Placebo Response in Randomized Controlled Trials of Antidepressants for Pediatric Major Depressive Disorder
Abstract
Objective: The authors examined characteristics and predictors of response to placebo in all available reports of short-term randomized controlled trials of antidepressants for pediatric major depressive disorder. Method: Response, defined as a score ≤2 on the improvement item of the Clinical Global Impression scale, and potential predictors were extracted from 12 published and unpublished randomized controlled trials of second-generation antidepressants in participants 6–18 years of age with major depression. Results: The single best predictor of the proportion of patients taking placebo who responded to treatment was the number of study sites. Baseline severity of illness also emerged as a significant inverse predictor of placebo response, although the strength of this relationship was diminished when number of sites was controlled for. After one large fluoxetine trial was excluded, younger participants showed a higher placebo response rate than older adolescents. Higher placebo response rates in more recent studies were explained by an increasing trend toward large multisite trials and by publication delays and failures to publish some negative trials. Conclusions: The recent shift toward large multisite trials of antidepressant medications for pediatric major depression may be contributing to an increasing incidence of response to placebo. Pharmacotherapy studies of pediatric depression that carefully recruit patients with at least moderately severe depression may be more informative and efficient than many trials conducted to date. Such studies should have sufficient power to determine whether age moderates medication and placebo response.
Major depressive disorder in children and adolescents is a common, impairing, frequently comorbid condition; it is associated with problems in family and social functioning, difficulties in school performance, and an increased risk of recurrence, substance abuse, and suicidality (1) . Effective treatment may reduce the impact of depression on psychosocial functioning and may lessen the risk of other adverse psychiatric sequelae. We previously reported the results of a meta-analysis of 27 randomized controlled trials of pediatric major depressive disorder, obsessive-compulsive disorder (OCD), and non-OCD anxiety disorders, presenting evidence that the benefits of antidepressants appear to be much greater than risks of suicidal ideation and suicide attempt across indications (2) . The efficacy of antidepressant treatment was greatest for non-OCD anxiety disorders, intermediate for OCD, and more modest for major depressive disorder, with numbers needed to treat of 3, 6, and 10, respectively. However, while the response to antidepressants was similar across diagnostic groups (range=46% to 63%), the main difference was that the response to placebo was higher for youths with major depressive disorder (50%) when compared with the placebo response for youths with OCD and non-OCD anxiety disorders (32% and 39%, respectively). Furthermore, in major depression trials, the placebo response rate appeared to be higher in studies with greater numbers of sites and in children under age 12 compared with adolescents.
Identification of the characteristics of youths most likely to respond to placebo could be useful for both research and clinical care. In research, youths who are highly likely to respond to placebo could be screened out, which might decrease the necessary sample size and increase the power of treatment trials to detect differences between active treatment and placebo. In clinical care, there are acute shortages of therapists trained in one of the indicated psychotherapies and of physicians skilled in the use of antidepressants (3 , 4) . Identification of those children least likely to respond to placebo could help to prioritize referrals. Finally, while the reported risks of selective serotonin reuptake inhibitors (SSRIs) appear to be outweighed by the benefits, this would clearly not be the case in someone who is just as likely to respond to placebo. Therefore, identification of youths who are likely to respond to placebo would substantially increase the benefit-to-risk ratio for the use of antidepressants in pediatric depression. Moreover, depressed youths who are likely to respond to placebo could be offered a brief psychosocial intervention rather than medication or other more intense types of psychotherapies (1 , 5 , 6) .
While the response to placebo in depressed youths has not been well studied (5) , predictors of higher placebo response rates in depressed adults have been identified, including younger age, male sex, longer duration of the antidepressant trial, shorter duration of illness, lower severity of depression, fewer prior episodes, and study location (with higher placebo response in major depression trials conducted in Europe compared with the United States) (7 – 12) .
In this study, we used summary data from all available published and unpublished trial reports to examine patient and methodological factors that may predict placebo response in pediatric depression. Secondary aims included quantifying the magnitude of response to active medication that can be explained by placebo response and the impact of placebo response on the drug-placebo difference in efficacy (13) , and determining whether the proportion of pediatric patients with major depression responding to placebo has increased in recent years, as has been reported for adults in a study of outpatients with major depression (14) .
