Enhanced Cortisol Suppression Following Dexamethasone Administration in Domestic Violence Survivors
Abstract
OBJECTIVE: The authors compared responses of female domestic violence survivors and a matched group of nontraumatized participants to a low-dose (0.5 mg) dexamethasone suppression test (DST). METHOD: Seventy female domestic violence survivors and 14 nontraumatized women matched for age and race were recruited. Participants were assessed for trauma severity, severity of PTSD and depressive symptoms, and DST cortisol response. Of the domestic violence survivors who were DST-compliant, comparisons were made among those with PTSD (N=15), those with PTSD plus depression (N=27), and those with no PTSD or depression diagnosis (N=8) along with the nontraumatized comparison subjects (N=14). RESULTS: Domestic violence survivors with PTSD, regardless of whether or not they had comorbid depression, had significantly lower baseline cortisol levels at 9:00 a.m. than the healthy subjects and trauma survivors with no diagnosis. Survivors with a sole diagnosis of PTSD showed significantly greater cortisol suppression to dexamethasone than did healthy subjects or the group diagnosed with PTSD plus depression. CONCLUSIONS: These findings agree with previous studies showing hypothalamic-pituitary-adrenal (HPA) axis abnormalities in PTSD. The findings suggest that the chronic nature of domestic violence leads to a severe dysregulation of the HPA axis.
The physical and sexual violence perpetrated against women by their intimate male partners has been identified as one of our nation’s most pressing public health concerns (1, 2). Yet, survivors of domestic violence have been largely unstudied with respect to biological alterations. The study of this chronically traumatized population could shed light on some of the discrepancies emerging in the neuroendocrine literature on PTSD. For example, trauma survivors with PTSD have generally been reported to have lower 24-hour urinary cortisol excretion (3–8), but some studies have found significantly higher cortisol excretion (9–12). It has been suggested that the disparate observations regarding cortisol levels in PTSD may reflect, among other things, the nature of the traumatic event that gave rise to PTSD, comorbid diagnoses, and gender.
There have been fewer discrepant findings in studies of PTSD that used a low-dose dexamethasone suppression test (DST). Although the majority of these studies have been performed with combat veterans, this test has provided relatively consistent findings of enhanced cortisol suppression after dexamethasone administration in other trauma populations with PTSD, including childhood sexual abuse victims (13), Desert Storm veterans (14), and adolescent earthquake survivors (15). Initial observations in Vietnam combat veterans showed that there is enhanced cortisol suppression during the low-dose (0.5 mg) dexamethasone suppression test in participants with PTSD (6, 16).
In the present study, we sought to examine negative feedback inhibition using the low-dose DST test in women subjected to domestic violence. This group is somewhat different from people with PTSD derived from a discrete event because it is typical for women who have been subjected to domestic violence to have been chronically traumatized over an extended period of time. Domestic violence survivors are also interesting to study in the context of biologic alterations in PTSD because they have an extremely high prevalence of comorbid depression. A recent meta-analysis of domestic violence survivors calculated a weighted mean prevalence of 64% for PTSD and 48% for depression (17).
In view of the different findings of negative feedback inhibition in PTSD and major depressive disorder, we hypothesized that negative feedback inhibition would be more enhanced in survivors of domestic violence with PTSD, but that women with both PTSD and depression might show a weaker negative feedback inhibition relative to comparison subjects.
Method
Participants
Seventy female domestic violence survivors (mean age=35.7 years [SD=8.1, range=18–51]; African American: 61% [N=43], white: 33% [N=23], other: 6% [N=4]) were recruited from established domestic violence shelters (N=36 [51%]) or were nonshelter participants recruited from community agencies (N=34 [49%]). In addition, we recruited 14 nontraumatized women matched for age and race (mean age=33.0 years [SD=8.4, range=18–48]; African American: 50% [N=7], white: 43% [N=6], other: 7% [N=1]) from the community. The nontraumatized comparison subjects denied experiencing any PTSD criterion A events, and major depression was ruled out with the Beck Depression Inventory.
