A Multidimensional Model of Obsessive-Compulsive Disorder
Abstract
OBJECTIVE: Obsessive-compulsive disorder (OCD) is a clinically heterogeneous condition. This heterogeneity can reduce the power and obscure the findings from natural history studies to genome scans, neuroimaging, and clinical trials. The authors review the evidence supporting a multidimensional model of OCD. METHOD: Computerized and manual literature searches were performed to identify factor-analytic studies of obsessive-compulsive symptoms before data from disciplines that bear on the potential usefulness of these dimensions were considered. Selection criteria included the novelty and importance of studies and their relevance to outcomes of interest to well-informed mental health professionals. RESULTS: Twelve factor-analytic studies involving more than 2,000 patients were identified that consistently extracted at least four symptom dimensions: symmetry/ordering, hoarding, contamination/cleaning, and obsessions/checking. These dimensions were associated with distinct patterns of comorbidity, genetic transmission, neural substrates, and treatment response. The evidence supporting the hoarding dimension is particularly robust. CONCLUSIONS: The complex clinical presentation of OCD can be summarized with a few consistent, temporally stable symptom dimensions. These can be understood as a spectrum of potentially overlapping syndromes that may 1) coexist in any patient, 2) be continuous with normal obsessive-compulsive phenomena, and 3) extend beyond the traditional nosological boundaries of OCD. Although the dimensional structure of obsessive-compulsive symptoms is imperfect, this quantitative approach to phenotypic traits has the potential to advance our understanding of OCD and may aid in the identification of more robust endophenotypes. The need for a dimensional rating scale and suggestions for future research aimed at reducing the burden of this disorder are discussed.
The idea of a disease entity is not an objective to be reached, but our most fruitful point of orientation.
—Karl Jaspers, 1923 (1)
The symptoms of obsessive-compulsive disorder (OCD) are remarkably heterogeneous to the extent that two patients with this diagnosis can display completely different nonoverlapping symptom patterns. Despite this phenotypic heterogeneity, standard nomenclatures (DSM-IV and ICD-10) regard OCD as a unitary nosological entity. While this parsimony has some esthetic appeal, it may be misleading. Moreover, with the exception of evolutionary-based models (2), most current models of OCD—neurobiological, developmental, or cognitive behavior—do not account for or put enough emphasis on this heterogeneity. Accordingly, most OCD research is based on comparisons between groups of OCD patients and healthy individuals, and global severity rating scales, such as the Yale-Brown Obsessive Compulsive Scale (3), are used.
Recognizing this heterogeneity, investigators have attempted to dissect the phenotype into homogeneous subtypes. For example, Falret made the distinction between folie du doute (“madness of doubt”) and délire du toucher (“delusion of touch”) in 1869 (4). Investigators frequently distinguish “washers” from “checkers” (5) and other symptom-based clusters (6–8). Other authors have classified patients into groups that represent extremes of a continuum of, for example, impulsivity (9) or insight (10, 11). Generally, these attempts had limited success in relating the identified subtypes to biological markers, genetic factors, or treatment response, in part because pure subtypes of patients are rare and the recruitment of sufficient sample sizes of each subtype is difficult and highly impractical. Other putative subtypes have been identified based on clinical characteristics, such as age at onset (12) and comorbid diagnoses, particularly tic disorders (13). Limitations of these approaches include knowing exactly when the obsessive-compulsive symptoms began and the difficulty of identifying “hidden” tic-related cases (individuals who have relatives with tic disorders but no tics of their own).
Factor-analytic approaches have been fruitful in the advancement of our understanding of other heterogeneous conditions, such as schizophrenia (14, 15), bipolar disorder (16), Tourette’s disorder (17), eating disorders (18), and learning disabilities (19). In OCD, too, recent factor-analytic studies have reduced its symptoms to a few fairly consistent and clinically meaningful symptom dimensions.
In this article, we critically review the evidence supporting a multidimensional model of OCD. We examine the studies aimed at identifying the structure of obsessive-compulsive symptoms using a variety of statistical methods before considering data from a range of disciplines that bear on the potential usefulness of these dimensions. Such review is timely because various research groups have begun to search for underlying genes and neural substrates of these symptom dimensions. We will argue that a dimensional approach can better account for the heterogeneity of OCD and has the potential of explaining a further portion of the variance from previous approaches. Ultimately, we aim to generate new clinical interpretations and stimulate further research in this promising field.