Method
Search Strategy and Study Selection
The search strategy has been described in detail elsewhere (2) . Briefly, we conducted a PubMed search, covering January 1988 through July 2006, using the MeSH keywords “SSRI,” “serotonin reuptake inhibitors,” “antidepressive agents: second-generation,” “child,” and “adolescent”; specific names of antidepressant medications; and “randomized controlled trial.” Relevant studies were also identified through references of originally identified articles, regulatory reports (15 – 18) , proceedings of three scientific meetings, clinical trial registries, and contact with individual investigators.
For the purposes of this study, we included only randomized controlled trials in youths (6–18 years of age) with major depressive disorder that studied SSRIs as well as other novel antidepressants because the response to placebo in major depression trials was higher than for other indications and appeared to be influenced by patient and trial characteristics (2) . Trials were included only if response data were available for both participants treated with placebo and those treated with antidepressants, as measured by the improvement item on the Clinical Global Impression (CGI) scale (19) . This requirement differed from our previous study (2) , in which the efficacy outcome was the drug-placebo difference in the primary study-defined measure of treatment response, but was necessary to reduce the likelihood of spurious associations that might occur if the primary response measure varied across studies. For example, studies using both the CGI improvement item and a score ≤28 (indicating complete symptom remission) on the Children’s Depression Rating Scale–Revised ( 20 ; CDRS-R) report lower placebo and medication response rates on the CDRS-R measure than on the CGI improvement measure (21 , 22) .
Twelve of 15 eligible trials fulfilled the inclusion criteria (21 – 32) . Of the three excluded trials, two failed to report any response data (33 , 34) , and one did not measure response using the CGI improvement item (35) .
Response Criteria
Treatment responders were defined by an end-of-treatment rating of ≤2 (“much improved” or “very much improved”) on the CGI improvement item (19) , indicating a clinically significant reduction in depressive symptoms to the point that the patient no longer met criteria for psychiatric disorder.
Predictors of Response
Potential predictors of placebo response included age (categorized as children [under age 12 or 13, depending on study definition] versus adolescents [age 12 or 13 and older]), sex (proportion of participants who were female), race (proportion of participants who were white/Caucasian), number of patients who underwent randomized assignment, number of study sites, average number of participants per study site, number of treatment weeks, location of study (U.S. only versus non-U.S. only), use of a placebo run-in period, duration of depressive episode, episode history (proportion of participants in a first depressive episode versus those who had more than one episode), and severity of illness as measured by the CDRS-R and the CGI severity item.
Statistical Analyses
Descriptive statistics were used to characterize the features of each of the included trials. Pearson correlation coefficients (r) and linear regression were used to assess the associations between continuous variables. Spearman rank correlations (r s ) were computed to assess associations between continuous and dichotomous variables (use of a placebo run-in period [yes/no], study location [U.S. versus non-U.S.]), and number of treatment weeks because of small variation across trials. All statistical tests were two-tailed, and p values ≤0.05 were considered statistically significant. The number of trials allowed us to detect only associations of large effect size between putative predictors and response; therefore, these analyses were strictly exploratory in nature. No correction was made for multiple comparisons. For sensitivity analysis, we iteratively deleted each trial and recomputed r and r s to confirm that no single trial unduly influenced the overall results. The impact of placebo response on the antidepressant-placebo difference in efficacy was tested using two measures of efficacy—risk differences (indicating the difference in the proportion of CGI improvement item responders) and Hedges’ g (indicating the scalar change in symptoms from baseline to end of treatment) (2) . Statistical analyses were conducted with SPSS, version 14.0 (SPSS, Inc., Chicago), and StatXact, version 7 (Cytel Software, Cambridge, Mass.).
Results
In the 12 studies we analyzed, 2,862 patients underwent randomized assignment to active medication or placebo ( Table 1 ). The median age was 12.3 years (range=12.0–15.6 years), and the median proportion of female participants was 0.53 (range=0.46–0.66). The mean proportion of participants per study responding to placebo was 0.46 (SD=0.08, range=0.33–0.57), and for active drugs, 0.59 (SD=0.07, range=0.47–0.69).