After complete description of the study to the participants, written informed consent was obtained. Women were eligible to participate in the study if they had been the victims of battering by an intimate partner and they had been in the intimate relationship for at least 3 months. In addition, the last episode of battering had to be within 6 months of the assessment.
Battering was defined by responses on the revised Conflict Tactics Scale–2 (18) as four or more incidents of minor violence (e.g., being pushed, shoved, or slapped; having their arm twisted or hair pulled; or having objects thrown at them), two or more severe incidents (e.g., being punched with a fist, choked, slammed into a wall, thrown down stairs, kicked, threatened or attacked with a weapon, or forced to have sex as well as any event that caused them to fear for their life or the lives of a family member), or any combination of four or more minor and severe incidents during the past year of the relationship.
Exclusionary criteria were apparent psychosis, intoxication, mental retardation, or illiteracy to ensure that informed consent could be given. A second exclusion was having a body mass index >35 to ensure that the dose of dexamethasone would have a comparable effect in all participants. Finally, women were excluded if they were taking any medication known to interfere with the DST such as oral corticosteroids.
Analyses were performed on this original group of 70 domestic violence survivors, and several additional criteria were used to define the other main analyses in this paper. A subset of 25 women were excluded from analyses of dexamethasone suppression because of issues of dexamethasone administration compliance. Reasons included improper dexamethasone administration (N=4) for taking dexamethasone earlier or later than instructed (as self-reported) or dexamethasone levels undetectable in bloodstream (N=21), which was an indication that they did not take the dexamethasone at all. The remaining 45 DST-compliant participants were included in analyses of the level of cortisol suppression to dexamethasone. A separate set of analyses examined the DST-compliant versus DST-noncompliant participants on clinical variables.
Clinical Instruments
All clinical interviews were administered by master’s- or doctoral-level clinicians experienced in working with trauma survivors.
PTSD symptoms were evaluated with the Clinician-Administered PTSD Scale (19), which gives both a continuous score of symptom frequency and intensity as well as the ability to make diagnostic determinations of PTSD status. Because of the chronic nature of domestic violence in which women typically report many incidents that might be legitimate criterion A events, the Clinician-Administered PTSD Scale assessment was based upon the women’s self-reported most traumatic event. The women in this study did not appear to have difficulty identifying a focal trauma as the most traumatic event from the relationship.
The Structured Clinical Interview for DSM-IV (SCID) (20) has been widely used in research to assess for psychopathology and consists of interviewer-based modules for different disorders. In this study, the mood disorders module was used to assess major depression.
The revised Conflict Tactics Scale–2 (18) is a 33-item self-report scale that has been widely used to measure the level of conflict among couples. Subscale scores provide information about physical aggression, psychological aggression, and injury. Internal consistency for these subscales is reported to be very good to excellent, ranging from 0.86 to 0.95.
The Beck Depression Inventory (21) is a 21-item self-report questionnaire widely used in research on depression (22). It has previously been used to assess depression in battered women (23). Split-half reliability reported by Beck was 0.93.
Blood Sampling and Low-Dose Dexamethasone Suppression Test
Blood samples were collected on an outpatient basis at the Center for Trauma Recovery by a registered nurse. Baseline blood samples were collected via venipuncture of the arm between 9:00–9:15 a.m. on day 1. Participants were given a 0.5-mg dose of dexamethasone to take at home that evening at 11:00 p.m.. This relatively low dose was used to avoid a “floor” effect in cortisol suppression based upon previous studies indicating that a hypersuppression of cortisol could be expected in a traumatized group (6, 16). Attempts were made to call participants on the phone the evening that they were to take the dexamethasone as a reminder. Participants returned the next morning, and blood was drawn again between 9:00–9:15 a.m. Immediately following blood collection samples were centrifuged at 300 g for 10 minutes and plasma was stored at –70°C for later assay.
Cortisol and Dexamethasone Assays
All plasma samples were sent to the laboratory of one of the authors (R.Y.) for assay of cortisol and dexamethasone levels. All assays were conducted blind to diagnostic status or group. Plasma cortisol levels were determined in both day 1 and day 2 samples by radioimmunoassay using a commercially available kit (IncStar Inc., Minneapolis) as previously described (5, 24) (inter- and intraassay coefficients of variation=6.8% and 4.0%, respectively). Dexamethasone levels were determined on the day 2 samples by radioimmunoassay using a commercially available antibody (IgG Corporation, Nashville, Tenn.) as previously described (25).
