Lamina-Specific Reductions in Dendritic Spine Density in the Prefrontal Cortex of Subjects With Schizophrenia
Abstract
OBJECTIVE: In a previous study the authors found that dendritic spine density was reduced on prefrontal pyramidal neurons in layer 3 of subjects with schizophrenia. From a neural circuitry perspective, understanding the pathophysiological significance of this finding requires knowledge of whether pyramidal neurons in other cortical layers are similarly affected. The authors’ goal was to determine whether their finding in layer 3 was also present in other cortical layers in the same group of subjects with schizophrenia. METHOD: Spine density and other dendritic measures were made for pyramidal neurons in layers 5 and 6 of prefrontal area 46 in the brains of deceased subjects with schizophrenia, subjects with other psychiatric disorders, and normal comparison subjects. RESULTS: None of the dendritic measures for layer 5 or 6 pyramidal neurons differed across the subject groups, but the within-subject differences in spine density between deep layer 3 and layer 5 or 6 pyramidal neurons were significantly greater in the patients with schizophrenia than in the comparison subjects. CONCLUSIONS: These findings are consistent with the idea that prefrontal pyramidal neurons involved in corticocortical and/or thalamocortical connections are preferentially affected in schizophrenia.
Dysfunction of the dorsolateral prefrontal cortex is thought to reflect abnormalities in the synaptic connectivity of this region in subjects with schizophrenia (1, 2). Consistent with this interpretation, we reported a reduced density of dendritic spines, the location of most excitatory inputs to pyramidal neurons, on dorsolateral prefrontal cortex pyramidal neurons in deep layer 3 of subjects with schizophrenia (3).
Because pyramidal neurons in each cortical layer tend to differ in their extrinsic connections (4), understanding the pathophysiological significance of these findings from a neural circuitry perspective requires knowledge of the laminar specificity of the reductions in spine density. Consequently, in this study we determined spine density on the basilar dendrites of pyramidal neurons in layers 5 and 6 of the same subjects.
Method
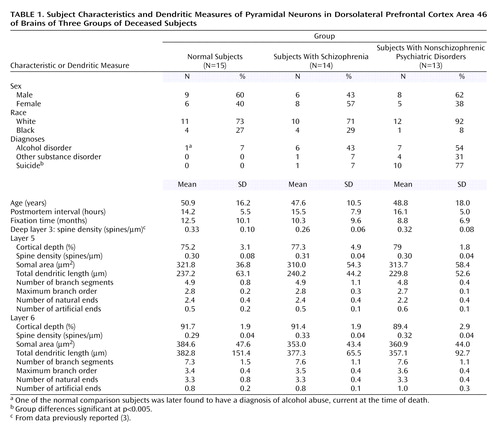
We used the same tissue sections containing dorsolateral prefrontal cortex area 46 from the brains of 15 deceased subjects with schizophrenia, 15 deceased normal comparison subjects, and 15 deceased subjects with other psychiatric disorders included in our previous Golgi study (3). However, for technical reasons, one subject with schizophrenia and two subjects with other psychiatric disorders could not be studied. All 14 of the remaining subjects with schizophrenia had a history of treatment with antipsychotic agents, as did nine of the 13 remaining psychiatric comparison subjects. The final subject groups did not differ significantly in sex, race, age, postmortem interval, or tissue fixation time (Table 1).
For each subject, Nissl-stained sections from adjacent tissue blocks were examined to determine the borders of layer 5 and layer 6 in area 46 as a percentage of the total cortical thickness. On the basis of these findings, neurons located between 65% and 80% or between 85% and 100% of the distance from the pial surface were considered to be located in layer 5 or layer 6, respectively. Inclusion criteria for Golgi-impregnated neurons were identical to those previously described (3), except in layer 6, where neurons with a modified pyramidal morphology were included. We attempted to reconstruct 15 neurons in both layers 5 and 6 in each subject, but this was not possible in some subjects because of overlying artifacts. Consequently, we report the findings for 42 subjects, independent of the number of neurons counted in each subject, and for the subset of subjects (10 per group) in whom 15 neurons were sampled.
For each selected neuron, we reconstructed the longest basilar dendrite including all branches using Neurolucida software (MicroBrightfield, Williston, Vt.) and an oil-immersion ×100 objective at a final on-screen magnification of ×4650. For each basilar dendrite and its branches, the mean diameter and total length, the number of spines, the total number of dendritic segments, and the maximum branch order of the dendrites were determined (3). Each dendritic branch was recorded as having either a natural end or an artificial end (i.e., cut dendrite). The cross-sectional area of each cell body was determined by tracing its outline. All neurons were reconstructed by the same investigator (N.K.) without knowledge of the subject number or the diagnostic group.
Identical statistical analyses were conducted for all 42 subjects and for the subset of subjects for whom data from 15 neurons were available. The primary model employed multivariate analysis of covariance with diagnostic group as the main effect and age, gender, postmortem interval, and storage time as covariates. In addition, within-subject differences in spine density (and other response variables) between layer 5 or 6 and deep layer 3 were compared by using analysis of covariance with diagnostic group as the main effect and the same covariates.
Results
The location of the reconstructed neurons, assessed as a percentage of cortical depth, did not differ across subject groups (Table 1), indicating that comparable populations of pyramidal neurons were sampled in each subject group. In layer 5, neither spine density, somal area, nor any of the dendritic parameters differed across subject groups, either for all of the subjects (Table 1) (F<1.5, df=2, 35, p>0.25) or for those with 15 neurons available (F<0.8, df=2, 23, p>0.47). Similarly, no between-group differences in any of these measures were found in layer 6 for either all of the subjects (Table 1) (F<1.27, df=2, 35, p>0.29) or those with 15 neurons available (F<0.78, df=2, 23, p>0.47).