Method
Some definitions may be useful to the reader. In this article, we distinguish between categorical and dimensional models of OCD. Categorical studies aim at identifying homogeneous and mutually exclusive subgroups of patients (e.g., washers versus checkers). “Subtype” will be used as a synonym for “subgroup.” In opposition, dimensions derive from factor-analytical studies and are not mutually exclusive because each patient can score on one or more symptom dimensions at any one time. “Factor” will be used as a synonym for dimension.
Keyword-driven PUBMED and PsychINFO searches were performed. We also searched the reference sections of the manuscripts for additional sources. First, we identified studies that evaluated the structure of obsessive-compulsive symptoms using factor analysis. Only studies that used comprehensive and nonbiased instruments to ascertain obsessive-compulsive symptoms were included, such as the Yale-Brown Obsessive Compulsive Scale symptom checklist (3) and the Obsessive-Compulsive Inventory (20). Other frequently used instruments were excluded because their items are heavily biased and are not representative of the complex phenomenology of OCD. Second, we searched for studies that examined the various sources of evidence to support the predictive validity of the identified symptom dimensions. Topics of interest included natural history, comorbidity (axis I and axis II), genetics, life-span development, neuroimaging, neuropsychology, and predictors of treatment outcome with medications and cognitive behavior therapy.
Results
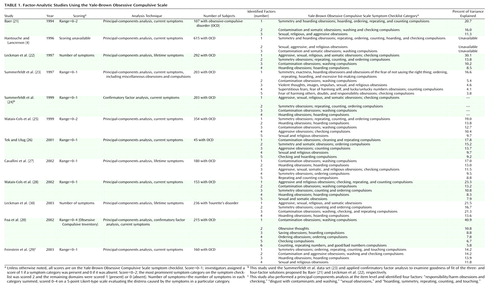
In the first factorial study of the Yale-Brown Obsessive Compulsive Scale symptom checklist, Baer (21) factor-analyzed its 13 major symptom categories in a sample of 107 patients and identified three factors, accounting for 48% of the variance, that were called “symmetry/hoarding,” “contamination/cleaning,” and “pure obsessions.” Since Baer’s seminal work, 10 studies corresponding to nine large OCD data sets and involving more than 2,000 patients have been identified (4, 20–29). One further study (30) that factor-analyzed the Yale-Brown Obsessive Compulsive Scale symptom checklist in a sample of patients with Tourette’s disorder and their first-degree relatives was also included (Table 1). Although these studies have used different methods (current versus lifetime symptoms, dichotomous versus ordinal versus interval scoring, a priori categories versus item-level analysis, exploratory versus confirmatory factor analysis) and instruments (Yale-Brown Obsessive Compulsive Scale versus Obsessive-Compulsive Inventory), an inspection of the factor content suggests more similarities than differences. Of note is that most studies that identified more than three factors explained more than 60% of the total variance. The most consistent factorial solutions were those of four or five dimensions.
Correlates of OCD Symptom Dimensions
Baer (21) reported that patients with high scores on his symmetry/hoarding factor were more likely to have a comorbid diagnosis of chronic tics and obsessive-compulsive personality disorder. Similarly, Leckman et al. (22) found that patients with high scores on the obsessions/checking and symmetry/ordering factors were more likely to have tics. Mataix-Cols et al. (25) reported that male but not female OCD patients with chronic tics scored higher than patients without tics on the symmetry/ordering dimension. These results are in accordance with earlier reports of elevated frequency of these symptoms in OCD patients with comorbid Tourette’s syndrome or a lifetime history of tics (31, 32).