Measurement of dexamethasone levels was particularly important to ensure compliance with instructions to take the dexamethasone at home. Findings from the dexamethasone assays revealed that 21 participants failed to take the dexamethasone at all and four more participants did not take it as instructed. These participants were not included in the main data analyses, but their clinical data were compared to the DST-compliant participants to determine if there were any significant differences.
Statistical Analysis
Group comparisons were made by using analysis of covariance (ANCOVA) with repeated measures (pre- versus postdexamethasone plasma cortisol levels with dexamethasone level as a covariate). Pearson product-moment correlations with Bonferroni corrections for multiple comparisons were used to examine linear relationships between variables. All statistical tests were two-tailed with alpha level set at 0.05 for statistical significance or for some analyses adjusted for multiple comparisons to 0.01. Observation of significant analysis of variance (ANOVA) findings were followed up with post hoc analyses using Tukey’s honestly significant difference procedure when more than two groups were involved.
Results
This was a severely and chronically victimized group that averaged 5 years of abuse. Overall, the level of physical violence was quite severe. Examination of the frequency of physical violence revealed that the women had suffered bruises to the head (81%), bruises to the body (84%), broken bones in the head (17%), broken bones in the body (22%), cuts to the face or head (64%), cuts to the body (41%), loss of consciousness (49%), damage to the teeth (26%), ruptured eardrums (19%), damage to internal organs (12%), transmission of sexually transmitted diseases from their abusive partner (25%), and miscarriages (13%) as a result of the violence.
Clinical interviews revealed that this group of domestic violence survivors was also a highly symptomatic group, with 38 (54.3%) out of 70 meeting criteria for major depression. In addition, 62 (88.6%) met criteria for full PTSD (N=52 [74.3%]) or subthreshold PTSD (N=10 [14.3%]). Subthreshold PTSD was defined here as participants who were a total of one symptom short of full PTSD (N=8) or two symptoms short but with a total Clinician-Administered PTSD Scale score over 45 (N=2). Previous research has demonstrated the diagnostic utility of using a total Clinician-Administered PTSD Scale score in this manner (26). Hereafter the term “PTSD” will be used to include those participants diagnosed with full and subthreshold PTSD. In the total group there were 25 (36%) out of 70 with PTSD only, 37 (53%) with comorbid PTSD and major depression, and eight (11%) with neither diagnosis. The clinical data from these three groups are presented in Table 1.
In the DST-compliant group, 42 (93%) out of 45 met criteria for full PTSD (N=36 [80%]) or subthreshold PTSD (N=6 [13%]), and 28 (62%) out of 45 met criteria for major depression. Of the DST-compliant subgroup with PTSD, there were a total of 15 participants with PTSD only and 27 with comorbid PTSD and major depression. There were only three compliant participants who did not have either diagnosis.
Overall there were no demographic or abuse characteristic differences between the three groups except for a tendency for the group with comorbid PTSD and depression having been the victim of a higher level of psychological aggression than those in the no diagnosis group. However this difference did not exceed our adjusted alpha level of 0.01 for multiple comparisons. There were significant group differences in total and subscale scores on the Clinician-Administered PTSD Scale. Significant group differences are noted in Table 1 but generally followed the tendency of the no diagnosis group having the lowest scores and the comorbid group displaying the highest scores. As expected, the PTSD-only group and the comorbid group had significantly higher scores on the Beck Depression Inventory than the no diagnosis group.
