Treatment-Resistant Bipolar Depression: A STEP-BD Equipoise Randomized Effectiveness Trial of Antidepressant Augmentation With Lamotrigine, Inositol, or Risperidone
Abstract
OBJECTIVE: Clinicians have few evidence-based options for the management of treatment-resistant bipolar depression. This study represents the first randomized trial of competing options for treatment-resistant bipolar depression and assesses the effectiveness and safety of antidepressant augmentation with lamotrigine, inositol, and risperidone. METHOD: Participants (N=66) were patients with bipolar I or bipolar II disorder enrolled in the NIMH Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). All patients were in a current major depressive episode that was nonresponsive to a combination of adequate doses of established mood stabilizers plus at least one antidepressant. Patients were randomly assigned to open-label adjunctive treatment with lamotrigine, inositol, or risperidone for up to 16 weeks. The primary outcome measure was the rate of recovery, defined as no more than two symptoms meeting DSM-IV threshold criteria for a mood episode and no significant symptoms present for 8 weeks. RESULTS: No significant between-group differences were seen when any pair of treatments were compared on the primary outcome measure. However, the recovery rate with lamotrigine was 23.8%, whereas the recovery rates with inositol and risperidone were 17.4% and 4.6%, respectively. Patients receiving lamotrigine had lower depression ratings and Clinical Global Impression severity scores as well as greater Global Assessment of Functioning scores compared with those receiving inositol and risperidone. CONCLUSIONS: No differences were found in primary pairwise comparison analyses of open-label augmentation with lamotrigine, inositol, or risperidone. Post hoc secondary analyses suggest that lamotrigine may be superior to inositol and risperidone in improving treatment-resistant bipolar depression.
Depression has emerged as the major challenge for the short- and long-term management of bipolar disorder (1–6). Guidelines support the use of antidepressants for bipolar depression (7–10), although one guideline gave this approach a relatively low priority because of the limited evidence base supporting it (11). A meta-analysis of the literature (12) revealed that remarkably few controlled studies have been published but nevertheless concluded that antidepressants can be effective for bipolar depression. A careful examination of the studies included in the meta-analysis reveals that most of the studies had significant methodological limitations. Moreover, this meta-analysis included multiple small studies, an approach that can introduce bias favoring positive outcomes (13). With regard to the adverse effects of antidepressants for bipolar depression, double-blind, placebo-controlled data suggest that antidepressant monotherapy (14) or the addition of a tricyclic antidepressant (15) may worsen the course of bipolar disorder. No data have suggested that this exacerbation will occur if the modern generation of antidepressants are prescribed in combination with at least one antimanic agent, as recommended by expert consensus (16).
Few studies are available that guide the next best treatment if a mood stabilizer plus an antidepressant fail to help patients with bipolar depression. Limited data suggest that patients can be switched to either ECT (17) or monoamine oxidase inhibitors (18, 19), but these treatments are commonly not acceptable to patients. Other options include combining mood stabilizers (20), switching to the combination of olanzapine and fluoxetine (21), switching to quetiapine (22), or adding novel treatments such as pramipexole (23, 24) or riluzole (25). Preliminary reports have suggested three potential candidates to augment other agents for bipolar depression: lamotrigine (a mood stabilizer approved for maintenance monotherapy in bipolar I disorder that appears more effective in preventing bipolar depression than mania [26–30]), inositol (a sugar derivative with effects on intracellular signaling [31]), and risperidone (an atypical antipsychotic approved for monotherapy and adjunctive therapy for acute mania [32]). No studies have compared the effectiveness and safety of these treatments after other approaches fail, leaving clinicians uncertain about the effectiveness of these options. This study is the first randomized trial of treatment-resistant bipolar depression to compare open-label adjunctive administration of lamotrigine, inositol, and risperidone for patients nonresponsive to a mood stabilizer plus one or two antidepressant trials during a current major depressive episode.
Method
The Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) is a multicenter NIMH-funded project designed to evaluate the longitudinal outcome of patients with bipolar disorder (see Sachs et al. [33] for details). After complete description of the study to the subjects, written informed consent was obtained.
