 467, or 500 Hz (data acquisition software and hard drive were upgraded during the course of data collection, and the digitization rate was increased). After the MEG session, structural magnetic resonance imaging (MRI) using a 1.5-T Picker Edge imager (Philips Health-care, Andover, Mass.) provided T1-weighted, three-dimensional anatomic images. Details regarding online artifact rejection and other data collection procedures have been provided previously (24).
Analyses of EEG Event-Related Potentials
Cz EEG P50 and N100 data were analyzed with custom MATLAB programs (MathWorks, Natick, Mass.). Individual S1 and S2 P50 and N100 averages were created by visually inspecting the raw data from each trial offiine and discarding trials with ocular or muscle artifacts or excessive alpha-band activity occurring from 300 msec preceding S1 to 300 msec after S2. The filter parameters for P50 were as follows: Fstop=3 Hz and Fpass=5 Hz for the high pass and Fpass=50 Hz and Fstop=60 Hz for the low pass. The filter parameters for N100 were as follows: Fstop=1 Hz and Fpass=2 Hz for the high pass and Fpass=38 Hz and Fstop=42 Hz for the low pass. Each filter was applied twice, once in the forward and once in the reverse direction, to increase roll-off and preserve latencies. Pre-stimulus baseline activity, computed as the mean amplitude from –100 to –10 msec, was removed.
The P50 response to S1 was defined as the most positive peak at electrode Cz occurring between 35 and 75 msec poststimulus. The P50 response to S2 was defined as the most positive peak that occurred within ±10 msec of the S1 peak. P50 S1 and S2 amplitudes were scored as the difference between the peak and the preceding negativity (N40) to ensure that the P30 component was not selected (3). The N100 response to S1 was defined as the most negative trough at Cz occurring between 75 and 130 msec post-stimulus. The N100 response to S2 was defined as the most negative trough that occurred within ±10 msec of the S1 peak. N100 responses to clicks 1 and 2 were scored as the difference between the trough and the preceding positive peak (P50). Ratio scores were calculated for P50 and N100 by dividing the scores for S2 by the scores for S1.
Magnetic Source Analysis
To coregister the MEG and structural MRI data, three anatomical landmarks (nasion and right and left preauriculars) as well as more than 50 additional points on the scalp were digitized for each subject by using the Probe Position Identification System (Polhemus, Colchester, Vt.). The three fiducials were identified in the subject's MRI, and a transformation matrix that involved rotation and translation between the MEG and MRI coordinate systems was used.
A trial was rejected automatically if there was magnetic activity greater than 1,750 fT/cm in any MEG channel or if there was electrical activity between –110 and 110 µV peak to peak in the EOG channel. A –100 to –10-sec baseline adjustment was applied to the averaged MEG data. Prior to source localization, a 4–55 Hz bandpass filter was applied for M50 and a 2–40 Hz bandpass was applied for M100. In a few subjects, the high-pass filter setting was adjusted ±1 Hz to improve source localization.
The strength, location, and peak latency of M50 sources (35–75 msec poststimulus) and M100 sources (75–130 msec poststimulus) in the left and right hemispheres were determined by fitting a dipole separately over the left and right hemispheres and using subsets of 34 planar gradiometers over the temporal lobe. For modeling the S1 M50 and M100, 10 msec of data surrounding the M50 and M100 peaks were selected. Equivalent current dipoles were determined separately for each hemisphere. Only equivalent current dipoles with goodness of fit values (measures of the correlation between calculated and measured signals) exceeding 70% for S1 were accepted. The peak strength of the source, measured in nano-ampere-meters (nAm), over the 10-msec period was then determined. The S2 M50 and M100 measures were identified by using a procedure (42) in which the location of the S2 dipole was assumed to be the same as that of the S1 dipole. To assure that the same component was chosen for both clicks, the S2 latency was required to be within ±10 msec for M50 and M100. In the event that no identifiable peak was available, the S2 amplitude was scored at the same latency as for S1. M50 and M100 ratio scores for each hemisphere and component were expressed as the S2 dipole peak source strength divided by the S1 dipole peak source strength.
Cognitive Measurements
To assess several cognitive domains, both groups were given the Wisconsin Card Sorting Test (43), the WAIS-III digit span back (44), Connors' version of the Continuous Performance Test (45), the Trail Making Test A (46), the Rey Auditory-Verbal Learning Test (47), and the Wechsler Memory Scale—Revised (WMS-R) visual reproduction measure (48). The Shipley Institute of Living Scale (49) was administered to estimate IQ. An attention composite was derived from the time for the Trail Making Test A time (inverse) and the Continuous Performance Test hit rate and d′. A working memory composite was derived from Wisconsin Card Sorting Test perseverative errors (inverse) (27) and digit span back total recall. Finally, a long-delay memory composite was computed from Rey's Auditory-Verbal Learning Test list A delayed recall and WMS-R visual reproduction delayed recall. The composite scores for attention, working memory, and long-delay memory were computed by z-scoring each test, adding the z-scores within each domain, and calculating the z-score mean.
Statistical Analyses
Analyses of variance (ANOVAs) and t tests examined group effects on Cz and MEG amplitude and ratio score measures. To examine how cognitive ability may differ as a function of psychiatric status and ratio score, hierarchical regression was done in which ratio score was entered first, group second, and their interaction last, with each cognitive composite measure (attention, working memory, delayed memory) analyzed separately. For each cognitive measure, regressions were run separately for the two Cz ratio scores (P50 and N100) and four MEG ratio scores (left- and right-hemisphere M50 and M100). In addition, for each cognitive measure, separate regressions were run for S1 and S2 amplitude scores to assess whether relationships were specific to the ratio score or were instead better refiected by S1 or S2. As the primary goal was to identify associations between ratio scores and cognitive performance, ratio score was entered first in the regressions. The results, however, were essentially the same when group was entered first and ratio score second. See the online data supplement for information on outliers and EEG and MEG interrater reliability.