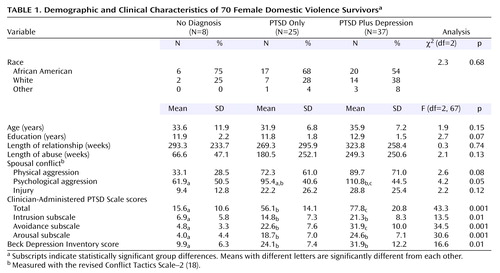
Mean plasma cortisol and dexamethasone levels for the domestic violence survivors with PTSD, PTSD plus depression, and no diagnosis and the nontraumatized healthy comparison subjects as well as the percent of cortisol suppression from day 1 to day 2 are presented in Table 2. Figure 1 presents the individual cortisol values for the subjects with PTSD, PTSD plus depression, and the healthy comparison group. Among the trauma survivors there were only three participants with no diagnosis who took dexamethasone, therefore they were not included in this graph or statistical analyses. A two-way ANCOVA examining the effect of day (day 1 [before DST], day 2 [after DST]) and group (PTSD, PTSD plus depression, healthy nontraumatized) on cortisol levels with dexamethasone level entered as a covariate revealed a significant day-by-group interaction effect (F=15.2, df=2, 52, p<0.001). Follow-up analyses of cortisol levels indicated a significant group difference on day 1 (F=32.8, df=2, 53, p<0.001); Tukey’s honestly significant difference test showed that the healthy group had a significantly higher baseline cortisol level than the two domestic violence groups, which did not differ from each other. A separate one-way ANOVA of only the day 1 cortisol values for all subjects (trauma survivors with PTSD [N=25], PTSD plus depression [N=37], and no diagnosis [N=8] and nontraumatized comparison subjects [N=14]) confirmed this finding in the larger study group (F=30.9, df=3, 80, p<0.001). Post hoc analyses revealed that baseline cortisol levels were not significantly different between the trauma survivors with PTSD (mean=6.6 μg/dl, SD=2.6) and those with PTSD plus depression (mean=7.7, SD=2.7) and that both were significantly lower than the baseline cortisol levels of the trauma survivors with no diagnosis (mean=17.7, SD=8.3) and the nontraumatized comparison group (mean=15.2, SD=4.3), which did not differ statistically from each other. Figure 2 shows a plot of the individual day 1 cortisol values against the total Clinician-Administered PTSD Scale score for all of the domestic violence survivors.
Analysis of cortisol level on day 2 after dexamethasone administration in the DST-compliant group showed that there was also a significant group difference (F=8.0, df=2, 53, p<0.001) such that the PTSD plus depression and healthy comparison groups did not differ from each other. Trauma survivors with a sole diagnosis of PTSD had significantly lower day 2 cortisol levels than did the healthy group but missed being significantly lower than the PTSD plus depression group (p=0.08, Tukey honestly significant difference). Analysis of dexamethasone levels across the three groups revealed no significant difference in dexamethasone metabolism.
Changes in cortisol level from day 1 to day 2 also were converted into percentages and reflected the relative amount of cortisol suppression to dexamethasone administration (Table 2). A one-way ANOVA of these percent change scores showed a tendency toward a greater percentage change in those subjects with a sole diagnosis of PTSD (F=2.7, df=2, 53, p=0.07).
Additional analyses were conducted that focused on the PTSD and comorbid depression group in an attempt to elucidate the role of depression in the hypothalamic-pituitary-adrenal (HPA) axis alterations. This group was split on the basis of Beck Depression Inventory score into those with more severe depression (score >29; mean=42.6, SD=7.1) and those with less severe depression (score ≤29; mean=22.1, SD=7.1). As seen in Figure 3, group comparisons of day 1 cortisol levels revealed no significant difference (t=0.6, df=25, p=0.53). However, the less severely depressed participants had a significantly lower day 2 mean cortisol level (t=2.1, df=25, p<0.05) and produced a significantly greater amount of cortisol suppression expressed as a percentage of baseline level (t=2.1, df=25, p<0.05) than did the more severely depressed participants. Levene’s test for equality of variance in the two groups revealed a significantly greater amount of variance in the scores of the more severely depressed participants for both the day 2 cortisol values and suppression percentage (Levene’s F=19.4 and 15.6, respectively, p<0.001).