Measures
Bipolar illness characteristics and comorbid conditions were identified using the Mini-International Neuropsychiatric Interview (MINI) (34). The Clinical Monitoring Form (35), administered to every STEP-BD participant at every clinic visit, determined treatment and current clinical status, including suicidal thoughts or behaviors and DSM-IV criteria for major depressive and mania symptoms. Within the Clinical Monitoring Form are measures that sum all associated depressive symptom scores (SUM-D) and all manic symptom scores (SUM-M). SUM-D scores have been found to strongly correlate with Montgomery-Åsberg Rating Scale (36) scores (r=0.87), and SUM-M scores strongly correlate with Young Mania Rating Scale (37) scores (r=0.84) (35).
Subjects
Subjects were included if they 1) were at least 18 years old, 2) met criteria for bipolar disorder type I or II with a current DSM-IV major depressive episode of at least 8 weeks before pathway entry, and 3) had not responded to treatment in first 12 weeks of standard or randomized care pathways for bipolar depression, or had a well-documented failure (e.g., a medical chart was available) to respond to at least two trials of antidepressants or an antidepressant and mood stabilizer regimen. Patients were required to be taking a mood stabilizer or agree to begin treatment with a mood stabilizer. All patients were offered treatment with ECT in the STEP-BD standard care pathway and were made aware of potential benefits. This procedure ensured that patients had the information necessary to make an informed decision regarding whether to immediately pursue ECT, since this treatment is effective but commonly rejected as an alternative for addressing treatment nonresponse. Only patients who refused ECT at this stage were eligible for randomization to the open-label treatment conditions (adjunctive lamotrigine, risperidone, or inositol). No patients elected to have ECT rather than enter the randomized trial. Sixty-seven patients were screened and 66 entered.
Subjects were excluded from participation if there was a history of nonresponse to, intolerance of, or any medical contraindications to at least two of the study medications. Patients were excluded if they met criteria for mixed episode or hypomania or if they met criteria for current substance abuse or dependence diagnosis.
Subjects were managed with an optimized mood stabilizer regimen (lithium, valproate, combined lithium and valproate, or carbamazepine) plus either one or two antidepressants. Additionally, patients were systematically monitored for symptoms of suicidality.
Treatments
Patients were randomly assigned to receive one of the refractory depression pathway interventions (lamotrigine, inositol, or risperidone) for up to 16 weeks in addition to their current open-label mood stabilizer treatment with active antidepressant(s). Since many patients had taken at least one of the three medications under study, or considered one of the options unacceptable, patients were assigned treatments using equipoise randomization (38). Equipoise randomization means that patients were allowed to be randomized to one of all three options (if all were acceptable) or to only one of two, resulting in four randomization strata: 1) accept all, 2) accept only lamotrigine or risperidone, 3) accept only lamotrigine or inositol, and 4) accept only risperidone or inositol. Patients were randomly assigned to receive one of the active agents under open-label conditions within the chosen strata.
Mood stabilizer therapy was optimized within the recommended range (lithium: 0.6–0.9 mmol/liter; valproate: 45–90 μg/ml; carbamazepine: 4–10 μg/ml). In addition, the treating psychiatrist could prescribe any adjunctive medication deemed necessary for appropriate clinical management, with the exception of additional antidepressant medications. Trazodone was not considered an antidepressant medication if used as a hypnotic at bedtime in doses up to 150 mg. Patients were scheduled for weekly follow-up evaluations during the first 4 weeks of the acute treatment phase.
Per clinical guidleines, lamotrigine doses started at 50 mg/day for 2 weeks, followed by 50 mg b.i.d. for 2 weeks, then increases in daily dose every week until the target dose of between 150 and 250 mg/day was reached. Inositol doses started at 2.5 to 5 g with a target dose of between 10 and 25 g. Risperidone doses started at between 0.5 and 1.0 mg with titration up to 6 mg as tolerated.
The primary outcome measure was the recovery rate within equipoise randomization strata. Recovery was defined as 1) no more than two symptoms meeting DSM-IV threshold criteria for a major depressive, manic, or hypomanic episode, as determined with the clinician-administered Clinical Monitoring Form, and 2) no significant symptoms present for 8 weeks, consistent with the DSM-IV definition of full remission (33). Secondary outcome measures included Clinical Global Impression (CGI) severity ratings, Clinical Monitoring Form SUM-D and SUM-M scores, and Global Assessment of Functioning scores; secondary analyses were done across equipoise randomization strata.