Results
P50 and N100 Ratio Scores and Amplitudes
P50 and N100 ratio and amplitude scores are shown in Table 2 and Figure 1. Cz P50 ratio scores were larger in patients than in comparison subjects (t=–2.17, df=140, p=0.03). No P50 S1 or S2 amplitude main effect or interaction was observed. Cz N100 ratio scores were also larger in the schizophrenia than comparison group (t=–2.63, df=144, p=0.01). The group-by-stimulus (S1 or S2) ANOVA on N100 source strength showed a significant interaction (F=12.08, df=1, 144, p<0.01), with a larger S1 response in the comparison subjects than in the patients (F=12.67, df=1, 144, p<0.01).
| Brain Activity Measure and Group | Log-Transformed Amplitude of Response (μV for EEG, nAm for MEG) | S2/S1 Ratio Scorea | ||||
|---|---|---|---|---|---|---|
| Stimulus 1 (S1) | Stimulus 2 (S2) | |||||
| Mean | SD | Mean | SD | Mean | SD | |
| EEG: Cz site | ||||||
| P50 | ||||||
| Comparison subjects (N=70) | 1.61 | 0.54 | 1.03 | 0.59 | 0.39 | 0.22 |
| Patients (N=72) | 1.47 | 0.55 | 1.04 | 0.49 | 0.48b | 0.28 |
| N100 | ||||||
| Comparison subjects (N=71) | 2.36 | 0.66 | 1.70 | 0.60 | 0.37 | 0.18 |
| Patients (N=75) | 1.92c | 0.84 | 1.56 | 0.60 | 0.45c | 0.19 |
| Magnetoencephalography (MEG): superior temporal gyrus | ||||||
| Left M50 | ||||||
| Comparison subjects (N=67) | 2.74 | 0.47 | 1.99 | 0.72 | 0.54 | 0.24 |
| Patients (N=70) | 2.61 | 0.44 | 2.05 | 0.65 | 0.61b | 0.21 |
| Right M50 | ||||||
| Comparison subjects (N=67) | 2.74 | 0.49 | 2.15 | 0.64 | 0.61 | 0.24 |
| Patients (N=70) | 2.68 | 0.52 | 2.06 | 0.68 | 0.61 | 0.27 |
| Left M100 | ||||||
| Comparison subjects (N=62) | 3.05 | 0.49 | 2.09 | 0.79 | 0.44 | 0.19 |
| Patients (N=61) | 2.79c | 0.58 | 2.03 | 0.88 | 0.56c | 0.27 |
| Right M100 | ||||||
| Comparison subjects (N=62) | 2.98 | 0.46 | 1.97 | 0.98 | 0.44 | 0.22 |
| Patients (N=61) | 2.89 | 0.51 | 2.11 | 0.85 | 0.56c | 0.29 |
TABLE 2. Paired-Click Amplitude and Ratio Scores for Comparison Subjects and Patients With Schizophrenia
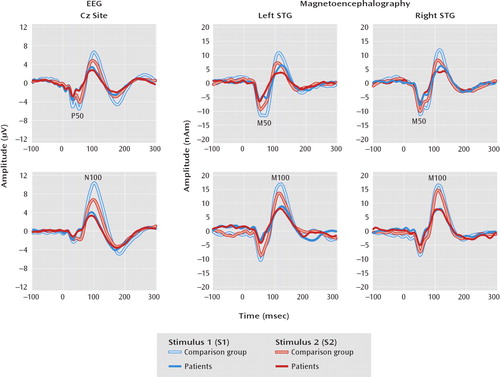
FIGURE 1. Paired-Click Waveforms at Electrode Cz and at Left and Right Superior Temporal Gyrus (STG) in 73 Comparison Subjects and 79 Patients With Schizophreniaa
aNumbers of subjects varied slightly because of outliers (see online data supplement and Results section for more information).
M50 and M100 Ratio Scores and Source Strength
Table 2 displays the M50 and M100 ratio and source strength scores; amplitudes are also shown in Figure 1. No M50 ratio score main effects or interactions were observed. Dissection of the group-by-hemisphere-by-stimulus M50 source strength interaction (F=3.97, df=1, 135, p=0.05) showed a larger difference between the M50 S1 and S2 responses (S1 minus S2) in the comparison subjects than in the patients in the left hemisphere (F=4.57, df=1, 135, p=0.03). A group-by-hemisphere ANOVA on M100 ratio scores showed a group main effect, with a lower ratio score for the comparison subjects than for the patients (F=11.92, df=1, 121, p<0.01). A group-by-hemisphere-by-stimulus ANOVA on M100 source strength showed a larger S1 response in the comparison subjects than the patients (F=4.67, df=1, 121, p=0.03).
Repeated-measures ANOVAs assessed differences in ratio scores between the paranoid and undifferentiated subtypes of schizophrenia. There were no significant differences in S1 amplitude, S2 amplitude, or ratio score (p>0.05 in all cases).