Mataix-Cols et al. (33) examined the presence of all DSM-III-R axis II diagnoses and their relation to obsessive-compulsive symptom dimensions in a sample of 75 OCD patients. They found that hoarding symptoms were strongly related to the presence and number of all personality disorders, especially from the anxious-fearful cluster. Similarly, Frost et al. (34) found that hoarding was associated with higher levels of comorbidity, as well as work and social disability, compared to nonhoarding OCD and other anxiety disorders. In another study (35), the presence of hoarding was associated with male gender, earlier age at onset, comorbid social phobia, personality disorders, and pathological grooming conditions (skin picking, nail biting, and trichotillomania). Although one study (36) found that hoarding was associated with greater overall illness severity (total Yale-Brown Obsessive Compulsive Scale scores), another study did not (37).
Taken together, these studies suggest that a symptom-based dimensional approach can integrate previous classification attempts based on age at onset, gender, or presence of comorbid conditions because it has the advantages of allowing each patient to have scores in one or more symptom dimension and of permitting studies that cut across traditional diagnostic boundaries.
Temporal Stability of OCD Symptom Dimensions
One potential challenge of the dimensional approach is the assumption that OCD patients experience drastic symptom changes over time. For a dimensional approach to be useful, some degree of symptom stability would be expected, but few longitudinal studies examined the evolution of symptoms per se. Rettew et al. (37) assessed the longitudinal course of obsessive-compulsive symptoms in 76 children and adolescents with OCD who were followed over a period of 2–7 years with the categories of the Yale-Brown Obsessive Compulsive Scale symptom checklist. They found that none of the patients maintained the same constellation of symptoms from baseline to follow-up. Nevertheless, these authors acknowledged that these changes could have occurred within rather than between symptom dimensions, although they did not test this hypothesis. In a later study (38), a large sample of adult patients was repeatedly administered the Yale-Brown Obsessive Compulsive Scale symptom checklist over a period of 2 years. For the most part, the patients maintained their symptoms across follow-up, and the strongest predictor of having a particular symptom was having had that symptom in the past. For the symptoms that changed across time, changes occurred within rather than between previously identified (25) symptom dimensions, suggesting that the symptoms of adult OCD patients are more stable than it is often assumed. Longitudinal studies following up patients from childhood to adulthood are needed to further understand the course of obsessive-compulsive symptoms over longer periods of time.
Genetic Studies
Twin and family studies suggest that genetic factors play a role in the expression of OCD. Although earlier studies have indicated that the vertical transmission of OCD in families is consistent with the effects of a single major autosomal gene (39, 40), it is likely that there are a number of vulnerability genes involved. Alsobrook et al. (41) found that the relatives of OCD probands who had high scores on the obsessions/checking and symmetry/ordering factors were at greater risk for OCD than were the relatives of probands who had low scores on those factors. Using similar methods, Leckman et al. (30) found that the obsessions/checking and symmetry/ordering factors were significantly correlated in sibling pairs that were concordant for Tourette’s disorder. They also observed that mother-child correlations—but not father-child correlations—were significant for these two factors.
Cavallini et al. (27) performed a candidate gene study with a functional polymorphism in the promoter region of the serotonin transporter locus at 17q11. They found a significant association of the long/long haplotype in patients with tics and high scores on the “repeating/counting” factor. Because their finding was based on a post hoc analysis in a case-control study, the authors considered their findings preliminary.
Using the same data set as Leckman et al. (30), Zhang et al. (42) observed significant allele sharing for the hoarding factor for loci at 4q34, 5q35.2, and 17q25. The 4q site is in proximity to a region previously linked to the Tourette’s disorder phenotype (43).
In sum, the use of quantitative traits may provide a powerful approach to detect the genetic susceptibility loci that contribute to OCD presentations. Thus far, this approach has provided especially promising leads with regard to the hoarding obsessive-compulsive phenotype. The next steps include, first, the use of these symptom dimensions in large multigenerational families in order to refine the initial genetic linkage results for the hoarding phenotype. If specific loci are identified, this will provide compelling evidence for the validity of this multidimensional approach to OCD. Second, genome scans also need to be conducted using the remaining obsessive-compulsive symptom dimensions. Families segregating for Tourette’s disorder or early-onset OCD may be especially valuable in this enterprise. Given the high mother-child correlations in the study by Leckman et al. (30), it may also be valuable to examine the linkage results for alleles that are identical by descent from the mother. Third, twin and cross-fostering studies are needed to evaluate the heritability of these symptom dimensions within the general population. Future genetic studies will also need to examine the relationship between these dimensions and other closely related phenotypes, including tics, eating disorders, and body dysmorphic disorder.