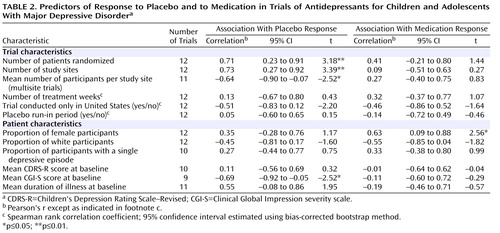
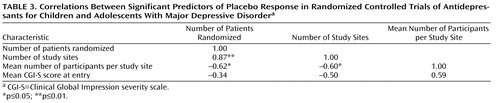
As shown in Table 2 , there were significant correlations between proportion of placebo responders and each of the three site selection variables—number of patients who underwent randomized assignment, number of study sites, and average number of participants per study site. Figure 1 plots the proportion of placebo responders by number of study sites. Severity of illness at entry, as assessed by the CGI severity item, showed an inverse relationship with placebo response, indicating a higher placebo response with decreasing severity ( Figure 2 ). Sensitivity analyses confirmed that these significant associations were generally insensitive to the exclusion of any single trial (number of patients randomized, r range: 0.59 to 0.76; number of study sites, r range: 0.64 to 0.79; average number of participants per site, r range: –0.48 to –0.75; CGI severity score, r range: –0.54 to –0.80). No correlations were evident between proportion of placebo responders and duration of the treatment period, duration of illness, study location, placebo run-in period, CDRS-R score, or proportion of female participants, white participants, or participants who had first-episode major depressive disorder. The predictors for response to placebo and response to active medication were distinct. Among the above-noted variables, only the proportion of participants who were female was positively correlated with antidepressant response.
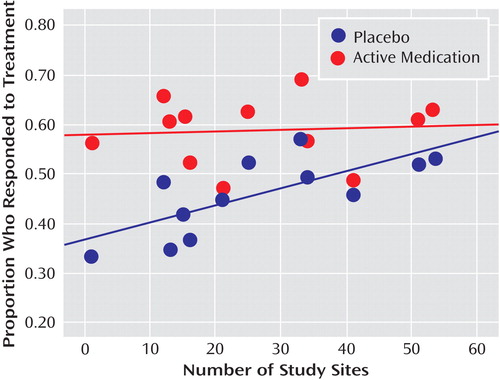
a Treatment response was defined as a score ≤2 (much improved or very much improved) on the improvement item of the Clinical Global Impression scale at end of treatment. The lines in the figure are the best-fitting straight lines indicating the relationship between proportion of participants responding and number of study sites for antidepressants (r=0.09, p=0.81) and placebo (r=0.73, p=0.007). This correlation remained significant after correction for severity of illness at baseline (partial r=0.71, p=0.05).
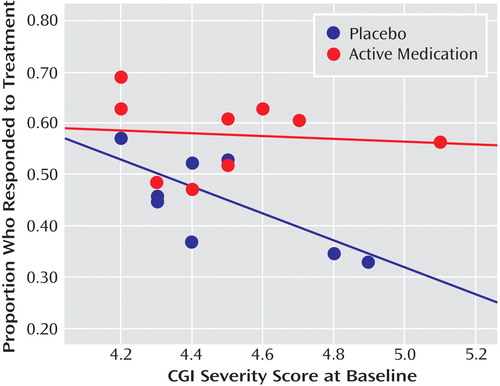
a Treatment response was defined as a score ≤2 (much improved or very much improved) on the improvement item of the Clinical Global Impression scale at end of treatment. The lines in the figure are the best-fitting straight lines indicating the relationship between proportion of participants responding and initial severity of illness for antidepressants (r=–0.11, p=0.78) and placebo (r=–0.69, p=0.04).
All three site selection variables were intercorrelated ( Table 3 ). In order to explore further the factors most associated with placebo response, we conducted a multiple linear regression analysis using a logit transformation of the proportion of placebo responders per study as the dependent variable and CGI severity score and number of study sites as predictor variables; number of study sites was selected for analysis because it was the only site selection variable to survive both a backward-stepping multiple regression analysis with control for other site selection variables and to enter a forward-stepping regression model predicting proportion of placebo response (data available on request). In the multiple regression analysis with control for number of study sites and initial severity, only number of study sites remained significant (β=0.59, t=2.46, df=6, p=0.05, partial r=0.71); severity of illness did not reach statistical significance (β=–0.40, t=1.69, df=6, p=0.14, partial r=–0.57), although the adjusted effect size for initial severity can be considered large (36) .