To determine whether the reduction in spine density previously observed in deep layer 3 of the subjects with schizophrenia was specific to that laminar location, we compared this measure across layers within subjects. There were significantly greater within-subject differences in spine density between deep layer 3 and layer 5 (F=17.6, df=1, 23, p=0.0003) and between deep layer 3 and layer 6 (F=7.33, df=1, 23, p=0.01) pyramidal neurons in the subjects with schizophrenia than in the normal comparison subjects. In contrast, the normal comparison subjects and those with schizophrenia did not differ in the between-layer measures for dendritic length (F<2.7, df=1, 23, p>0.11) or somal area (F<0.1, df=1, 23, p>0.79).
Discussion
In contrast to our findings (3) and those of others (5) of reduced spine density of pyramidal neurons in deep layer 3 of subjects with schizophrenia, neither spine density nor any other measures of basilar dendrites were altered on pyramidal neurons in layers 5 or 6 of area 46. These findings are consistent with the idea that neuronal morphological alterations in schizophrenia may be restricted to, or are at least more prominent in, pyramidal neurons located in deep layer 3. This interpretation is supported by reports of reduced pyramidal cell somal volume in deep layer 3, but not in layer 5, of dorsolateral prefrontal cortex area 9 (6, 7).
The axons of pyramidal neurons in layer 3 project principally to other cortical association areas, although a minority project to the striatum, whereas the proportions of neurons furnishing these projections are reversed for pyramidal neurons in layer 5 (4). In addition, neurons in layer 6 and, to a lesser extent, in layer 5 project to the thalamus, whereas neurons in layer 3 do not. Finally, the axonal projections from the mediodorsal nucleus of the thalamus densely innervate deep layer 3 but only pass through layers 5 and 6, where they exhibit few synaptic boutons (8). Thus, it would appear that the anatomical substrate for corticocortical and/or thalamocortical connections may be more disturbed than that for corticostriatal or corticothalamic connections, at least in this group of subjects with schizophrenia.
Consistent with the idea of altered thalamocortical circuitry in schizophrenia, some (but not all) studies have found a reduced number of neurons in the mediodorsal nucleus of the thalamus in subjects with schizophrenia (see reference 9 for review). Since the basilar dendrites of dorsolateral prefrontal cortex pyramidal neurons in deep layer 3 are major targets of the cortical projections from the mediodorsal thalamus, a reduction in spine density restricted to these layers might be expected.
The apparent specificity of the spine changes to pyramidal neurons in layer 3 has interesting implications for developmental hypotheses of schizophrenia that propose abnormalities in the adolescence-related pruning of exuberant synapses produced earlier in development (10). In the monkey dorsolateral prefrontal cortex, the overproduction and subsequent pruning of synapses are most prominent in layers 1–3 and less marked in layers 5 and 6 (11). Thus, the reduction of spine density in schizophrenia on pyramidal neurons in layer 3, and not those in layers 5 and 6, may be related to these laminar differences in synaptic pruning. For example, excessive synaptic pruning in schizophrenia may result from deficits in the expression of genes that encode proteins involved in the machinery of neurotransmitter release, giving rise to synapses with reduced efficacy of neurotransmission and thus a greater susceptibility to pruning (1). The present findings suggest that such aberrant pruning may be most marked on neurons with the greatest dynamic range in synaptic number during postnatal development.
 |
Received June 21, 2004; revision received Aug. 5, 2004; accepted Aug. 19, 2004. From the Departments of Psychiatry, Neuroscience, and Statistics, University of Pittsburgh. Address correspondence and reprint requests to Dr. Lewis, Department of Psychiatry, W1651 BST, 3811 O’Hara St., Pittsburgh, PA 15213; [email protected] (e-mail). Supported by NIMH Conte Center for the Neuroscience of Mental Disorders grant MH-45156.
1. Mirnics K, Middleton FA, Lewis DA, Levitt P: Analysis of complex brain disorders with gene expression microarrays: schizophrenia as a disease of the synapse. Trends Neurosci 2001; 24:479–486Crossref, Medline, Google Scholar
2. Selemon LD, Goldman-Rakic PS: The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 1999; 45:17–25Crossref, Medline, Google Scholar
3. Glantz LA, Lewis DA: Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 2000; 57:65–73Crossref, Medline, Google Scholar
4. Jones EG: Laminar distribution of cortical efferent cells, in Cerebral Cortex, vol 1. Edited by Peters A, Jones EG. New York, Plenum, 1984, pp 521–552Google Scholar
5. Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TRE, Hirsch SR: Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry 1998; 65:446–453Crossref, Medline, Google Scholar
6. Rajkowska G, Selemon LD, Goldman-Rakic PS: Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry 1998; 55:215–224Crossref, Medline, Google Scholar
7. Pierri JN, Volk CLE, Auh S, Sampson A, Lewis DA: Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex in subjects with schizophrenia. Arch Gen Psychiatry 2001; 58:466–473Crossref, Medline, Google Scholar
8. Erickson SE, Lewis DA: Cortical connections of the lateral mediodorsal thalamus in cynomolgus monkeys. J Comp Neurol 2004; 473:107–127Crossref, Medline, Google Scholar
9. Dorph-Petersen K-A, Pierri JN, Sun Z, Sampson AR, Lewis DA: Stereological analysis of the mediodorsal thalamic nucleus in schizophrenia: volume, neuron number, and cell types. J Comp Neurol 2004; 472:449–462Crossref, Medline, Google Scholar
10. Feinberg I: Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res 1982; 17:319–334Crossref, Medline, Google Scholar
11. Bourgeois J-P, Goldman-Rakic PS, Rakic P: Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex 1994; 4:78–96Crossref, Medline, Google Scholar