Cognitive Measures
Hierarchical regression analyses examined the relationship between Cz and MEG ratio scores and the three cognitive composites (zero-order correlations between the ratio scores and the individual cognitive tests are reported in the online data supplement). As expected, group (entered second) accounted for significant incremental variance and showed that the patients with schizophrenia performed more poorly than the comparison subjects on all three cognitive composites (p<0.001 in all cases; the percentages of variance accounted for by group differ slightly depending on whether P50, N100, M50, or M100 was the predictor added second, because the number of subjects excluded in the analyses of P50, N100, M50, and M100 differed slightly) even after variance due to ratio score was accounted for (Table 3). As the N100 and M100 S1 amplitude and M50 S1 and S2 values contributed to group differences in ratio scores (Table 2), hierarchical regressions also examined the relationship between S1 and S2 amplitude and the cognitive composites to characterize the ratio score findings (Table 4).
| Gating Predictor and Brain Activity Measure | Ratio Score (S2/S1) | Interaction of Ratio Score and Group | ||
|---|---|---|---|---|
| R2 Change | p | R2 Change | p | |
| Attention | ||||
| EEG: Cz site | ||||
| P50 | 0.09 | <0.01 | 0.01 | 0.31 |
| N100 | 0.04 | 0.03 | 0.02 | 0.13 |
| Magnetoencephalography: superior temporal gyrus | ||||
| Left M50 | 0.07 | <0.01 | 0.02 | 0.10 |
| Right M50 | 0.01 | 0.36 | 0.03 | 0.04 |
| Left M100 | 0.15 | <0.001 | 0.02 | 0.10 |
| Right M100 | 0.11 | <0.01 | 0.05 | <0.01 |
| Working memory | ||||
| EEG: Cz site | ||||
| P50 | 0.10 | <0.01 | 0.00 | 0.53 |
| N100 | 0.04 | 0.04 | 0.00 | 0.56 |
| Magnetoencephalography: superior temporal gyrus | ||||
| Left M50 | 0.07 | <0.01 | 0.01 | 0.36 |
| Right M50 | 0.06 | 0.02 | 0.00 | 0.64 |
| Left M100 | 0.15 | <0.001 | 0.00 | 0.92 |
| Right M100 | 0.05 | 0.04 | 0.03 | 0.09 |
| Long-delay memory | ||||
| EEG: Cz site | ||||
| P50 | 0.03 | 0.10 | 0.00 | 0.93 |
| N100 | 0.01 | 0.32 | 0.01 | 0.24 |
| Magnetoencephalography: superior temporal gyrus | ||||
| Left M50 | 0.01 | 0.30 | 0.00 | 0.79 |
| Right M50 | 0.01 | 0.35 | 0.00 | 0.44 |
| Left M100 | 0.07 | 0.01 | 0.01 | 0.27 |
| Right M100 | 0.06 | 0.03 | 0.00 | 0.69 |
TABLE 3. Hierarchical Regressions With Group and Paired-Click Ratio Scores Predicting Cognitive Scores for 73 Comparison Subjects and 79 Patients With Schizophreniaa, b
| Gating Predictor, Brain Activity Measure, and Stimulus (S1 or S2) | Amplitude | Interaction of Amplitude and Group | ||
|---|---|---|---|---|
| R2 Change | p | R2 Change | p | |
| Attention | ||||
| EEG: Cz site | ||||
| P50 S1 | 0.06 | <0.01 | 0.01 | 0.35 |
| P50 S2 | 0.00 | 0.63 | 0.00 | 0.46 |
| N100 S1 | 0.15 | <0.001 | 0.01 | 0.19 |
| N100 S2 | 0.03 | 0.04 | 0.01 | 0.24 |
| Magnetoencephalography: superior temporal gyrus | ||||
| Left M50 S1 | 0.06 | 0.01 | 0.02 | 0.07 |
| Left M50 S2 | 0.00 | 0.82 | 0.00 | 0.44 |
| Right M50 S1 | 0.02 | 0.13 | 0.02 | 0.06 |
| Right M50 S2 | 0.00 | 0.93 | 0.00 | 0.96 |
| Left M100 S1 | 0.06 | 0.03 | 0.04 | 0.02 |
| Left M100 S2 | 0.01 | 0.50 | 0.01 | 0.32 |
| Right M100 S1 | 0.00 | 0.68 | 0.01 | 0.39 |
| Right M100 S2 | 0.02 | 0.22 | 0.02 | 0.12 |
| Working memory | ||||
| EEG: Cz site | ||||
| P50 S1 | 0.07 | <0.01 | 0.00 | 0.70 |
| P50 S2 | 0.00 | 0.92 | 0.02 | 0.20 |
| N100 S1 | 0.06 | 0.02 | 0.00 | 0.78 |
| N100 S2 | 0.02 | 0.21 | 0.00 | 0.60 |
| Magnetoencephalography: superior temporal gyrus | ||||
| Left M50 S1 | 0.01 | 0.39 | 0.01 | 0.35 |
| Left M50 S2 | 0.01 | 0.44 | 0.00 | 0.65 |
| Right M50 S1 | 0.02 | 0.16 | 0.01 | 0.41 |
| Right M50 S2 | 0.00 | 0.98 | 0.00 | 0.92 |
| Left M100 S1 | 0.01 | 0.41 | 0.01 | 0.47 |
| Left M100 S2 | 0.03 | 0.15 | 0.00 | 0.88 |
| Right M100 S1 | 0.01 | 0.33 | 0.00 | 0.65 |
| Long-delay memory | ||||
| EEG: Cz site | ||||
| P50 S1 | 0.01 | 0.29 | 0.00 | 0.91 |
| P50 S2 | 0.00 | 0.84 | 0.00 | 0.84 |
| N100 S1 | 0.04 | 0.04 | 0.00 | 0.87 |
| N100 S2 | 0.02 | 0.16 | 0.00 | 0.53 |
| Magnetoencephalography: superior temporal gyrus | ||||
| Left M50 S1 | 0.00 | 0.63 | 0.00 | 0.62 |
| Left M50 S2 | 0.00 | 0.91 | 0.00 | 0.55 |
| Right M50 S1 | 0.00 | 0.86 | 0.02 | 0.08 |
| Right M50 S2 | 0.00 | 0.93 | 0.02 | 0.17 |
| Left M100 S1 | 0.00 | 0.55 | 0.00 | 0.65 |
| Left M100 S2 | 0.01 | 0.38 | 0.00 | 0.95 |
| Right M100 S1 | 0.01 | 0.46 | 0.00 | 0.88 |
| Right M100 S2 | 0.01 | 0.28 | 0.00 | 0.86 |
TABLE 4. Hierarchical Regressions With Group and Paired-Click Amplitude Scores Predicting Cognitive Scores for 73 Comparison Subjects and 79 Patients With Schizophreniaa, b
Attention.