In the nine trials for which age-grouped data were available (22 – 26 , 28 – 30 , 37) , the rate of placebo response was not significantly different between children and adolescents (49.6% [95% CI=43.7–55.5] and 44.5% [95% CI=40.9–48.2%], respectively). (See the figure in the data supplement that accompanies the online edition of this article.) Systematically deleting one study at a time revealed that the overall results were substantively altered by excluding one fluoxetine trial (24) , yielding placebo response rates of 54.3% (95% CI=47.7–60.9) in children and 44.9% (95% CI=41.1–48.7) in adolescents (χ 2 =5.87, p=0.02). The rate of response to active medication was not significantly different between children and adolescents (58.4% [95% CI=52.5–64.3] and 61.5% [95% CI=58.2–64.8], respectively), and the exclusion of any single trial from the analysis did not substantively change the overall result.
The magnitude of placebo response appeared to play a larger role in predicting the drug-placebo difference in efficacy than did the magnitude of active medication response, despite a nonsignificant correlation between response to placebo and response to active medication (N=12; r=0.47; 95% CI=–0.14 to 0.82, p=0.13). The proportion of patients responding to placebo was strongly related to both the risk difference in CGI improvement response (N=12; r=–0.61; 95% CI=–0.88 to –0.06, p=0.03) and to the scalar change in symptoms from baseline to end of treatment in active medication versus placebo (Hedges’ g, N=12; r=–0.78; 95% CI=–0.93 to –0.37, p=0.003); however, neither the association with risk difference in response nor Hedges’ g was significant after correcting for number of sites. On the other hand, the proportion of responders to active medication was not related to either of these measures of efficacy (risk difference, N=12; r=0.41; 95% CI=–0.21 to 0.79, p=0.18; Hedges’ g, N=12; r=–0.18; 95% CI=–0.68 to 0.44, p=0.58).
Studies included in this report were published between 1997 and 2007. The proportion of patients responding to placebo was significantly correlated with year of publication (N=10; r=0.64; 95% CI=0.02 to 0.91, p=0.05), indicating an increase in placebo response over time. The number of study sites increased significantly with year of publication (N=10, r=0.69, p=0.03); in multiple linear regression analyses, neither year of publication nor number of study sites was a significant predictor of the proportion of patients responding to placebo. Because the nefazodone and mirtazapine trials (31 , 32) were unpublished, we also examined the association between treatment response and year of study completion, which occurred between 1995 and 2004. The association between year of study completion and placebo response was not statistically significant, suggesting that the increase in placebo response over time is due to a publication artifact. The average time between year of study completion and year of publication for the five trials that found evidence of efficacy for antidepressants (21 , 22 , 24 – 26) was around 2 years (mean=28 months [SD=10]), compared with an average of almost 5 years to publication for negative trials (mean=59 months [SD=25]; p=0.03). No significant correlations were found between the proportion of patients responding to active medication and year of publication or year of study completion.
Discussion
In this study of all available reports of randomized clinical trials of antidepressant treatment for pediatric major depression, we found that the proportion of participants responding to placebo, but not the proportion responding to active medication, was strongly related to the number of study sites. Initial severity of illness was inversely related to the proportion of patients responding to placebo, although this association was reduced when number of sites was controlled for. The proportion of placebo response may also be higher in younger participants, as we found after excluding one trial of fluoxetine that showed a low rate of placebo response across the age range of participants (22) . While the placebo and active medication responses were intercorrelated within studies, the placebo response explained more of the variance in efficacy than did the response to active medication. Consistent with previous meta-analyses, the placebo response rate was higher in more recently published studies, although this effect was explained in part by a change in study characteristics over time and a publication bias. Before we discuss each of these findings, we first place the results of this study within the context of its limitations.
This study is restricted to an analysis of trial-level summary data, which may fail to identify important individual patient factors influencing the response to placebo (38) . In addition, even trial-level data were inconsistently reported, and thus we were unable to examine the impact of several other factors that have been shown to be relevant to clinical response and presumably are relevant to placebo response, such as history of nonresponse to an SSRI, history of abuse, family history of psychiatric disorder, and comorbid psychiatric disorders (23 , 39 – 42) . Because the relatively small number of studies gave us low statistical power, statistical interactions were not examined, and null findings should be interpreted with caution. Finally, participants in clinical trials may differ from real-world clinical populations, which may limit generalizability (43) .
