Association of Genetic Variants in Alcohol Dehydrogenase 4 With Alcohol Dependence in Brazilian Patients
Abstract
OBJECTIVE: The authors evaluated the association of three functional promoter polymorphisms of the ADH4 gene with alcohol dependence. METHOD: DNA from 92 alcohol-dependent patients and 92 healthy comparison subjects was genotyped for all three polymorphisms. RESULTS: Variants at the –75 base-pair (bp) (C allele) and –159 bp (A allele) positions were associated with alcohol dependence. Individuals with haplotypes carrying both risk alleles showed an odds ratio of 2.9. CONCLUSIONS: These preliminary results suggest that ADH4 may play a role in the etiology of alcohol dependence. The association requires further study and replication but is functionally plausible and has a large effect size.
Alcohol metabolism strongly influences drinking behavior and the likelihood of being dependent (1). Most ethanol is oxidized to acetaldehyde and acetate, mainly by alcohol dehydrogenases (ADH types 1–4) and aldehyde dehydrogenase (ALDH) (2). Functional allelic variants of ADH1B (*2) and ADH1C (*1) giving ADHs with higher maximum velocity of an enzyme-catalyzed reaction for a given value of enzyme concentration reduce the risk for alcohol dependence (3–5).
The gene for ADH4 (ADH4/*ADH) is mapped to 4q22 (6). Human ADH4 enzyme is found mainly in the liver, and at intoxicating levels of alcohol it may account for as much as 40% of the total ethanol oxidation rate (7). Edenberg et al. (8) reported three polymorphisms in the promoter of ADH4: –192 base-pair (bp) (T/A), –159 bp (G/A), and –75 bp (A/C). These authors analyzed the effects of four different haplotypes (T,A,C; T,G,A; A,G,C; and T,A,A) on gene expression. Two promoters with A at –75 bp had higher activity than the promoters with C at the same position. Edenberg et al. hypothesized that the –75A allele should have a protective effect against alcohol dependence like the protective effect of the alleles of ADH1B and ADH1C genes with higher maximum velocity.
To our knowledge, this is the first report of an association study with these polymorphisms comparing a group of Brazilian alcohol-dependent patients with a group of normal subjects.
Method
Ninety-two patients with ICD-10-defined alcohol dependence were ascertained at the Institute of Psychiatry, Sao Paulo. Fifty-one of the patients were men and 41 were women; their mean age was 47.3 years (SD=9.7). The 92 comparison subjects were enrolled at the Human Genome Research Center, University of Sao Paulo. Fifty-one of the comparison subjects were men, and 41 were women; their mean age was 45.3 years (SD=11.7). All comparison subjects were screened with CAGE questionnaire (9) for potential alcohol-related disorders. The recommended cutoff score on this instrument for alcohol problems is 2, but we adopted a score of 1 for the selection of the comparison subjects. The healthy subjects were matched for ethnic background with the patients: 76% were Caucasian, and 24% were African Brazilian. All subjects gave written informed consent.
Genomic DNA was extracted from whole blood according to standard procedures (10) and submitted to polymerase chain reaction (PCR) amplification (8). The PCR products were sequenced using ABI-BigDye kit on an Applied Biosystems Model 377 (Applied Biosystems, Foster City, Calif.). Fifty healthy individuals were typed for ADH1B polymorphism as described elsewhere (3).
We used both chi-square tests and Fisher’s exact tests to determine the significance of differences between groups. GENECOUNTING software (version 1.3, March 2003) (11) was used to estimate the haplotype frequencies and to evaluate pairwise measures of linkage disequilibrium with Cramer’s V statistics. Corresponding odds ratios were calculated by using the method of Woolf (12).