Hierarchical regression results for the cognitive composites are shown in Table 3. Smaller Cz P50 ratio scores predicted better performance on the attention composite measure (F=10.03, df=1, 107, p<0.01). Examination of the P50 S1 and S2 amplitudes revealed that larger P50 S1 amplitude predicted better performance on the attention tests (F=7.05, df=1, 111, p<0.01) (Table 4). Smaller Cz N100 ratio scores also predicted better performance on attention tests (F=4.61, df=1, 111, p=0.03). A combination of S1 and S2 amplitudes seemed to contribute to the N100 ratio score finding, as larger N100 S1 amplitude (F=19.78, df=1, 113, p<0.001) and smaller S2 amplitude (F=4.14, df=1, 117, p=0.04) predicted better performance on the attention tests. Regressions run by entering S1 first and S2 second or vice versa indicated that only S1 accounted for significant unique variance in attention.
Similar to the Cz P50 ratio scores, smaller left M50 ratio scores predicted better performance on the attention composite (F=8.53, df=1, 107, p<0.001). Dissection of a marginal interaction between group and left M50 S1 (F=5.71, df=1, 84, p=0.02) source strength (F=3.34, df=1, 107, p=0.07) indicated that left M50 S1 source strength correlated with better performance on the attention composite only in the patients (r=0.29, df=55, p=0.03). The interaction between group and right M50 ratio score (F=4.17, df=1, 102, p=0.04) indicated that higher right M50 ratio scores were associated with worse performance on the attention tests only for the patients (r=–0.27, df=53, p=0.04).
Similar to the Cz N100 ratio scores, smaller left M100 ratio scores predicted better performance on the attention composite (F=14.81, df=1, 86, p<0.001). An interaction between group and source strength for the left M100 S1 (F=5.71, df=1, 84, p=0.02) indicated a relationship between S1 source strength and better attention performance only in the patients (r=0.31, df=43, p=0.04). An interaction between group and right M100 ratio score (F=7.33, df=1, 84, p<0.01) indicated a relationship between smaller right M100 ratio scores and better performance on attention tests only for the patients (r=–0.39, df=43, p<0.01). Right M100 S1 and S2 amplitude did not predict variance in attention performance. Figure 2 shows scatter plots representing the relationship between S1 amplitude and the attention composite for the 50-msec and 100-msec Cz S1 responses and for the 50-msec and 100-msec left STG responses (for working memory and long-delay memory, scatter plots are included in the online data supplement).
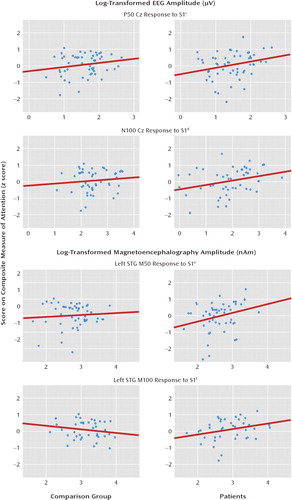
FIGURE 2. Relation of Attention to First-Click Amplitude (S1) at Electrode Cz and at Left and Right Superior Temporal Gyrus (STG) in 73 Comparison Subjects and 79 Patients With Schizophreniaa, b
aAttention performance was predicted by the patient's ability to encode auditory information, as represented by associations with S1.
bNumbers of subjects and degrees of freedom varied because of missing data and outliers (see online data supplement and Results section for more information).
cThe variance (R2) for the comparison subjects and patients was 0.04 and 0.07, respectively.
dThe variance (R2) for the comparison subjects and patients was 0.01 and 0.10, respectively.
eThe variance (R2) for the comparison subjects and patients was 0.01 and 0.08, respectively.
fThe variance (R2) for the comparison subjects and patients was 0.04 and 0.10, respectively.
Working memory.
As shown in Table 3, smaller P50 ratio scores predicted better working memory performance (F=10.25, df=1, 95, p<0.01), with greater P50 S1 amplitude accounting for this relationship (F=7.05, df=1, 95, p<0.01) (Table 4). Smaller N100 ratio scores also predicted better working memory performance (F=4.22, df=1, 102, p=0.04), with increased N100 S1 amplitude accounting for this relationship (F=5.93, df=1, 100, p=0.02).