Neuroimaging Studies
Functional neuroimaging studies have greatly increased our understanding of the neural mechanisms underlying OCD. Although the replicability among these studies has been imperfect, they strongly link obsessive-compulsive symptoms with activation of the orbitofrontal cortex, with less consistent involvement of the anterior cingulate gyrus, the striatum, the thalamus, the lateral frontal and temporal cortices, the amygdala, and the insula (44).
Most previous studies lumped together patients with mixed symptoms. Only a limited number of studies used patients with one predominant type of symptom or compared mutually exclusive groups of patients. In one positron emission tomography study, Rauch et al. (45) found that checking symptoms correlated with increased—and symmetry/ordering with reduced—regional cerebral blood flow (rCBF) in the striatum, whereas washing symptoms correlated with increased rCBF in the bilateral anterior cingulate and the left orbitofrontal cortex. Phillips et al. (46) compared OCD patients with mainly washing symptoms with OCD patients with mainly checking symptoms while viewing pictures of either normally disgusting scenes or washing-relevant pictures using functional magnetic resonance imaging (fMRI). When viewing washing-related pictures, only washers demonstrated activations in regions implicated in emotion and disgust perception (i.e., visual regions and the insular cortex), whereas checkers demonstrated activations in frontostriatal regions and the thalamus. In a similar study, OCD patients with predominantly washing symptoms demonstrated greater activation than comparison subjects in the right insula, the ventrolateral prefrontal cortex, and the parahippocampal gyrus when viewing disgust-inducing pictures (47). Saxena et al. (48) found that 12 patients with predominant hoarding symptoms showed reduced glucose metabolism in the posterior cingulate gyrus (versus comparison subjects) and the dorsolateral prefrontal cortex (versus nonhoarding OCD subjects) and that the severity of hoarding in the whole patient group (N=45) correlated negatively with metabolism in the latter region. Limitations of these studies included the artificial division between washers, checkers, and hoarders and in the symptom-provocation studies (46, 47), the exclusive use of washing-related material. A recent fMRI study (49, 50) used a symptom-provocation paradigm to examine, within the same patients, the neural correlates of washing, checking, and hoarding symptom dimensions of OCD. Each of these dimensions was mediated by distinct but partially overlapping neural systems. Although both patients and comparison subjects activated similar brain regions in response to symptom provocation, the patients showed greater activation in bilateral ventromedial prefrontal regions (the washing experiment); the putamen/globus pallidus, the thalamus, and dorsal cortical areas (the checking experiment); and the left precentral gyrus and the right orbitofrontal cortex (the hoarding experiment). These results were further supported by correlation analyses within the patient group, which revealed highly specific positive associations between subjective anxiety, questionnaire scores, and neural response in each experiment (50).
Although preliminary, these studies suggest that different symptoms may be mediated by distinct neural systems and that previous discrepant findings may result from phenotypic variations in the studied samples. Because of the “neural promiscuity” within the frontostriatothalamic loops (51), it is not surprising that different symptom dimensions often coexist in any given patient.
Much research is needed on the common and distinct neural correlates of various obsessive-compulsive symptom dimensions with symptom-provocation paradigms, as well as combining neuropsychological tasks and neuroimaging techniques. Structural neuroimaging studies have been remarkably inconsistent (44), and no studies to date, to our knowledge, have examined the relationship between gray and white matter abnormalities and symptom dimensions. Finally, the addition of neuroimaging protocols to treatment studies should be particularly rewarding.
Neuropsychological Studies
Remarkably few studies reported the relationship between obsessive-compulsive subgroups and performance in neuropsychological tests, and when reported, the results were negative. One study (52) compared neuropsychological performance in groups of categorically defined washers (N=8), checkers (N=8), and pure obsessionals (N=11) and found no differences between them on a battery of frontal lobe tests. Deckersbach et al. (53) examined the relationship between obsessive-compulsive symptom dimensions (25) and performance on the California Verbal Learning Test, a measure of verbal episodic memory, and found no significant associations. At least two possible explanations for these negative results are possible. First, cognitive dysfunctions could be a general feature of OCD and probably of other related disorders. Second, classic neuropsychological tests may be insensitive to subtle variations in symptom profiles. More experimental approaches may yield more meaningful results.