A comparison of women living in domestic violence shelters versus those not living in shelters at the time of the DST revealed no significant difference in baseline cortisol values (mean=8.0 μg/dl [SD=2.8] and 8.9 μg/dl [SD=6.6], respectively). Examination of intercorrelations in the domestic violence survivors among the clinical variables from the Clinician-Administered PTSD Scale (the reexperiencing, avoidance, and arousal symptom clusters), Beck Depression Inventory scores, abuse severity variables (subscale scores from the Conflict Tactics Scale), and the endocrine measures (cortisol levels on day 1 and day 2 and cortisol suppression percentage) revealed only one significant correlation that indicated an inverse relationship between Beck Depression Inventory score and cortisol suppression percentage (r=–0.43, df=42, p=0.01).
Comparisons of the domestic violence participants who were compliant with the dexamethasone administration instructions (N=45) versus those who were noncompliant (N=25), using an adjusted alpha level for multiple comparisons (0.01), indicated that there were no significant differences in the demographic variables, abuse variables, or continuously scored clinical variables. In addition there was not a significant difference in the frequency of PTSD diagnosis (χ2=2.8, df=1, p=0.10) or major depression (χ2=1.8, df=1, p=0.18). Analysis of the baseline cortisol level from day 1 also showed no significant difference (t=0.9, df=68, p=0.37) between the compliant (mean=8.9 μg/dl, SD=5.4) and the noncompliant participants (mean=7.7 μg/dl, SD=4.0).
The noncompliant participants who did not take dexamethasone at all (N=21) provided a built-in test-retest measure of baseline cortisol levels on successive days. Specifically, the noncompliant group allowed for an examination of the consistency of the very low cortisol levels observed in the larger sample of domestic violence survivors with a psychiatric diagnosis. The correlation between day 1 and day 2 cortisol values was high (r=0.80, df=19, p<0.01), which confirmed a high degree of within-participant consistency in cortisol output as expected. Figure 4 shows a plot of the individual cortisol values on days 1 and 2 in the noncompliant group organized by psychiatric diagnosis. A one-way ANOVA on the average cortisol value from day 1 and day 2 revealed that the domestic violence survivors with PTSD and those with comorbid PTSD and depression had significantly lower baseline cortisol values than the trauma victims with no PTSD or depression diagnosis (F=13.8, df=2, 22, p<0.001). This pattern mirrored the findings in the larger sample of domestic violence survivors.
Discussion
This is the first study, to our knowledge, to report plasma cortisol response to dexamethasone in a group of battered women. The main finding from our study was that domestic violence survivors with a sole diagnosis of PTSD show a very strong hypersuppression of cortisol following administration of a low (0.5 mg) dexamethasone dose. This finding joins a growing number of previous studies showing enhanced suppression in PTSD (6, 13–16). In particular, the findings lend support to the idea of a dysregulation in the HPA axis and perhaps to enhanced negative feedback inhibition in PTSD (27, 28). The findings also provide additional evidence that women with PTSD show a similar type of dysregulation of the HPA axis as men (29).
It is important to point out that our findings of enhanced suppression were specific to those domestic violence survivors with a sole diagnosis of PTSD, since the group with comorbid PTSD and major depression displayed significantly less cortisol suppression. Indeed, the PTSD plus depression group showed cortisol suppression that was comparable to the nontraumatized comparison group, and there was a negative correlation between the level of depression as indexed on the Beck Depression Inventory and amount of cortisol suppression. Previous studies that have used the DST in trauma populations have not always shown a clear link to PTSD diagnosis but sometimes have shown that enhanced suppression is also linked to trauma group membership (13). In our study, we had a very small number of participants (N=3) who were DST compliant and did not have PTSD or depression. While this group was small, the observed cortisol response to dexamethasone in these participants looked very much like the response observed in the nontraumatized comparison group.
The analysis of the comorbid PTSD and depression group indicated that those participants with severe depression showed less cortisol suppression as a group. However, inspection of Figure 3 also reveals that there was greater variability in the response of this group to dexamethasone and that some individuals who were severely depressed still showed very strong cortisol suppression. More data are needed to uncover the role of comorbid depression in the dysregulation of HPA axis.