Left and right M50 ratio scores accounted for variance in scores on the working memory composite (left: F=7.56, df=1, 94, p<0.01; right: F=5.39, df=1, 93, p=0.02). Left and right M100 ratio scores also accounted for significant variance (left: F=15.51, df=1, 86, p<0.001; right: F=4.58, df=1, 86, p=0.04). In both groups, lower left and right M50 and M100 ratio scores were associated with better working memory performance. None of the MEG amplitude measures were associated with working memory.
Verbal and visual long-delay memory.
P50 and N100 ratio scores did not predict variance in the long-delay memory composite (Table 3). Of the amplitude measures, only larger N100 S1 amplitude predicted better long-delay memory composite score (F=4.30, df=1, 99, p=0.04).
Left and right M50 ratio scores also did not predict delayed memory performance. Smaller left and right M100 ratio scores, however, were associated with better performance on the delayed memory tests (left: F=4.41, df=1, 83, p=0.01; right: F=0.06, df=1, 84, p=0.03). None of the S1 or S2 source strength values predicted additional variance in long-delay memory.
IQ and hierarchical regressions.
To assess the degree to which performance in cognitive domains predicted ratio scores after variance associated with general cognitive ability was removed, hierarchical multiple regressions were conducted with IQ entered first. In separate tests, in the second block the 1) composite measure of attention, 2) composite measure of working memory, or 3) composite measure of delayed memory was entered. Group was entered third. The group-by-composite interaction term was entered fourth.
Regression results for the second block (cognitive composite measure) and fourth block (group-by-composite interaction) are reported in Table 5. With IQ variance removed, the attention composite predicted additional variance in P50 ratio scores (F=5.44, df=1, 106, p=0.02). The attention composite score did not predict additional variance in N100 or in left or right M50 ratio scores. The attention composite, however, predicted additional variance in left (F=5.26, df=1, 85, p=0.02) and right (F=9.46, df=1, 85, p<0.01) M100 ratio scores. The group-by-attention composite interaction added 5% of the variance in the right M100 ratio score (F=5.34, df=1, 83, p=0.02), with simple-effects analysis showing that attention continued to predict significant variance in the ratio score only in the patient group (R2=0.17, p<0.01).
| Gating Predictor and Brain Activity Measure | Cognitive Composite Score | Interaction of Cognitive Score and Group | ||
|---|---|---|---|---|
| R2 Change | p | R2 Change | p | |
| Attention | ||||
| EEG: Cz site | ||||
| P50 | 0.05 | 0.02 | 0.01 | 0.34 |
| N100 | 0.01 | 0.32 | 0.01 | 0.25 |
| Magnetoencephalography: superior temporal gyrus | ||||
| Left M50 | 0.03 | 0.07 | 0.00 | 0.97 |
| Right M50 | 0.02 | 0.21 | 0.03 | 0.10 |
| Left M100 | 0.05 | 0.02 | 0.02 | 0.19 |
| Right M100 | 0.10 | <0.01 | 0.05 | 0.02 |
| Working memory | ||||
| EEG: Cz site | ||||
| P50 | 0.02 | 0.15 | 0.00 | 0.82 |
| N100 | 0.02 | 0.15 | 0.00 | 0.93 |
| Magnetoencephalography: superior temporal gyrus | ||||
| Left M50 | 0.03 | 0.10 | 0.00 | 0.75 |
| Right M50 | 0.04 | 0.05 | 0.00 | 0.88 |
| Left M100 | 0.04 | 0.04 | 0.00 | 0.73 |
| Right M100 | 0.02 | 0.21 | 0.03 | 0.10 |
| Long-delay memory | ||||
| EEG: Cz site | ||||
| P50 | 0.00 | 0.63 | 0.00 | 0.88 |
| N100 | 0.00 | 0.90 | 0.01 | 0.25 |
| Magnetoencephalography: superior temporal gyrus | ||||
| Left M50 | 0.00 | 0.99 | 0.00 | 0.54 |
| Right M50 | 0.00 | 0.66 | 0.03 | 0.08 |
| Left M100 | 0.01 | 0.39 | 0.01 | 0.52 |
| Right M100 | 0.02 | 0.20 | 0.00 | 0.85 |
TABLE 5. Hierarchical Regressions With IQ, Cognitive Scores, and Group Predicting Paired-Click Ratio Scores for 73 Comparison Subjects and 79 Patients With Schizophreniaa, b
With IQ variance removed, working memory did not predict variance in P50 or N100 ratio score. The working memory composite predicted additional variance in the right M50 ratio score (F=3.94, df=1, 92, p=0.05) and the left M100 ratio score (F=4.39, df=1, 85, p=0.04). The working memory composite did not predict variance in left M50 or right M100 ratio score. With IQ variance removed, the delayed memory composite did not predict variance in the P50, N100, M50, or M100 ratio score.
Discussion
As hypothesized, patients with schizophrenia had larger P50 Cz ratio scores. Although the group main effect for M50 ratio score was not significant, group variation in the difference between S1 and S2 amplitudes (S1 minus S2) for left but not right M50 was observed, supporting a left M50 paired-click deficit (hypothesis 1). Whereas the 50-msec S1 and S2 values reported in Table 2 as well as the Figure 1 source waveforms suggest a left M50 S1 effect, analyses indicated that group differences in the ratio scores were not explained solely by either a pure encoding deficit (driven by S1) or a pure gating deficit (driven by S2). Therefore, the present findings do not resolve the 50-msec ratio score debate. The patients also showed larger N100 Cz ratio scores. For M100, group differences in the ratio score were observed bilaterally. As hypothesized, group differences in the 100-msec ratio score were due to a smaller S1 response in patients (hypothesis 2). Thus, ratio score group differences at 100 msec were explained by an encoding deficit.