Predictors of Response to Somatic Treatments
Numerous placebo-controlled studies have demonstrated the efficacy of clomipramine and selective serotonin reuptake inhibitors (SSRIs) in the treatment of OCD. However, as many as 40%–60% of patients may not respond or may have only a partial response to these medications. Recent studies have suggested that a symptom-based dimensional approach may prove to be valuable in identifying significant predictors of treatment response. For instance, several studies have shown that patients with high scores on the hoarding dimension respond worse to SSRIs (25, 36, 54–56). High scores on the sexual/religious dimension (25) were associated with poorer long-term outcome with SSRIs and behavior therapy in 66 adult outpatients who were followed up from 1 to 5 years (57). Another study (56) reported that patients with somatic obsessions had poorer insight and responded less well to SSRIs. Alternative treatments may also help patients with specific symptoms. For instance, one study (58) found that patients with symmetry and unusual somatic obsessions might respond well to monoamine oxidase inhibitors. In another study (59), the presence of symmetry/ordering and hoarding symptoms predicted better response in refractory cases treated with cingulotomy.
Predictors of Compliance With and Response to Cognitive Behavior Therapy
The efficacy of cognitive behavior therapy for OCD has been well established in controlled trials. However, a significant number of patients still remain unimproved or simply refuse or drop out from this treatment. Some studies have suggested that checking rituals may respond less well to cognitive behavior therapy (60), but others found no differences in outcome between washers and checkers (8, 28, 61). It is often assumed that patients with “pure” obsessions and mental rituals respond less well to classic behavioral interventions, although data supporting these assumptions is sparse. In a meta-analysis, patients with primary obsessive thoughts without rituals tended to improve less with cognitive behavior therapy than those who had overt motor rituals (62). In a study by Alonso et al. (57), the presence of sexual and/or religious obsessions predicted poorer long-term outcome, but because most patients received both SSRIs and cognitive behavior therapy, it was not clear from this study whether these symptoms predicted poorer outcome with SSRIs, cognitive behavior therapy, or both.
Patients with hoarding symptoms have been described as having poor compliance with and response to cognitive behavior therapy (63), but little empirical evidence is available from large patient samples. Using a dimensional approach, Mataix-Cols et al. (28) examined 153 OCD participants in a randomized, controlled trial of cognitive behavior therapy. High scorers on the hoarding dimension were more likely to drop out prematurely from the trial and to improve less than nonhoarding OCD patients. In addition, high scorers on the sexual/religious dimension responded less well to behavior therapy. Of interest, patients with mental rituals did as well as other OCD patients in this study. Another study showed that categorically defined hoarders improved less with cognitive behavior therapy (8). Therefore, cognitive behavior therapy might be better indicated for patients with contamination/washing, aggressive/checking, and symmetry/ordering symptoms.
Cognitive interventions, alone or in combination with traditional exposure techniques, have shown promising results in treating patients without overt compulsions (64). Similarly, some case series (65) suggest that hoarding symptoms can be successfully treated with a multifaceted cognitive behavior therapy intervention consisting of training in decision making, exposure, and cognitive restructuring. Because these interventions in their present form are very long and labor intensive, group treatments may be a more cost-effective alternative (66), although this promising approach requires further testing.
Discussion
Multiple Disorders or One Multidimensional Disorder?
The heterogeneity of OCD can potentially reduce the power and obscure the findings from gene-localization methods, neuroimaging studies, and clinical trials. Previous attempts to subdivide OCD into mutually exclusive subtypes of patients proved relatively fruitless with regard to identifying biological markers, patterns of genetic transmission, or prediction of treatment response. We have reviewed data from large factor-analytic studies involving more than 2,000 patients suggesting remarkably consistent and temporally stable symptom dimensions. These dimensions, albeit imperfect, have been able to explain a significant part of the variance of previous studies. Does this mean that OCD is not one but multiple separate disorders? We regard this idea as premature. Subtyping OCD into smaller, mutually exclusive entities could be an endless process and would have the same limitations as some of the categorical approaches we described. Rather, we conceptualize OCD as a spectrum of potentially overlapping syndromes that can co-occur in any given patient. This view accords with current dimensional views of psychiatric nosology (67, 68).