The other main finding was the extremely low baseline cortisol level in these domestic violence survivors with a diagnosis of PTSD or PTSD and depression. The mean morning level on day 1 of the DST was only about half the level observed in our nontraumatized comparison group and our group of trauma survivors with no diagnosis. The finding of low baseline cortisol in trauma victims replicates this finding from a number of the other DST studies (6, 14, 15), but not all previous studies have shown low baseline cortisol (13, 16).
The DST in this study was conducted on an outpatient basis, and therefore blood samples were drawn about 1 hour later than has typically been reported in the literature (9:00 a.m. instead of 8:00 a.m.). This is normally a time of day when the circadian pattern for cortisol release should be reaching peak levels. These low levels must be interpreted with some caution, since this is a single measurement rather than an aggregate over an entire circadian cycle, but these findings deserve to be assessed again in a more thorough manner in a sample of domestic violence survivors. In our DST-noncompliant subjects we did see that there was consistency in the low baseline levels at least on consecutive morning measurements. Unfortunately, we did not collect any information about the sleep/wake cycle of these women. Approximately half of the women were living in domestic violence shelters at the time of our assessment, and it may be that the normal sleep/wake cycle is altered in that environment. However, the analysis comparing women living in shelters versus those not living in shelters revealed no significant difference in baseline cortisol values. Additionally, the possibility of differential dexamethasone metabolism between groups is critical to the interpretation of DST studies (25). However, in this study that was not an issue because we did not observe significant differences in dexamethasone levels between our groups.
We found extremely high rates of PTSD and depression in this sample of battered women. These high rates were likely due, at least partially, to the stringent abuse criteria that we used for admission to the study but also reflect the highly traumatic nature of domestic violence (30, 31). Our rates of psychiatric disorder are in general agreement with previous studies of domestic violence survivors (17).
There were several implications to having such a traumatized and symptomatic sample. First, we had only a very small group of domestic violence victims with no diagnosis to compare to our victims with PTSD and PTSD plus depression. Second, this group may not have had a wide enough range of scores on a number of variables to allow us to see correlations between variables. For example, some previous studies have found a significant linear relationship between trauma symptom severity and baseline cortisol levels or cortisol suppression to dexamethasone (14, 15). However, other studies with a greater restriction of range on trauma severity, which leads to a more homogeneous group of severely traumatized subjects, have not found a significant linear relationship (6, 13).
Inspection of Figure 1 reveals that members of the PTSD-only group uniformly had extremely low postdexamethasone cortisol values. This raises the possibility of a “floor effect” in terms of the ability to achieve even greater suppression. It would be interesting to repeat this study using 0.25 mg dexamethasone instead of 0.50 mg. In the only previous study using this lower dose of dexamethasone in combat veterans (6), it was found that the lower dose of dexamethasone produced a difference in cortisol suppression between groups of a greater magnitude than the higher dose.
The DST in this study was conducted on an outpatient basis and therefore required that participants remember to take the dexamethasone at home at 11:00 p.m. We clearly had a sizable portion of the sample (36%) who were not compliant with these instructions. Analyses of the clinical data and baseline cortisol data suggested that there were no significant differences between the DST-compliant and DST-noncompliant groups. However, there was clearly a cost in terms of losing power. Reminder calls were regularly made to participants, but it was not always possible to reach participants; this may be an unavoidable issue when dealing with a domestic violence sample.
In summary, our findings suggest that the chronic nature of domestic violence may produce profound neuroendocrine alterations in the HPA axis that are as severe as any reported in the literature.
 |
 |
Received Sept. 10, 2003; revision received April 19, 2004; accepted July 8, 2004. From the Department of Psychology and Center for Trauma Recovery, University of Missouri-St. Louis; the National Center for PTSD, Veterans Affairs Boston Healthcare System, Boston; the Psychiatry Department, Mount Sinai School of Medicine, New York; and the Division of Traumatic Stress Studies, Bronx Veterans Affairs Medical Center, Bronx, N.Y. Address correspondence and reprint requests to Dr. Griffin, University of Missouri-St. Louis, Center for Trauma Recovery, Kathy J. Weinman Bldg., 8001 Natural Bridge Rd., St. Louis, MO 63121; [email protected] (e-mail). Supported by NIMH grant MH-55542 (Dr. Resick).