Results
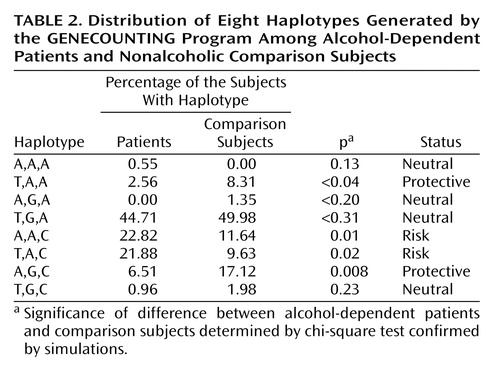
Genotype frequencies for the ADH4 gene among alcohol-dependent patients and comparison subjects were significantly different for the –75 bp and –159 bp polymorphisms. The differences were still significant after taking ethnicity into account (Table 1) and after logistic regression analyses controlling for ethnicity were carried out (data not shown). No association was observed for the –192 bp polymorphism (p>0.05). The homogeneity of the study group for the ADH4 polymorphisms allows the grouping of the data from the two ethnic groups. The –75C allele (odds ratio=1.6, 95% CI=2.4–1.05, p<0.05) and the –159A allele (odds ratio=2.2, 95% CI=3.3–1.4, p<0.001) were associated with alcohol dependence (data not shown). The GENECOUNTING program generated eight different haplotypes among patients and comparison subjects (Table 2). The haplotypes A,A,C and T,A,C were significantly more frequent among patients (p=0.01 and p=0.02, respectively) and were considered risk haplotypes. Among comparison subjects the haplotypes T,A,A, and A,G,C were overrepresented (p=0.01 and p=0.02, respectively) and were considered protective.
Haplotypes with the allele –75C and the allele –75A were then compared. The same analysis was done with the –159A and –159G alleles and finally for haplotypes with both –75C and –159A alleles versus all others. The combination of both risk alleles on one haplotype showed an odds ratio of 2.9 (95% CI=4.73–1.89, p<0.00001).
To test linkage disequilibrium between ADH1B functional polymorphism and ADH4 single nucleotide polymorphisms (SNPs), 50 healthy individuals were genotyped. No significant linkage disequilibrium was observed among them (Cramer’s V=0.095 for –75 bp, 0.136 for –159 bp, and 0.062 for –192 bp polymorphisms) (p>0.05) (data not shown).
Discussion
Two ADH4 polymorphisms and their haplotypes were associated with greater risk of developing alcohol dependence. In our study group, individuals who carried a cytosine at –75 bp of the ADH4 gene and adenine at –159 bp were three times more likely to develop alcohol dependence. All other probable haplotypes showed a protective or neutral association. We investigated the possibility that the association of these ADH4 polymorphisms with alcohol dependence could be attributable to linkage disequilibrium with the ADH1B locus. This seems not to be the case, given the low linkage disequilibrium between the SNPs. Additionally, published data have shown that there is no significant linkage disequilibrium between the ADH1 and ADH4 loci (13).
Edenberg et al. (8) reported a higher expression for the –75A allele and suggested it should be protective against alcohol dependence. Moreover, they reported that the T,A,C and T,A,A promoters had lower and higher levels of expression, respectively. We agree with these authors because T,A,C—found mainly among patients—was a risk factor in our study and T,A,A—significantly overrepresented in the comparison group—was protective. The effect of our other risk haplotype—A,A,C—on promoter function was not measured by Edenberg et al. The only exception was that the haplotype A,G,C, reported by them to lower expression, was apparently protective in our sample.
In short, our results suggest a role for common ADH4 promoter polymorphisms in the etiology of alcoholism. Given that our study group is small, and there is a possibility of genetic stratification, these findings need to be replicated in larger and different populations. In addition, further genotyping of the other ADH gene polymorphisms will be necessary in large samples to investigate the possibility of linkage disequilibrium. However, this association is with common alleles/haplotypes and is functionally plausible.