The number of study sites proved to be the strongest predictor of placebo response (but not response to active medication), which expands on our earlier finding that the magnitude of antidepressant treatment efficacy decreased as the number of study sites increased (2) . The correlation between mean study severity and number of sites (r=–0.50) indicates that studies with many sites recruited participants with less severe illness, suggesting that screening for participants may be less stringent in trials with more sites. When possible, limiting the number of investigative sites to a few larger centers with tighter control may improve the ability to screen out likely placebo responders. Development of a treatment research network of a small number of sites that are experienced in clinical research for child and adolescent mood disorders might provide an optimal strategy for assessing the relative short-term and long-term efficacy of pharmacological and nonpharmacological treatments. Alternatively, when there is a need for many sites, screening carefully for illness severity is essential. Given that site differences have been shown to increase the likelihood of negative or failed antidepressant trials in adults, efforts to reduce potential sources of variability (e.g., amount of investigator experience, procedures used to recruit subjects) within and between study sites are needed (44) .
Consistent with a recent meta-analysis (12) , a higher proportion of participants responding to placebo but not to antidepressants was predicted by decreased baseline CGI severity. These results should be interpreted with caution because three trials did not assess severity using CGI criteria and were excluded from analyses. If confirmed, these findings raise questions about the benefit-to-risk profile of antidepressants in treating depressed pediatric patients with mild functional impairment. It is unclear why CDRS-R symptom scores did not correlate with placebo response when we found a strong relationship between the CGI severity score and placebo response on univariate analysis. However, at a trial level, the low correlation between the mean baseline CDRS-R and mean CGI severity score (r=0.05, p=0.91) suggests that these two rating scales are measuring different aspects of illness severity. Moreover, while the majority of studies (10/12) had a minimum CDRS-R entry requirement, only three of the trials used a cutoff on the CGI severity score. This selection strategy may explain the high placebo response, since the CGI severity score is a stronger correlate of placebo response than is baseline CDRS-R. From a clinical perspective, these data suggest a role for supportive therapy or a brief (4- to 6-week) trial of more specialized psychotherapy as first-line treatment options for mild depression (1 , 5 , 45) .
We found no significant overall age effect in placebo response, but a sensitivity analysis that excluded one fluoxetine trial revealed that children younger than age 12 had a higher placebo response rate than adolescents age 12 and older. This finding is consistent with our previous report (2) showing that the lack of a significant treatment effect for antidepressants other than fluoxetine in children may be due in part to a higher placebo response in children than in adolescents. However, a recent post hoc analysis of two fluoxetine trials (22 , 24) showed a vigorous antidepressant response and lower placebo response in children under age 12 compared with adolescents (46) . Thus, it appears premature to conclude that the placebo response rate is higher in children than in adolescents.
Despite some covariation between response to medication and to placebo in this study (r=0.47), higher placebo response emerged as a potent negative correlate of treatment efficacy, an association that was attenuated when number of study sites was controlled for. Conversely, in some trials, it was clearly possible to recruit depressed individuals for whom a placebo intervention was not very efficacious, and not surprisingly, it was in these studies that the effects of antidepressants were most robust (22 , 26) . Patient-level regression tree analyses that use participants’ demographic and clinical characteristics to develop models of response prediction may offer one promising approach for future work aimed at identifying candidate variables associated with optimum drug response on the one hand and the lowest placebo response on the other (47 – 49) .
In published placebo-controlled trials of adult depression, response rates increased between 1980 and 2000 among both placebo and active medication groups (14) . We found that the reported relationship between placebo response and date of publication was actually explained by the increasing trend over time toward large multisite trials and an artifact of publication bias. When the date of study completion was taken into account, there was no relationship between the proportion of participants responding to placebo and the temporal order of study completion, which highlights the need for prompt reporting of study results regardless of outcome (50) . A recent study found that selective reporting of adult antidepressant medication results exaggerated their effectiveness, which could mislead physicians and patients about the relative efficacy of antidepressant treatment (51) .
In summary, our findings indicate that the placebo response in controlled antidepressant trials of pediatric depression is strongly correlated with the number of study sites. Restricting the number of sites to a few select centers with experience in clinical assessment of pediatric mood disorders may allow for more careful selection of participants and improved quality assurance over the methods individual sites use to approach, recruit, and retain the patients under study. Since many young patients with an episode of mild depression may respond to brief supportive therapy (5 , 6 , 52) , future work should aim to identify the level of clinical severity at which first-line treatments with medication, psychotherapy, or their combination are warranted.