Data Analysis
Three of 66 subjects were willing to accept all three medications. None of these three were randomly assigned to lamotrigine; one was randomly assigned to inositol and the other two to risperidone. The remaining subjects were willing to be assigned to two of the three adjunctive treatment options.
For each two-drug comparison, analyses were conducted twice: once including only those patients willing to accept the two drugs in the comparison (i.e., within equipoise randomization strata) and a second time including those patients willing to accept all three drugs who were randomly assigned to the drugs being compared in the pairwise comparison (i.e., across equipoise randomization strata). When patients who were willing to accept assignment to any of the three treatments were included, no differences in any of the comparisons were found. Thus, the results are presented with all patients included. Ideally, one would analyze the data separately for these two instances and then combine the results using the Mantel-Haenszel approach. However, because only three subjects were willing to accept all three treatments, this was not feasible.
For the discrete outcome measures, Fisher’s exact tests were used to compare rates of recovery across treatments. For the ordinal SUM-D, SUM-M, and CGI measures at baseline and at exit, nonparametric analysis of variance tests were used to compare treatments. At baseline assessment, some patients had missing SUM-D, SUM-M, or CGI scores because a Clinical Monitoring Form was not obtained within 7 days of enrolling in the refractory depression randomized care pathway. SUM-D, SUM-M, and CGI scores were taken from the Clinical Monitoring Form closest to the exit assessment but within 1 week before exiting the refractory depression randomized care pathway. If no Clinical Monitoring Form was available within this time frame, the data were considered to be missing.
The proportion and 95% confidence interval for patients who recovered with each augmenting agent was estimated by pooling all of the patients assigned to each augmenting agent, regardless of randomization strata.
Results
Patients and Medication Doses
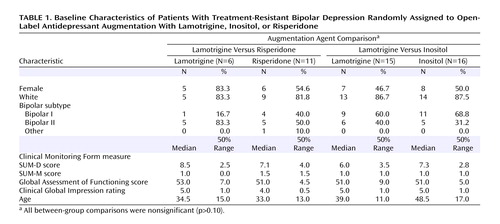
Overall, 66 subjects were enrolled in the study and were randomly assigned to one of three augmentation agent comparisons: lamotrigine versus risperidone (N=17), lamotrigine versus inositol (N=31), or risperidone versus inositol (N=21). Three subjects were willing to accept random assignment to any of the three treatments and therefore are included in two strata and are counted twice in the pairwise comparisons. As for differences in the groups that chose each option, younger patients chose lamotrigine (mean age=39.4 years, SD=10.7), and older patients chose inositol (mean age=45.0 years, SD=10.7), but overall there were no statistically significant differences for any demographic or clinical variable between the groups assigned each medication (Table 1).
Comparisons Within Equipoise Randomization Strata
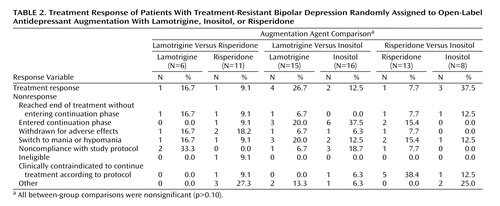
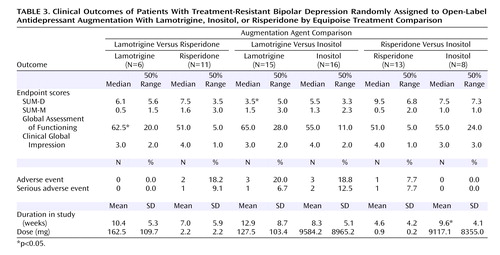
No differences in treatment response (Table 2) or secondary outcome measures (Table 3) were found for any paired comparison with the exception of lower exit SUM-D scores for lamotrigine compared with inositol and a higher exit Global Assessment of Functioning score for lamotrigine compared with risperidone. In the risperidone versus inositol comparison, patients assigned to inositol remained in the randomized phase of the study significantly longer. No differences were seen in the rate of adverse events or serious adverse events for any treatment comparison.