 |
 |
Received June 20, 2003; revisions received March 12 and July 1, 2004; accepted July 28, 2004. From the Human Genome Research Center, Department of Biology, Institute of Biosciences, and GREA, Interdisciplinary Group of Studies on Alcohol and Drugs, Institute and Department of Psychiatry, Medicine Faculty; and Department of Parasitology, Institute of Biomedical Sciences, University of Sao Paulo, Brazil; and the Section of Genetics, Division of Psychological Medicine and SGDP Research Centre, Institute of Psychiatry, Kings College London, De Crespigny Park, London. Address correspondence and reprint requests to Dr. Zatz, Human Genome Research Center, Department of Biology, Institute of Biosciences, University of Sao Paulo, Rua do Matão, Travessa 13, Number 106, Cidade Universitária CEP:05508-090, Sao Paulo SP, Brazil; [email protected] (e-mail). Supported in part by the Fundação de Amparo a Pesquisa do Estado de São Paulo and Conselho Nacional de Desenvolvimento Cientifico e Tecnológico. The authors thank Constância Urbani, Patrícia Hochgraf, Antonia Cerqueira, and Elisangela Quedas.
1. Ramchandani VA, Bosron WF, Li TK: Research advances in ethanol metabolism: Pathol Biol (Paris) 2001; 49:676–682Google Scholar
2. Bosron WF, Li TK: Catalytic properties of human liver alcohol dehydrogenase isoenzymes. Enzyme 1987; 37:19–28Crossref, Medline, Google Scholar
3. Borras E, Coutelle C, Rosell A, Fernandez-Muixi F, Broch M, Crosas B, Hjelmqvist L, Lorenzo A, Gutierrez C, Santos M, Szczepanek M, Heilig M, Quattrocchi P, Farres J, Vidal F, Richart C, Mach T, Bogdal J, Jornvall H, Seitz HK, Couzigou P, Pares X: Genetic polymorphism of alcohol dehydrogenase in Europeans: the ADH2*2 allele decreases the risk for alcohol dependence and is associated with ADH3*1. Hepatology 2000; 31:984–989Crossref, Medline, Google Scholar
4. Thomasson HR, Edenberg HJ, Crabb DW, Mai XL, Jerome RE, Li TK, Wang SP, Lin YT, Lu RB, Yin SJ: Alcohol and aldehyde dehydrogenase genotypes and alcohol dependence in Chinese men. Am J Hum Genet 1991; 48:677–681Medline, Google Scholar
5. Wall TL, Carr LG, Ehlers CL: Protective association of genetic variation in alcohol dehydrogenase with alcohol dependence in Native American Mission Indians. Am J Psychiatry 2003; 160:41–46Link, Google Scholar
6. McPherson JD, Smith M, Wagner C, Wasmuth JJ, Hoog JO: Mapping of the class II alcohol dehydrogenase gene locus to 4q22 (abstract). Cytogenet Cell Genet 1989; 51:1043Google Scholar
7. Ditlow CC, Holmquist B, Morelock MM, Vallee BL: Physical and enzymatic properties of a class II alcohol dehydrogenase isozyme of human liver: pi-ADH. Biochemistry 1984; 23:6363–6368Crossref, Medline, Google Scholar
8. Edenberg HJ, Jerome RE, Li M: Polymorphism of the human alcohol dehydrogenase 4 (ADH4) promoter affects gene expression. Pharmacogenetics 1994; 9:25–30Crossref, Google Scholar
9. Masur J, Monteiro MG: Validation of the “CAGE” alcoholism screening test in a Brazilian psychiatry impatient hospital setting. Braz J Med Biol Res 1983; 16:215–218Medline, Google Scholar
10. Miller SA, Dykes DD, Polesky HF: A simple salting out procedure for extracting DNA from nucleated cells. Nucleic Acids Res 1988; 16:1215Crossref, Medline, Google Scholar
11. Zhao JH, Lissarrague S, Essioux L, Sham PC: GENECOUNTING: haplotype analysis with missing genotypes. Bioinformatics 2002; 18:1694–1695Crossref, Medline, Google Scholar
12. Woolf B: On estimating the relation between blood groups and disease. Ann Hum Genet 1955; 19:251–253Crossref, Medline, Google Scholar
13. Edman K, Maret W: Alcohol dehydrogenase genes: restriction fragment length polymorphisms for ADH4 (pi-ADH) and ADH5 (chi-ADH) and construction of haplotypes among different ADH classes. Hum Genet 1992; 90:395–401Crossref, Medline, Google Scholar