A dimensional model of OCD provides clinicians and researchers with a more complete picture. Because monosymptomatic patients are rare, dividing OCD into mutually exclusive subtypes is unreasonable and impractical. From a dimensional perspective, each patient can score in one or more symptom dimensions. The focus is on symptoms or behaviors, not on groups of patients. As Krueger and Piasecki (67) have proposed for psychiatric nosology in general, a dimensional approach allows for reconciliation between a “lumping” perspective, in which all symptom dimensions are mere manifestations of a single broad disorder, i.e., OCD, and a “splitting” perspective, in which each symptom subtype is considered to be an entirely separate entity. These perspectives are not incompatible because it is likely that there are both shared and distinct etiological factors within the OCD phenotype. OCD research should concentrate on identifying the general and specific etiological factors that contribute to the development of each symptom dimension.
DSM-IV diagnostic criteria are arbitrary in that they require a specific number of symptoms to be met. In addition, sufferers need to spend more than 1 hour daily on their symptoms. A dimensional approach assumes that obsessive-compulsive phenomena are normally distributed in the general population (69). This implies broadening the diagnostic boundaries of OCD to include subsyndromal cases, thus dramatically increasing the population available for study. For instance, it is well known that risk for a more broadly defined OCD is increased among the parents of OCD probands but not among the parents of normal comparison subjects (e.g., references 70 and 71). In addition, obsessions and compulsions can also co-occur along with a variety of neurological and psychiatric conditions. For instance, hoarding behavior has been related to brain injury, dementia, schizophrenia, obsessive-compulsive personality disorder, eating disorders, and autism, among other symptoms (65). It would be reductionistic to limit the study of hoarding exclusively to the context of OCD. Future genetic studies involving patients from across a broad spectrum of disease or involving population-based samples may be particularly informative if these dimensions are stable traits.
Evolutionary and Developmental Perspectives
An evolutionary perspective may provide a fruitful vantage point to consider the multidimensional nature of OCD as well as other forms of psychopathology (2). The human brain is a remarkable product of evolution. In the struggle for life, certain traits have come to predominate. Natural selection is likely to have shaped not only the internal biology of our brains but, indirectly, also our mental processes and overt behavior. Elements in our mental and behavioral repertoire that were certainly the focus of the greatest selective pressures are those related to successful reproduction and survival in the face of external threats. Remarkably, each of the obsessive-compulsive symptom dimensions identified thus far can be seen in a distinctive and plausible relationship to successful aspects of our capacity to reproduce and survive as a species.
First, intrusive aggressive and egodystonic thoughts, impulses, and images relating to close family members are not uncommon among adults (69) and may be especially frequent during the perinatal period (72). Viewed from an evolutionary perspective, it seems nearly self-evident that the behavioral repertoires associated with early parenting would be subject to intense selective pressure (73). This line of thought would support the conclusion that further exploration of the factors that underlie the emergence and resolution of these behaviors in normal parents and parents experiencing postpartum OCD (74, 75) may provide valuable insight into the neurobiological substrates and evolutionary origins of these behaviors in normal adults as well as OCD patients whose illness is characterized by symptoms in this dimension (76).