1. Birmaher B, Brent D, Bernet W, Bukstein O, Walter H, Benson RS, Chrisman A, Farchione T, Greenhill L, Hamilton J, Keable H, Kinlan J, Schoettle U, Stock S, Ptakowski KK, Medicus J: Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry 2007; 46:1503–1526Google Scholar
2. Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Ren L, Brent DA: Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA 2007; 297:1683–1696Google Scholar
3. Thomas CR, Holzer CE 3rd: The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry 2006; 45:1023–1031Google Scholar
4. Koppelman J: The provider system for children’s mental health: workforce capacity and effective treatment. NHPF Issue Brief 2004(801):1–18Google Scholar
5. Renaud J, Brent DA, Baugher M, Birmaher B, Kolko DJ, Bridge J: Rapid response to psychosocial treatment for adolescent depression: a two-year follow-up. J Am Acad Child Adolesc Psychiatry 1998; 37:1184–1190Google Scholar
6. Goodyer I, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, Breen S, Ford C, Barrett B, Leech A, Rothwell J, White L, Harrington R: Selective serotonin reuptake inhibitors (SSRIs) and routine specialist care with and without cognitive behaviour therapy in adolescents with major depression: randomised controlled trial. BMJ 2007; 335:142Google Scholar
7. Wilcox CS, Cohn JB, Linden RD, Heiser JF, Lucas PB, Morgan DL, DeFrancisco D: Predictors of placebo response: a retrospective analysis. Psychopharmacol Bull 1992; 28:157–162Google Scholar
8. Posternak MA, Zimmerman M: Therapeutic effect of follow-up assessments on antidepressant and placebo response rates in antidepressant efficacy trials: meta-analysis. Br J Psychiatry 2007; 190:287–292Google Scholar
9. Stein DJ, Baldwin DS, Dolberg OT, Despiegel N, Bandelow B: Which factors predict placebo response in anxiety disorders and major depression? an analysis of placebo-controlled studies of escitalopram. J Clin Psychiatry 2006; 67:1741–1746Google Scholar
10. Charney DS, Nemeroff CB, Lewis L, Laden SK, Gorman JM, Laska EM, Borenstein M, Bowden CL, Caplan A, Emslie GJ, Evans DL, Geller B, Grabowski LE, Herson J, Kalin NH, Keck PE Jr, Kirsch I, Krishnan KR, Kupfer DJ, Makuch RW, Miller FG, Pardes H, Post R, Reynolds MM, Roberts L, Rosenbaum JF, Rosenstein DL, Rubinow DR, Rush AJ, Ryan ND, Sachs GS, Schatzberg AF, Solomon S; Consensus Development Panel: National Depressive and Manic-Depressive Association consensus statement on the use of placebo in clinical trials of mood disorders. Arch Gen Psychiatry 2002; 59:262–270Google Scholar
11. Khan A, Dager SR, Cohen S, Avery DH, Scherzo B, Dunner DL: Chronicity of depressive episode in relation to antidepressant-placebo response. Neuropsychopharmacology 1991; 4:125–130Google Scholar
12. Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT: Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med 2008; 5(2):e45Google Scholar
13. Khan A, Detke M, Khan SR, Mallinckrodt C: Placebo response and antidepressant clinical trial outcome. J Nerv Ment Dis 2003; 191:211–218Google Scholar
14. Walsh BT, Seidman SN, Sysko R, Gould M: Placebo response in studies of major depression: variable, substantial, and growing. JAMA 2002; 287:1840–1847Google Scholar
15. Medicines and Healthcare Products Regulatory Agency Committee on Safety of Medicines (CSM): Report of the CSM Expert Working Group on the Safety of Selective Serotonin Reuptake Inhibitor Antidepressants, December 2004 (http://www.mhra.gov.uk/home/groups/pl-p/documents/drugsafetymessage/con019472.pdf)Google Scholar
16. Hammad TA: Review and evaluation of clinical data. Washington, DC, US Food and Drug Administration, Aug 16, 2004 (http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4065b1-10-TAB08-Hammads-Review.pdf)Google Scholar
17. Hammad TA, Laughren T, Racoosin J: Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry 2006; 63:332–339Google Scholar
18. Medicines and Healthcare Products Regulatory Agency Committee on Safety of Medicines (CSM): selective serotonin reuptake inhibitors (SSRIs): overview of regulatory status and CSM advice relating to major depressive disorder (MDD) in children and adolescents including a summary of available safety and efficacy data. http://www.mhra.gov.uk/Safetyinformation/Safetywarningsalertsandrecalls/Safetywarningsandmessagesformedicines/CON019494Google Scholar
19. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 218–222Google Scholar
20. Poznanski E, Mokros H: Children’s Depression Rating Scale—Revised (CDRS-R). Los Angeles, Calif, Western Psychological Services, 1995Google Scholar
21. Wagner KD, Robb AS, Findling RL, Jin J, Gutierrez MM, Heydorn WE: A randomized, placebo-controlled trial of citalopram for the treatment of major depression in children and adolescents. Am J Psychiatry 2004; 161:1079–1083Google Scholar
22. Emslie GJ, Rush AJ, Weinberg WA, Kowatch RA, Hughes CW, Carmody T, Rintelmann J: A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry 1997; 54:1031–1037Google Scholar
23. Keller MB, Ryan ND, Strober M, Klein RG, Kutcher SP, Birmaher B, Hagino OR, Koplewicz H, Carlson GA, Clarke GN, Emslie GJ, Feinberg D, Geller B, Kusumakar V, Papatheodorou G, Sack WH, Sweeney M, Wagner KD, Weller EB, Winters NC, Oakes R, McCafferty JP: Efficacy of paroxetine in the treatment of adolescent major depression: a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 2001; 40:762–772Google Scholar
24. Emslie GJ, Heiligenstein JH, Wagner KD, Hoog SL, Ernest DE, Brown E, Nilsson M, Jacobson JG: Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial. J Am Acad Child Adolesc Psychiatry 2002; 41:1205–1215Google Scholar
25. Wagner KD, Ambrosini P, Rynn M, Wohlberg C, Yang R, Greenbaum MS, Childress A, Donnelly C, Deas D; Sertraline Pediatric Depression Study Group: Efficacy of sertraline in the treatment of children and adolescents with major depressive disorder: two randomized controlled trials. JAMA 2003; 290:1033–1041Google Scholar
26. March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S, Vitiello B, Severe J; Treatment for Adolescents With Depression Study (TADS) Team: Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA 2004; 292:807–820Google Scholar
27. Wagner KD, Jonas J, Findling RL, Ventura D, Saikali K: A double-blind, randomized, placebo-controlled trial of escitalopram in the treatment of pediatric depression. J Am Acad Child Adolesc Psychiatry 2006; 45:280–288Google Scholar
28. Berard R, Fong R, Carpenter DJ, Thomason C, Wilkinson C: An international, multicenter, placebo-controlled trial of paroxetine in adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 2006; 16:59–75Google Scholar
29. Emslie GJ, Wagner KD, Kutcher S, Krulewicz S, Fong R, Carpenter DJ, Lipschitz A, Machin A, Wilkinson C: Paroxetine treatment in children and adolescents with major depressive disorder: a randomized, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry 2006; 45:709–719Google Scholar
30. Emslie GJ, Findling RL, Yeung PP, Kunz NR, Li Y: Venlafaxine ER for the treatment of pediatric subjects with depression: results of 2 placebo-controlled trials. J Am Acad Child Adolesc Psychiatry 2007; 46:479–488Google Scholar
31. Emslie GJ, Findling RL, Rynn MA, Marcus RN: Efficacy and safety of nefazodone in the treatment of adolescents with major depressive disorder (abstract). J Child Adolesc Psychopharmacol 2002; 12:299Google Scholar
32. Dubitsky GM: Review and evaluation of clinical data: placebo-controlled antidepressant studies in pediatric patients. Washington, DC, US Food and Drug Administration, August 6, 2004. http://www.fda.gov/OHRMS/DOCKETS/ac/04/briefing/2004–4065b1–08-TAB06-Dubitsky-Review.pdfGoogle Scholar
33. Simeon JG, Dinicola VF, Ferguson HB, Copping W: Adolescent depression: a placebo-controlled fluoxetine treatment study and follow-up. Prog Neuropsychopharmacol Biol Psychiatry 1990; 14:791–795Google Scholar
34. US Food and Drug Administration: Review and Evaluation of Clinical Data, NDA: 20-152. S-032, nefazodone hydrochloride (Serzone). Washington, DC, 2002. http://www.fda.gov/cder/foi/esum/2004/20152s032_Serzone_clinical_BPCA_FIN.pdfGoogle Scholar