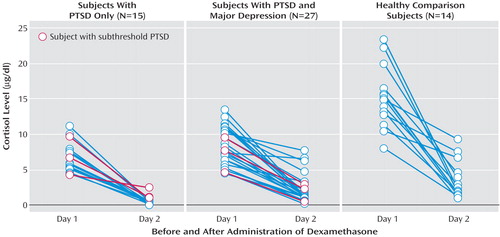
Figure 1. Individual Cortisol Blood Levels in Female Survivors of Domestic Violence and Demographically Matched Nontraumatized Women Before and After a Low-Dose (0.5 mg) Dexamethasone Suppression Test
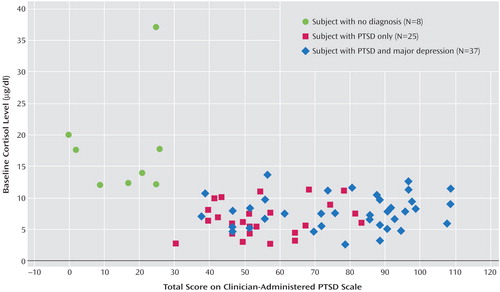
Figure 2. Baseline Cortisol and PTSD Severity Levels for 70 Female Domestic Violence Survivors
Figure 3. Cortisol Measurements for Female Domestic Violence Survivors With PTSD and Comorbid Major Depression, by Depression Severitya
aHorizontal black bars represent mean levels for each condition.
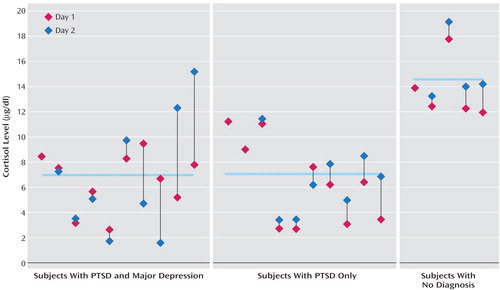
Figure 4. Individual Cortisol Values for Female Domestic Violence Survivors Noncompliant With a Low-Dose (0.5 mg) Dexamethasone Suppression Test, by Diagnosisa
aDay 2 scores are repeat measurements of baseline morning cortisol levels for participants who did not take dexamethasone (N=21). The four participants with only a day 1 value took dexamethasone later or earlier than directed. Horizontal bars represent group mean values.
1. Biden JR: Violence against women: the congressional response. Am Psychol 1993; 48:1059–1061Crossref, Medline, Google Scholar
2. Goodman LA, Koss MP, Russo NF: Violence against women: physical and mental health effects, II: research findings. Appl Prev Psychol 1993; 2:79–89Crossref, Google Scholar
3. Mason JW, Giller EL, Kosten TR, Ostroff RB, Podd L: Urinary free-cortisol levels in posttraumatic stress disorder patients. J Nerv Ment Dis 1986; 174:145–149Crossref, Medline, Google Scholar
4. Kosten TR, Wahby V, Giller E Jr, Mason J: The dexamethasone suppression test and thyrotropin-releasing hormone stimulation test in posttraumatic stress disorder. Biol Psychiatry 1990; 28:657–664Crossref, Medline, Google Scholar
5. Yehuda R, Southwick SM, Nussbaum G, Wahby VS, Giller EL, Mason JW: Low urinary cortisol excretion in patients with posttraumatic stress disorder. J Nerv Ment Dis 1990; 178:366–369Crossref, Medline, Google Scholar
6. Yehuda R, Boisoneau D, Lowy MT, Giller EL: Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry 1995; 52:583–593Crossref, Medline, Google Scholar
7. Yehuda R, Bierer LM, Schmeidler J, Aferiat DH, Breslau I, Dolan S: Low cortisol and risk for PTSD in adult offspring of Holocaust survivors. Am J Psychiatry 2000; 157:1252–1259Link, Google Scholar
8. Thaller V, Vrkljan M, Hotujac L, Thakore J: The potential role of hypercortisolism in the pathophysiology of PTSD and psoriasis. Coll Antropol 1999; 23:611–619Medline, Google Scholar
9. Pitman RK, Orr SP: Twenty-four hour urinary cortisol and catecholamine excretion in combat-related posttraumatic stress disorder. Biol Psychiatry 1990; 27:245–247Crossref, Medline, Google Scholar
10. Lemieux AM, Coe CL: Abuse-related posttraumatic stress disorder: evidence for chronic neuroendocrine activation in women. Psychosom Med 1995; 57:105–115Crossref, Medline, Google Scholar
11. Maes M, Lin A, Bonaccorso S, van Hunsel F, Van Gastel A, Delmeire L: Increased 24-hour urinary cortisol excretion in patients with post-traumatic stress disorder and patients with major depression, but not in patients with fibromyalgia. Acta Psychiatr Scand 1998; 98:328–335Crossref, Medline, Google Scholar