What is the functional significance of the paired-click findings? As 50-msec activity is thought to refiect sensory encoding, a relationship of the 50-msec and perhaps also 100-msec paired-click activity to attention was hypothesized, such that the less that redundant sensory information was inhibited (i.e., larger S2), the greater the attentional impairment (hypothesis 3). In the patients and comparison subjects, higher Cz P50 and N100 ratio scores were indeed associated with poorer performance on attention tests. For M50 and M100 STG measures, paired-click ratio scores were also associated with the attention composite, primarily in the patients. The second part of hypothesis 3 was not supported, as worse performance on attention tests was associated with a smaller S1 response rather than a greater S2 response (with the M50 relationship again observed only in patients). Thus, attention performance was predicted by the patients' ability to encode auditory information rather than their ability to filter redundant information. The lack of an association between STG S1 amplitude and attention in the comparison subjects may refiect the fact that most of the comparison subjects had normal STG activity. In any case, after the variance in ratio scores associated with general cognitive ability was removed, many of the associations of the 50- and 100-msec ratio scores with attention remained, suggesting that the association with attention was somewhat unique.
Ratio scores and amplitudes were also associated with working memory and verbal and visual long-delay memory. Only working memory added significant variance beyond that accounted for by general cognitive ability (IQ). Thus, the findings also reveal a nonspecific association of 50- and 100-msec electrophysiological measures (primarily S1) with general cognitive ability. It is interesting that associations were observed only with the ratio score or S1, again generally suggesting an association between encoding ability and cognitive performance.
Finally, hypothesis 4 received moderate support. First, to the extent that the Cz paired-click measure refiects brain activity only from primary and secondary auditory areas (especially true of 50-msec activity; see reference 23), present results indicate that the STG findings provide more information about paired-click group differences. For example, although a group difference in the P50 ratio score was observed, analyses of STG sources suggested that this was due to left and not right STG abnormality in the patients. In contrast, as group differences in the M100 STG ratio score were observed in both hemispheres, the Cz N100 findings more directly mirrored the STG findings. Second, whereas associations of the P50 and N100 Cz ratio scores and amplitudes with cognitive abilities were observed, the STG sources again provided more information. For example, although P50 and N100 S1 amplitudes were associated with attention (even after we removed IQ variance), STG analyses suggested S1 associations only in the left hemisphere and only in patients. To the extent that a detailed understanding of the specific brain areas associated with clinical measures is important, the present findings suggest the need to examine source rather than scalp activity.
Although in the present study MEG provided more specific information, the MEG and EEG findings were often similar. Thus, it is somewhat puzzling that so few studies have shown associations between paired-click activity and cognitive ability. There may be insufficient power in most EEG studies. The most extreme Cz example involves N100: although N100 was consistently associated with cognitive measures here, N100 ratio scores explained, at most, 5% of the variance in the cognitive scores. For a correlation of 0.22 (approximately 5% of the variance), a minimum of about 150 subjects would be needed to obtain a significant correlation (alpha=0.05, power=0.80), in line with the present subjects (comparison subjects plus patients) but far larger than groups in previous studies (see reference 3). The present findings suggest a larger effect size and thus a greater chance of observing associations when source activity is examined. For example, R2 values of 15% for left M100 were consistently observed for attention and working memory measures, for which a group of 50 subjects would suffice (alpha=0.05, power=0.80). It should be noted that the present M50 results in the comparison subjects did not replicate our earlier finding (26) in a much smaller but overlapping group of comparison subjects where worse M50 gating was associated with better working memory performance. The number of comparison subjects has increased fourfold since that study, and the present findings underscore the need to recruit relatively large subject groups to test relationships between paired-click measures and cognitive performance.
Although in the present study associations between auditory brain processes and cognitive ability were observed, the study design precludes causal claims. In addition, it is possible that a third, unstudied measure could account for electrophysiological and cognitive abnormalities. In particular, there is evidence to suggest that abnormal STG paired-click activity and impairment on cognitive tests in patients with schizophrenia may both be related to abnormal STG anatomy. In particular, low STG gray matter volume has been observed in many studies (for instance, 50–54). As 50- and 100-msec paired-click activity directly refiects gray matter activity, an examination of the relationship of 50- and 100-msec paired-click activity to STG structural measures is of interest. Available evidence also provides support for a relationship between STG gray matter structural abnormalities and functional impairment as assessed by psychophysiological measures as well as clinical measures. For example, lower than normal left temporal auditory P300 amplitude has been associated with smaller left posterior STG gray matter volume in chronic (55) and first-episode (56) schizophrenia. In addition, low left posterior STG gray matter volume has been associated with severity of thought disorder (57), and in an MRI study the severity of thought disorder was negatively correlated with activation changes in left Brodmann's area 22 (58). Thus, gray matter abnormalities may contribute to abnormal electrophysiology measures as well as to patient symptoms and cognitive abilities. In the present study, the observed left hemisphere M50 group differences may be due to structural STG hemisphere differences. Finally, it is worth noting that in the present study only S1 was associated with cognitive ability. To the extent that S1 activity primarily refiects local processes, whereas S2 may refiect local activity as well as inhibitory activity from the reticular formation and the thalamus (59–61), hippocampus (62, 63), or frontal cortex (35), S1 and S2 processes would be expected to be differentially associated with cognitive measures.