Second, developmental studies indicate that young children engage in a significant amount of ritualistic, repetitive, and compulsive-like activities, as part of their normal behavioral repertoire (77). Using a parent-report questionnaire, two groups of investigators assessed more than 2,000 children ages 8–72 months (78, 79). They found the early emergence of specific behaviors resembling the symptom dimensions observed in OCD patients. For example, parents reported that their children “arranged objects” or performed certain behaviors until they seemed “just right,” beginning on average, at ages 22–25 months. These children “lined up objects in straight lines or in symmetrical patterns,” beginning on average at ages 24–25 months. Behaviors resembling those associated with the contamination/washing dimension identified with such statements as “seemed very concerned with dirt or cleanliness” were found to have their mean age at onset from 22 to 24 months. Finally, parents reported that their children, on average, began to “collect or store objects” (resembling the hoarding dimension) from ages 25 to 27 months. Although direct evidence linking the emergence of these behaviors to the later development of OCD is lacking, investigators have found that aspects of these ritualistic and compulsive-like behaviors are correlated with children’s fears and phobias (79, 80). Further research in normally developing children may provide valuable insights into the neurobiological substrates and evolutionary emergence and resolution of these behaviors.
Limitations of the Dimensional Approach
The structure of obsessive-compulsive symptoms is not yet definitive. Any empirical research on obsessive-compulsive symptoms necessarily relies on an instrument of measure. While the Yale-Brown Obsessive Compulsive Scale symptom checklist is a comprehensive list of the most common obsessive-compulsive symptoms and improves the problem of symptom bias of other instruments, its psychometric properties are yet to be determined. For example, it is crucial to establish its interrater reliability because different clinicians may score this scale differently.
Although the factorial studies available to date have been fairly consistent, the number of factors has ranged from three to six. Some of the symptom dimensions were consistently replicated across studies (e.g., contamination/washing, symmetry/ordering, hoarding), but the aggressive/checking and sexual/religious dimensions need further study since it is unclear whether they form a single factor (22, 24, 27, 30) or can be broken down into two separate dimensions (4, 20, 21, 23, 25, 26, 28, 29). Similarly, it is unclear how to regard somatic obsessions because they loaded on the contamination/washing factor in two studies (4, 21), on the obsessions/checking factor in three other studies (22, 27, 30), and with sexual obsessions in other studies (28, 29).
Other problems relate to the method of analysis itself. Principal components analysis is limited in that there is no probability model, it is sensitive to variable scaling, and it depends on the decision rules to retain the factors. As Summerfeldt et al. (24) noted, most factorial studies of the Yale-Brown Obsessive Compulsive Scale symptom checklist used symptom groupings defined a priori rather than individual symptoms. In addition, miscellaneous obsessions and compulsions were not included in these analyses. The dichotomous (or ordinal—when a 0, 1, and 2 scoring system was used) nature of the Yale-Brown Obsessive Compulsive Scale symptom checklist data is problematic. Bayesian factor analysis could represent an alternative to conventional principal-components analysis.
It is clear that new reliable instruments need to be developed to confirm the dimensional structure of obsessive-compulsive symptoms and measure the resulting dimensions in a dimensional manner. These instruments should permit the development of better quantitative traits for genetic analyses (based on lifetime symptoms) as well as more discriminating data for use in clinical trials.
Conclusions and Future Directions
The complex clinical presentation of OCD can be summarized by using a few consistent and temporally stable symptom dimensions. These can be understood as a spectrum of potentially overlapping syndromes that may extend beyond the traditional nosological boundaries of OCD and closely related phenotypes. Although this symptom structure is far from definitive and is still subject to revision, from the studies we reviewed, we conclude that a dimensional approach may advance our understanding of the disorder and explain further part of the variance in our data sets. Preliminary evidence supporting the validity of these dimensions comes from clinical, longitudinal, developmental, genetic, neuroimaging, and treatment response studies. The evidence is strongest for the hoarding dimension, which correlates with increased comorbidity and has consistently been associated with poor treatment response to both medications and cognitive behavior therapy. Much research remains to be done, starting with the development of better instruments of measure that fully capture the complex phenomenology of the disorder. Research on the common and distinct genetic and neural substrates of the various dimensions has already started and is likely to develop even further. In addition, research on the development of these behaviors in normal populations across the life span is warranted. Finally and more important, much research is needed to refine existing treatments or develop new treatments to meet all patients’ needs. Many patients have trouble complying with or responding to conventional treatments. In this regard, considering OCD a unitary disorder is especially ill advised. The study of these dimensions, viewed from evolutionary and developmental perspectives, may be clinically valuable as it will reinforce the notion that obsessive-compulsive symptoms are little more than extreme and time-consuming versions of anxious intrusive thoughts and harm-avoidant behaviors that are common to most of us, particularly during periods of life in which a heightened sensitivity to threats is adaptive. This insight may also provide therapists with a greater empathic understanding of their patients’ plights to the degree that their patients’ symptoms resemble aspects of the therapist’s own internal experiences.