Comparisons Across Equipoise Randomization Strata
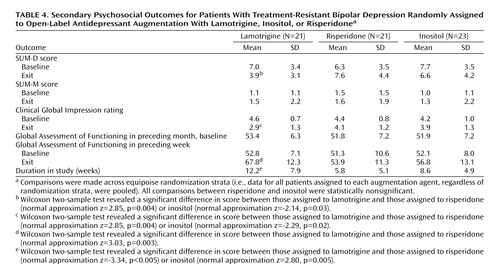
As shown in Table 4, subjects assigned to lamotrigine had significantly lower SUM-D exit scores compared with subjects receiving either inositol or risperidone. Similar results were found for exit CGI and Global Assessment of Functioning scores for the preceding week. Subjects randomly assigned to lamotrigine stayed in the randomized phase significantly longer than did those assigned to inositol or risperidone. For the more stringent definition of recovered for 8 weeks (Figure 1), the overall recovery rates were 23.8% (95% CI=5.8 to 41.8) for lamotrigine, 17.4% (95% CI=2.4 to 32.4) for inositol, and 4.6% (95% CI=0 to 14.6) for risperidone.
Discussion
This study is the first randomized, open-label medication augmentation trial for treatment-resistant bipolar depression. For the primary outcome measure of protocol-defined recovery within equipoise randomization strata, no statistically significant between-group differences were found for lamotrigine, inositol, and risperidone. Using a rigorous definition of sustained response (recovered) for 8 weeks, secondary analyses pooled across equipoise randomization strata showed differences in recovery rates with lamotrigine (24%), inositol (17%), and risperidone (5%) that were nonsignificant. However, several secondary outcome measures in the pooled analyses converge to suggest that lamotrigine may be more effective than either inositol or risperidone.
Equipoise randomization, which allowed patients and their clinician to pick at least two competing options, resulted in only three (4.5%) out of 66 patients accepting all three options. If this study had been conducted with conventional forced randomization, then only those who accepted or were eligible for all three options would have been included, and the generalizability of the results would have been limited because the majority of patients had already tried one of the treatments or had other reasons for not accepting all three (38). Equipoise randomization, the alternative solution, resulted in a fragmented sample size and limited statistical power to assess differences in response rates for each paired comparison.
In the secondary pooled analyses across equipoise randomization strata, the overall proportion of responders to each medication included different subjects across each randomization stratum. Because these are different groups, the results cannot be formally compared for hypothesis testing. An estimate of variability of recovery rates, with 95% confidence intervals around the proportions, is possible for descriptive purposes. The overlap of the confidence intervals for the three treatments suggests lack of significant differences. Overall, regardless of treatment assignment, the absolute rates of sustained recovery for 8 weeks were low, confirming the seriousness and persistence of treatment-resistant bipolar depression.
Post hoc analyses of relevant continuous outcomes suggest that subjects randomly assigned to lamotrigine had greater improvements in depressive symptoms, overall severity, and functioning at exit. Another signal that favored lamotrigine was that patients randomly assigned to lamotrigine elected to stay on this medication significantly longer than either inositol or risperidone. This difference in treatment duration emphasizes the “effectiveness” aspect of the study. STEP-BD study participants were treated in specialty clinics by clinicians who were trained to provide systematic evidence-based care and assess patients’ progress at every clinical visit. In this context, patients who met criteria for treatment-resistant bipolar depression were eligible to participate in the randomized trial. Patients could not only choose a pair of preferred treatments for randomization (equipoise randomization) but could also choose to stop treatment if they perceived a lack of benefit. If they stopped the randomized treatment, patients were still treated by their STEP-BD clinician. Thus, the longer duration of treatment for those who were randomly assigned to lamotrigine can be interpreted as a signal that patients perceived more benefit with lamotrigine than did those randomly assigned to either inositol or risperidone. Alternatively, the need to increase lamotrigine dose slowly could be a contributing factor to the longer duration of treatment.
This study has several strengths. We assessed in a multicenter, randomized study the effectiveness of adjunctive medication therapy for treatment-resistant bipolar depression in a heterogeneous sample of bipolar disorder patients with diverse psychiatric and medical comorbidity and concurrent medications. As such, our findings are more generalizable than those from conventional registration studies of monotherapy in homogeneous patients lacking comorbid psychiatric or medical disorders. Our primary outcome measure (recovery rate within randomization strata) reflected improvement to a euthymic state, which is more clinically meaningful than measures such as change in symptom ratings or response rates (proportion with at least 50% decrease in symptom ratings) commonly cited in conventional registration studies.