As detailed in the introduction, a few other studies have demonstrated associations between Cz P50 and attention (4, 5). In the present study a clear association between 50-and 100-msec ratio scores and performance on attention tests was observed, although primarily accounted for by S1 amplitude. To our knowledge, few studies have examined relationships between Cz N100 paired-click activity and cognitive measures (and we found no studies of associations with attention). Examining comparison subjects and patients with schizophrenia, Boutros et al. (64) reported a relationship between N100 ratio scores and prefrontal cortex function (performance on the Wisconsin Card Sorting Test). In the present study, although no significant association was observed between performance on the Wisconsin Card Sorting Test and N100 ratio scores, an association between test performance and left and right STG M100 ratio scores was observed in the patients (see online data supplement). Another study examining only healthy subjects showed associations of N100 S1 amplitude and ratio score with a measure of working memory (6), a relationship related more directly to stimulus processing properties (S1) than to N100 sensory gating. The present results generally replicated this finding, demonstrating an association between Cz N100 S1 amplitude and working memory performance.
In sum, the present findings indicate that the paired-click abnormalities predict cognitive deficits. In many instances, the cognitive impairments were more closely associated with encoding processes. The use of MEG source localization alongside scalp EEG provides larger effect sizes and more inferential specificity, which may explain why EEG-only studies have been inconclusive regarding an association between paired-click activity and clinical measures.
1. : Neurophysiological evidence for a deficit in inhibitor mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry 1982; 17:639–654Medline, Google Scholar
2. : P50 sensory gating ratios in schizophrenics and controls: a review and data analysis. Psychiatry Res 2008; 158:226–247Crossref, Medline, Google Scholar
3. : Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophr Bull 2006; 32:692–700Crossref, Medline, Google Scholar
4. : P50 abnormalities in schizophrenia: relationship to clinical and neuropsycho-logical indices of attention. Schizophr Res 1998; 33:157–167Crossref, Medline, Google Scholar
5. : Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophr Res 1993; 10:131–141Crossref, Medline, Google Scholar
6. : P50, N100, and P200 sensory gating: relationships with behavioral inhibition, attention, and working memory. Psychophysiology 2009; 46:1–10Crossref, Medline, Google Scholar
7. : Sensory gating deviance in schizophrenia in the context of task related effects. Int J Psychophysiology 1994; 18:1–12Crossref, Medline, Google Scholar
8. : Memory impairment and auditory evoked potential gating deficit in schizophrenia. Psychiatry Res 2004; 130:161–169Crossref, Medline, Google Scholar
9. : Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Exp Brain Res 2001; 139:377–390Crossref, Medline, Google Scholar
10. : Effects of P50 temporal variability on sensory gating in schizophrenia. Psychiatry Res 1997; 70:71–81Crossref, Medline, Google Scholar
11. : Contributions of subtype and spectral frequency analyses to the study of P50 ERP amplitude and suppression in schizophrenia. Schizophr Res 2005; 78:269–284Crossref, Medline, Google Scholar
12. : A fuzzy clustering approach to study the auditory P50 component in schizophrenia. Psychiatry Res 1997; 69:169–181Crossref, Medline, Google Scholar
13. : Sensory gating deficits during the mid-latency phase of information processing in medicated schizophrenia patients. Psychiatry Res 2004; 126:203–215Crossref, Medline, Google Scholar
14. : Ear of stimulation determines schizophrenia-normal brain activity differences in an auditory paired-stimuli paradigm. Eur J Neurosci 2003; 18:2853–2858Crossref, Medline, Google Scholar
15. : Contribution of different EEG frequencies to auditory evoked potential abnormalities in schizophrenia. Clin Neurophysiology 2004; 115:523–533Crossref, Medline, Google Scholar
16. : A meta-analysis of P50 studies in patients with schizophrenia and relatives: differences in methodology between research groups. Schizophr Res 2007; 97:137–151Crossref, Medline, Google Scholar
17. : Event-related potential abnormalities in schizophrenia: a failure to “gate in” salient information? Schizophr Res 2009; 113:332–338Crossref, Medline, Google Scholar
18. : Interpreting abnormality: an EEG and MEG study of P50 and the auditory paired-stimulus paradigm. Biol Psychol 2003; 65:1–20Crossref, Medline, Google Scholar
19. : Test-retest reliability of the P50 mid-latency auditory evoked response. Psychiatry Res 1991; 39:181–192Crossref, Medline, Google Scholar
20. : Reliability of P50 event-related potential indices of sensory gating. Psychophysiology 1994; 31:495–502Crossref, Medline, Google Scholar
21. : Improved test-retest reliability of 50-ms paired-click auditory gating using magnetoencephalography source modeling. Psychophysiology 2007; 44:86–90Crossref, Medline, Google Scholar
22. : Heritability and reliability of P300, P50 and duration mismatch negativity. Behav Genet 2006; 36:845–857Crossref, Medline, Google Scholar
23. : Predicting EEG responses using MEG sources in superior temporal gyrus reveals source asynchrony in patients with schizophrenia. Clin Neurophysiol 2003; 114:835–850Crossref, Medline, Google Scholar
24. : Distinct M50 and M100 auditory gating deficits in schizophrenia. Psychophysiology 2005; 42:417–427Crossref, Medline, Google Scholar