12. De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM: AE Bennett Research Award: developmental traumatology, part I: biological stress symptoms. Biol Psychiatry 1999; 45:1259–1270Crossref, Medline, Google Scholar
13. Stein MB, Yehuda R, Koverola C, Hanna C: Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol Psychiatry 1997; 42:680–686Crossref, Medline, Google Scholar
14. Kellner M, Baker DG, Yehuda R: Salivary cortisol in Operation Desert Storm returnees. Biol Psychiatry 1997; 42:849–850Crossref, Medline, Google Scholar
15. Goenjian AK, Yehuda R, Pynoos RS, Steinberg AM, Tashjian M, Yang RK, Najarian LM, Fairbanks LA: Basal cortisol, dexamethasone suppression of cortisol, and MHPG in adolescents after the 1988 earthquake in Armenia. Am J Psychiatry 1996; 153:929–934Link, Google Scholar
16. Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW: Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry 1993; 150:83–86Link, Google Scholar
17. Golding JM: Intimate partner violence as a risk factor for mental disorders: a meta-analysis. J Fam Violence 1999; 14:99–132Crossref, Google Scholar
18. Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB: The revised Conflict Tactics Scale (CTS2). J Fam Issues 1996; 17:283–316Crossref, Google Scholar
19. Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM: The development of a Clinician-Administered PTSD Scale. J Trauma Stress 1995; 8:75–90Crossref, Medline, Google Scholar
20. First MB, Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-IV—Patient Edition (SCID-P). Washington, DC, American Psychiatric Press, 1995Google Scholar
21. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
22. Beck AT, Steer RA, Garbin MG: Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988; 8:77–100Crossref, Google Scholar
23. Campbell J: A test of two explanatory models of women’s responses to battering. Nurs Res 1989; 38:18–24Crossref, Medline, Google Scholar
24. Yehuda R, Lowy MT, Southwick SM, Shaffer D, Giller EL Jr: Lymphocyte glucocorticoid receptor number in posttraumatic stress disorder. Am J Psychiatry 1991; 148:499–504Link, Google Scholar
25. Lowy MT, Meltzer HY: Dexamethasone bioavailability: implications for DST research. Biol Psychiatry 1987; 22:373–385Crossref, Medline, Google Scholar
26. Orr SP: Psychophysiologic reactivity to trauma-related imagery in PTSD: diagnostic and theoretical implications of recent findings. Ann NY Acad Sci USA 1997; 821:114–124Crossref, Medline, Google Scholar
27. Yehuda R: Biology of posttraumatic stress disorder. J Clin Psychiatry 2000; 61:14–21Medline, Google Scholar
28. Yehuda R: Current status of cortisol findings in post-traumatic stress disorder. Psychiatr Clin North Am 2002; 25:341–368Crossref, Medline, Google Scholar
29. Heim C, Ehlert U, Rexhausen J, Hanker JP, Hellhammer DH: Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosom Med 1998; 60:309–318Crossref, Medline, Google Scholar
30. Houskamp BM, Foy DW: The assessment of posttraumatic stress disorder in battered women. J Interpers Violence 1991; 6:367–375Crossref, Google Scholar
31. Astin MC, Lawrence KJ, Foy DW: Posttraumatic stress disorder among battered women: risk and resiliency factors. Violence Vict 1993; 8:17–28Medline, Google Scholar