This study also has important limitations. First, in view of the sample size (N=66) and the assessment of three treatments, overall statistical power was limited, and the equipoise randomization strata design yielded even lower statistical power within randomization strata. Although this effect was attenuated in our secondary analyses pooled across equipoise randomization strata, this approach raises aforementioned methodological concerns. Even with the latter approach, the confidence intervals for the recovery rates overlapped, although for several other measures lamotrigine appeared superior to the other treatments. But since these were secondary outcome measures and a correction for multiple comparisons was not applied, our observations need to be considered preliminary. Using a Bonferroni correction and setting the power at 80%, the sample sizes necessary to have adequate power to find a significant difference, given the effect size observed for equipoise randomization response rates, would be N=431 per group for lamotrigine versus risperidone; N=176 per group for lamotrigine versus inositol; and N=46 per group for risperidone versus inositol.
In summary, for augmentation of antidepressant treatment in patients with resistant bipolar depression, no statistically significant differences were found for lamotrigine versus risperidone, lamotrigine versus inositol, or risperidone versus inositol. The overall recovery rate was low, indicating that treatment-resistant bipolar depression is a serious clinical problem. The results suggest that few patients would be expected to recover with the addition of risperidone, while adjunctive lamotrigine and inositol may have some potential in treatment-resistant bipolar depression. Lamotrigine was superior to either inositol or risperidone on relevant post hoc secondary measures. Future studies are needed that compare other possible augmenting agents, studies that compare augmentation to switching strategies, and studies that compare competing switching strategies.
 |
 |
 |
 |
Received Aug. 31, 2005; revision received Nov. 15, 2005; accepted Nov. 28, 2005. From the Massachusetts General Hospital Bipolar Clinic and Research Program; Case Western Reserve University, Cleveland; Stanford University School of Medicine, Stanford, Calif.; the Department of Psychiatry, Baylor College of Medicine, Houston; the Departments of Psychology and Psychiatry, University of Colorado, Boulder and Denver; Epidemiological Data Center, University of Pittsburgh Graduate School of Public Health, Pittsburgh; Brown Medical School, Providence, R.I.; and the Western Psychiatric Institute and Clinic, Pittsburgh. Address correspondence and reprint requests to Dr. Nierenberg, MGH Bipolar Clinic and Research Program, Suite 580, 50 Staniford St., Boston, MA 02114; [email protected] (e-mail).This project has been funded in part with federal funds from the National Institute of Mental Health under contract N01 MH-80001. Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of NIMH. This article was approved by the publication committee of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). The authors thank Stephanie Gironde for her help in manuscript preparation.
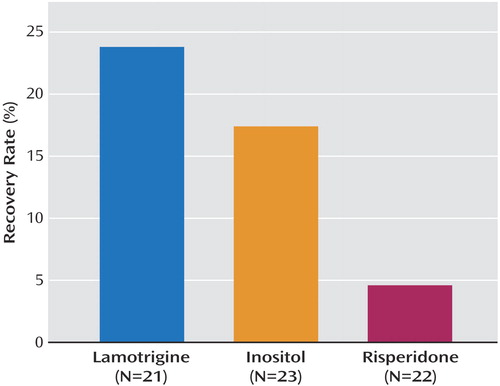
Figure 1. Recovery Rates of Patients With Treatment-Resistant Bipolar Depression Randomly Assigned to Open-Label Antidepressant Augmentation With Lamotrigine, Inositol, or Risperidonea
aComparisons were made across equipoise randomization strata (i.e., data for all patients assigned to each augmentation agent, regardless of randomization strata, were pooled). Recovery was defined as no more than two symptoms meeting DSM-IV threshold criteria for a mood episode and no significant symptoms present for 8 weeks.
1. Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, Leon AC, Rice JA, Keller MB: The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry 2002; 59:530–537Crossref, Medline, Google Scholar
2. Judd LL, Akiskal HS, Schettler PJ, Coryell W, Endicott J, Maser JD, Solomon DA, Leon AC, Keller MB: A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry 2003; 60:261–269Crossref, Medline, Google Scholar
3. Altshuler LL, Gitlin MJ, Mintz J, Leight KL, Frye MA: Subsyndromal depression is associated with functional impairment in patients with bipolar disorder. J Clin Psychiatry 2002; 63:807–811Crossref, Medline, Google Scholar
4. Post RM, Denicoff KD, Leverich GS, Altshuler LL, Frye MA, Suppes TM, Rush AJ, Keck PE, McElroy SL, Luckenbaugh DA, Pollio C, Kupka M, Nolen WA: Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH Life Chart Method. J Clin Psychiatry 2003; 64:680–690Crossref, Medline, Google Scholar
5. Post RM, Leverich GS, Nolen WA, Kupka RW, Altshuler LL, Frye MA, Suppes T, McElroy S, Keck P, Grunze H, Walden J: A re-evaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Foundation Bipolar Network. Bipolar Disord 2003; 5:396–406Crossref, Medline, Google Scholar
6. Joffe RT, MacQueen GM, Marriott M, Young TL: A prospective, longitudinal study of percentage of time spent ill in patients with bipolar I or bipolar II disorders. Bipolar Disord 2004; 6:61–66Crossref, Google Scholar
7. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Bipolar Disorder (Revision). Am J Psychiatry 2002; 159(April suppl)Google Scholar
8. Goodwin GM (Consensus Group of the British Association for Psychopharmacology): Evidence-based guidelines for treating bipolar disorder: recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2003; 2:149–173Crossref, Google Scholar
9. Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for Bipolar Disorder: Australian and New Zealand clinical practice guidelines for the treatment of bipolar disorder. Aust NZ J Psychiatry 2004; 38:280–305Crossref, Medline, Google Scholar
10. Calabrese JR, Kasper S, Johnson G, Tajima O, Vieta E, Yatham LN, Young AH: International Consensus Group on Bipolar I Depression Treatment Guidelines. J Clin Psychiatry 2004; 65:571–579Crossref, Medline, Google Scholar
11. Suppes T, Dennehy EB, Hirschfeld RM, Altshuler LL, Bowden CL, Calabrese JR, Crismon ML, Ketter TA, Sachs GS, Swann AC: The Texas Implementation of Medication Algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry 2005; 66:870–886Crossref, Medline, Google Scholar
12. Gijsman HJ, Geddes JR, Rendell JM, Nolen WA, Goodwin GM: Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry 2004; 161:1537–1547Link, Google Scholar
13. Kraemer HC, Gardner C, Brooks JO, Yesavage JA: Advantages of excluding underpowered studies in meta-analysis: inclusionist versus exclusionist viewpoints. Psychol Methods 1998; 3:23–31Crossref, Google Scholar
14. Prien RF, Klett CJ, Caffey EM: Lithium carbonate and imipramine in prevention of affective episodes: a comparison in recurrent affective illness. Arch Gen Psychiatry 1973; 29:420–425Crossref, Medline, Google Scholar
15. Nemeroff CB, Evans DL, Gyulai L, Sachs GS, Bowden CL, Gergel IP, Oakes R, Pitts CD: Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry 2001; 158:906–912Link, Google Scholar
16. Yatham LN, Kennedy SH, O’Donovan C, Parikh S, MacQueen G, McIntyre R, Sharma V, Silverstone P, Alda M, Baruch P, Beaulieu S, Daigneault A, Milev R, Young LT, Ravindran A, Schaffer A, Connolly M, Gorman CP: Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: consensus and controversies. Bipolar Disord 2005; 3(suppl 7):5-69Google Scholar
17. Homan S, Lachenbruch PA, Winkur G, Clayton P: An efficacy study of electroconvulsive therapy and antidepressants in the treatment of primary depression. Psychol Med 1982; 12:615–624Crossref, Medline, Google Scholar
18. Himmelhoch JM, Thase ME, Mallinger AG, Houck P: Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry 1991; 148:910–916Link, Google Scholar
19. Thase ME, Mallinger AG, McKnight D, Himmelhoch JM: Treatment of imipramine-resistant recurrent depression, IV: a double-blind crossover study of tranylcypromine for anergic bipolar depression. Am J Psychiatry 1992; 149:195–198Link, Google Scholar