35. von Knorring AL, Olsson GI, Thomsen PH, Lemming OM, Hultén A: A randomised double-blind placebo-controlled study of citalopram in adolescents with major depressive disorder. J Clin Psychopharmacol 2006; 26:311–315Google Scholar
36. Cohen J: Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988Google Scholar
37. Emslie GJ, Findling RL, Rynn MA, Marcus RN, Fernandes LA, D’Amico MF, Hardy SA: Efficacy and safety of nefazodone in the treatment of adolescents with major depressive disorder [abstract], in 42nd Annual Meeting of the National Institute of Mental Health: New Clinical Drug Evaluation Unit (NCDEU). Boca Raton, Fla, 2002Google Scholar
38. Berlin JA, Santanna J, Schmid CH, Szczech LA, Feldman HI: Individual patient- versus group-level data meta-regressions for the investigation of treatment effect modifiers: ecological bias rears its ugly head. Statist Med 2002; 21:371–387Google Scholar
39. Birmaher B, McCafferty JM, Bellew KM, et al: Comorbid ADHD and other disruptive behavior disorders as predictors of response in adolescents treated for major depression, poster, 153rd annual meeting of the American Psychiatric Association, Chicago, 2000Google Scholar
40. Curry J, Rohde P, Simons A, Silva S, Vitiello B, Kratochvil C, Reinecke M, Feeny N, Wells K, Pathak S, Weller E, Rosenberg D, Kennard B, Robins M, Ginsburg G, March J, Team TT: Predictors and moderators of acute outcome in the Treatment for Adolescents With Depression Study (TADS). J Am Acad Child Adolesc Psychiatry 2006; 45:1427–1439Google Scholar
41. Barbe RP, Bridge JA, Birmaher B, Kolko DJ, Brent DA: Lifetime history of sexual abuse, clinical presentation, and outcome in a clinical trial for adolescent depression. J Clin Psychiatry 2004; 65:77–83Google Scholar
42. Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP Jr, Weiss PM, Dunner DL, Rothbaum BO, Kornstein S, Keitner G, Keller MB: Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci USA 2003; 100:14293–14296Google Scholar
43. Greenhill LL, Vitiello B, Abikoff H, Levine J, March JS, Riddle MA, Capasso L, Cooper TB, Davies M, Fisher P, Findling RL, Fried J, Labellarte MJ, McCracken JT, McMahon D, Robinson J, Skrobala A, Scahill L, Varipatis E, Walkup JT, Zito JM: Developing methodologies for monitoring long-term safety of psychotropic medications in children: report on the NIMH conference, September 25, 2000. J Am Acad Child Adolesc Psychiatry 2003; 42:651–655Google Scholar
44. Amsterdam JD, Brunswick DJ: Site variability in treatment outcome in antidepressant trials. Prog Neuropsychopharmacol Biol Psychiatry 2002; 26:989–993Google Scholar
45. Mufson L, Dorta KP, Wickramaratne P, Nomura Y, Olfson M, Weissman MM: A randomized effectiveness trial of interpersonal psychotherapy for depressed adolescents. Arch Gen Psychiatry 2004; 61:577–584Google Scholar
46. Mayes TL, Tao R, Rintelmann JW, Carmody T, Hughes CW, Kennard BD, Stewart SM, Emslie GJ: Do children and adolescents have differential response rates in placebo-controlled trials of fluoxetine? CNS Spectr 2007; 12:147–154Google Scholar
47. Breiman L, Friedman JH, Olshen RA, Stone CJ: Classification and Regression Trees. Belmont, Calif, Wadsworth International, 1984Google Scholar
48. Noda A, Kraemer HC, Taylor JL, Schneider B, Ashford JW, Yesavage JA: Strategies to reduce site differences in multisite studies: a case study of Alzheimer disease progression. Am J Geriatr Psychiatry 2006; 14:931–938Google Scholar
49. Andreescu C, Mulsant BH, Houck PR, Whyte EM, Mazumdar S, Dombrovski AY, Pollock BG, Reynolds CF 3rd: Empirically derived decision trees for the treatment of late-life depression. Am J Psychiatry 2008; 165:855–862Google Scholar
50. Vieta E, Cruz N: Increasing rates of placebo response over time in mania studies. J Clin Psychiatry 2008; 69:681–682Google Scholar
51. Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R: Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med 2008; 358:252–260Google Scholar
52. March JS, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S, Vitiello B, Severe J: The Treatment for Adolescents With Depression Study (TADS): long-term effectiveness and safety outcomes. Arch Gen Psychiatry 2007; 64:1132–1143Google Scholar