25. : M50 sensory gating predicts negative symptoms in schizophrenia. Schizophr Res 2005; 73:311–318Crossref, Medline, Google Scholar
26. : Lateralization of auditory sensory gating and neuropsychological dysfunction in schizophrenia. Am J Psychiatry 2003; 160:1595–1605Link, Google Scholar
27. : Both random and perseverative errors underlie WCST deficits in prefrontal patients. Neuropsychologia 2002; 40:349–356Crossref, Medline, Google Scholar
28. : Information processing and attention dysfunctions in schizophrenia. Schizophr Bull 1993; 19:233–259Crossref, Medline, Google Scholar
29. : Auditory attention affects two different areas in the human supratemporal cortex. Electroencephalogr Clin Neurophysiol 1991; 79:464–472Crossref, Medline, Google Scholar
30. : Modulation of early sensory processing in human auditory cortex during auditory selective attention. Proc Natl Acad Sci USA 1993; 90:8722–8726Crossref, Medline, Google Scholar
31. : P50 sensitivity to physical and psychological state influences. Psychophysiology 2006; 43:320–328Crossref, Medline, Google Scholar
32. : Attentional modulation of the P50 suppression deficit in recent-onset and chronic schizophrenia. J Abnormal Psychol 2010; 119:31–39Crossref, Medline, Google Scholar
33. : Comparison of four components of sensory gating in schizophrenia and normal subjects: a preliminary report. Psychiatry Res 1999; 88:119–130Crossref, Medline, Google Scholar
34. : Neuronal substrates of sensory gating within the human brain. Biol Psychiatry 2003; 53:511–519Crossref, Medline, Google Scholar
35. : Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychol (Amst) 1999; 101:159–178Crossref, Medline, Google Scholar
36. : Effects of attentional and stressor manipulations on the P50 gating response. Psychophysiology 1997; 34:703–711Crossref, Medline, Google Scholar
37. : Electrical signs of selective attention in the human brain. Science 1973; 182:177–180Crossref, Medline, Google Scholar
38. : Superior temporal gyrus spectral abnormalities in schizophrenia. Psycho-physiology 2008; 45:812–824Crossref, Medline, Google Scholar
39. : Abnormal auditory N100 amplitude: a heritable endophenotype in first-degree relatives of schizophrenia probands. Biol Psychiatry 2008; 64:1051–1059Crossref, Medline, Google Scholar
40. : Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry 1993; 150:1856–1861Link, Google Scholar
41. : The linked reference issue in EEG and ERP recording. Psychophysiology 1991; 5:273–276Google Scholar
42. : The reliability of P50 suppression as measured by the conditioning/testing ratio is vastly improved by dipole modeling. Biol Psychiatry 1993; 33:335–344Crossref, Medline, Google Scholar
43. : Wisconsin Card Sorting Test—64-Card Version, Research Edition Version 3.1.0.8. Lutz, Fla, Psychological Assessment Resources, 2000Google Scholar
44. : Manual for the Wechsler Adult Intelligence Scale, 3rd ed (WAIS-III). San Antonio, Tex, Psychological Corp, 1997Google Scholar
45. : Connors' Continuous Performance Test Computer Program 3.0: User Manual. Toronto, Multi-Health Systems, 1994Google Scholar
46. : The Halstead-Reitan Neuropsychological Test Battery. Tucson, Ariz, Neuropsychology Press, 1971Google Scholar
47. : L'Examen Clinique Psychologie (The Clinical Exam in Psychology). Paris, Presses Universitaires de France, 1964Google Scholar
48. : Wechsler Memory Scale—Revised Manual. San Antonio, Tex, Psychological Corp, 1987Google Scholar
49. : Shipley Institute of Living Scale: Revised Manual. Los Angeles, Western Psychological Services, 1967Google Scholar
50. : Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am J Psychiatry 2004; 161:1603–1611Link, Google Scholar
51. : A review of MRI findings in schizophrenia. Schizophrenia Res 2001; 49:1–52Crossref, Medline, Google Scholar
52. : MRI anatomy of schizophrenia. Biol Psychiatry 1999; 45:1099–1119Crossref, Medline, Google Scholar
53. : Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry 1998; 155:1384–1391Link, Google Scholar
54. : Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry 2003; 60:766–775Crossref, Medline, Google Scholar
55. : Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Arch Gen Psychiatry 1993; 50:190–197Crossref, Medline, Google Scholar
56. : Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Arch Gen Psychiatry 2002; 59:321–331Crossref, Medline, Google Scholar
57. : Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604–612Crossref, Medline, Google Scholar
58. : Neural correlates of formal thought disorder in schizophrenia. Arch Gen Psychiatry 2001; 58:769–774Crossref, Medline, Google Scholar
59. : Availability of peripheral input to the midbrain reticular formation. Exp Neurol 1961; 4:358–376Crossref, Medline, Google Scholar
60. : Facilitation of tachistoscopic performance by stimulation of midbrain tegmental points in the monkey. Exp Neurol 1962; 6:384–406Crossref, Medline, Google Scholar
61. : Reticular modification of somatosensory cortical recovery function. Electroencephalogr Clin Neurophysiol 1963; 15:265–271Crossref, Medline, Google Scholar
62. : Auditory sensory gating in the rat hippocampus: modulation by brainstem activity. Brain Res 1993; 607:33–38Crossref, Medline, Google Scholar
63. : Inhibitory gating of an evoked response to repeated auditory stimuli in schizophrenic and normal subjects: human recordings, computer simulation, and an animal model. Arch Gen Psychiatry 1996; 53:1114–1121Crossref, Medline, Google Scholar
64. : Sensory-gating deficit of the N100 mid-latency auditory evoked potential in medicated schizophrenia patients. Schizophr Res 2009; 113:339–346Crossref, Medline, Google Scholar