20. Young LT, Joffe RT, Robb JC, MacQueen GM, Marriott M, Patelis-Siotis I: Double-blind comparison of addition of a second mood stabilizer versus an antidepressant to an initial mood stabilizer for treatment of patients with bipolar depression. Am J Psychiatry 2000; 157:124–126Link, Google Scholar
21. Tohen M, Vieta E, Calabrese J, Ketter TA, Sachs G, Bowden C, Mitchell PB, Centorrino F, Risser R, Baker RW, Evans AR, Beymer K, Dube S, Tollefson GD, Breier A: Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003; 60:1079–1088Crossref, Medline, Google Scholar
22. Calabrese JR, Keck PE Jr, Macfadden W, Minkwitz M, Ketter TA, Weisler RH, Cutler AJ, McCoy R, Wilson E, Mullen J (BOLDER Study Group): A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry 2005; 162:1351–1360Link, Google Scholar
23. Goldberg JF, Burdick KE, Endick CJ: Preliminary randomized, double-blind, placebo-controlled trial of pramipexole added to mood stabilizers for treatment-resistant bipolar depression. Am J Psychiatry 2004; 161:564–566Link, Google Scholar
24. Zarate CA Jr, Payne JL, Singh J, Quiroz JA, Luckenbaugh DA, Denicoff KD, Charney DS, Manji HK: Pramipexole for bipolar II depression: a placebo-controlled proof of concept study. Biol Psychiatry 2004; 56:54–60Crossref, Medline, Google Scholar
25. Zarate CA Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, Charney DS, Manji HK: An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry 2005; 57:430–432Crossref, Medline, Google Scholar
26. Bowden CL, Calabrese JR, Sachs G, Yatham LN, Asghar SA, Hompland M, Montgomery P, Earl N, Smoot TM, DeVeaugh-Geiss J (Lamictal 606 Study Group): A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry 2003; 60:392–400Crossref, Medline, Google Scholar
27. Calabrese JR, Bowden CL, Sachs GS, Ascher JA, Monaghan E, Rudd GD (Lamictal 602 Study Group): A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry 1999; 2:79–88Crossref, Google Scholar
28. Calabrese JR, Suppes T, Bowden CL, Sachs GS, Swann AC, McElroy SL, Kusumakar V, Ascher JA, Earl NL, Greene PL, Monaghan ET (Lamictal 614 Study Group): A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder. J Clin Psychiatry 2000; 11:841–850Crossref, Google Scholar
29. Calabrese JR, Bowden CL, Sachs G, Yatham LN, Behnke K, Mehtonen OP, Montgomery P, Ascher J, Paska W, Earl N, DeVeaugh-Geiss J (Lamictal 605 Study Group): A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry 2003; 9:1013–1024Crossref, Google Scholar
30. Goodwin GM, Bowden CL, Calabrese JR, Grunze H, Kasper S, White R, Greene P, Leadbetter R: A pooled analysis of 2 placebo-controlled 18-month trials of lamotrigine and lithium maintenance in bipolar I disorder. J Clin Psychiatry 2004; 3:432–441Crossref, Google Scholar
31. Chengappa KN, Levine J, Gershon S, Mallinger AG, Hardan A, Vagnucci A, Pollock B, Luther J, Buttenfield J, Verfaille S, Kupfer DJ: Inositol as an add-on treatment for bipolar depression. Bipolar Disord 2000; 2:47–55Crossref, Medline, Google Scholar
32. Hirschfeld RMA, Keck PE Jr, Kramer M, Karcher K, Canuso C, Eerdekens M, Grossman F: Rapid antimanic effect of risperidone monotherapy: a 3-week multicenter, double-blind, placebo-controlled trial. Am J Psychiatry 2004; 161:1057–1065Link, Google Scholar
33. Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, Lavori P, Lebowitz B, Rudorfer M, Frank E, Nierenberg AA, Fava M, Bowden C, Ketter T, Marangell L, Calabrese J, Kupfer D, Rosenbaum JF: Rationale, design, and methods of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Biol Psychiatry 2003; 53:1028–1042Crossref, Medline, Google Scholar
34. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC: The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl 20):22-33Google Scholar
35. Sachs GS, Guille C, McMurrich SL: A clinical monitoring form for mood disorders. Bipolar Disord 2002; 4:323–327Crossref, Medline, Google Scholar
36. Montgomery SA, Åsberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
37. Young RC, Biggs JT, Ziegler VE, Meyer DA: A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
38. Lavori PW, Dawson R, Rush AJ: Flexible treatment strategies in chronic disease: clinical and research implications. Biol Psychiatry 2000; 48:605–614Crossref, Medline, Google Scholar