 |
Received May 9, 2003; revision received Feb. 17, 2004; accepted April 23, 2004. From the Institute of Psychiatry, London; Yale University School of Medicine; and the Department of Psychiatry, University of São Paulo Medical School, São Paulo, Brazil. Address correspondence and reprint requests to Dr. Leckman, Child Study Center, Yale University School of Medicine, 230 South Frontage Rd., New Haven, CT 06520-7900; [email protected] (e-mail).
1. Jaspers K: General Psychopathology (1923). Translated from German. Chicago, University of Chicago Press, 1968Google Scholar
2. Leckman JF, Mayes LC: Understanding developmental psychopathology: how useful are evolutionary perspectives? J Am Acad Child Adolesc Psychiatry 1998; 37:1011–1021Crossref, Medline, Google Scholar
3. Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS: The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Crossref, Medline, Google Scholar
4. Hantouche EG, Lancrenon S: Modern typology of symptoms and obsessive-compulsive syndromes: results of a large French study of 615 patients. Encephale 1996; 22:9–21Medline, Google Scholar
5. Khanna S, Mukherjee D: Checkers and washers: valid subtypes of obsessive compulsive disorder. Psychopathology 1992; 25:283–288Crossref, Medline, Google Scholar
6. Khanna S, Kaliaperumal VG, Channabasavanna SM: Clusters of obsessive-compulsive phenomena in obsessive-compulsive disorder. Br J Psychiatry 1990; 156:51–54Crossref, Medline, Google Scholar
7. Calamari JE, Wiegartz PS, Janeck AS: Obsessive-compulsive disorder subgroups: a symptom-based clustering approach. Behav Res Ther 1999; 37:113–125Crossref, Medline, Google Scholar
8. Abramowitz JS, Franklin ME, Schwartz SA, Furr JM: Symptom presentation and outcome of cognitive-behavioral therapy for obsessive-compulsive disorder. J Consult Clin Psychol 2003; 71:1049–1057Crossref, Medline, Google Scholar
9. Hoehn-Saric R, Barksdale VC: Impulsiveness in obsessive-compulsive patients. Br J Psychiatry 1983; 143:177–182Crossref, Medline, Google Scholar
10. Insel TR, Akiskal HS: Obsessive-compulsive disorder with psychotic features: a phenomenologic analysis. Am J Psychiatry 1986; 143:1527–1533Link, Google Scholar
11. Foa EB, Kozak MJ: DSM-IV field trial: obsessive-compulsive disorder. Am J Psychiatry 1995; 152:90–96; correction, 152:654Link, Google Scholar
12. Rosario-Campos MC, Leckman JF, Mercadante MT, Shavitt RG, Prado HS, Sada P, Zamignani D, Miguel EC: Adults with early-onset obsessive-compulsive disorder. Am J Psychiatry 2001; 158:1899–1903Link, Google Scholar
13. Leckman JF, Grice DE, Barr LC, deVries ALC, Martin C, Cohen DJ, Goodman WK, Rasmussen SA: Tic-related vs non-tic related obsessive compulsive disorder. Anxiety 1995; 1:208–215Google Scholar
14. Andreasen NC, Arndt S, Alliger R, Miller D, Flaum M: Symptoms of schizophrenia: methods, meanings, and mechanisms. Arch Gen Psychiatry 1995; 52:341–351Crossref, Medline, Google Scholar
15. Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RS: Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry 1992; 160:179–186Crossref, Medline, Google Scholar
16. Cassidy F, Forest K, Murry E, Carroll BJ: A factor analysis of the signs and symptoms of mania. Arch Gen Psychiatry 1998; 55:27–32Crossref, Medline, Google Scholar
17. Alsobrook JP II, Pauls DL: A factor analysis of tic symptoms in Gilles de la Tourette’s syndrome. Am J Psychiatry 2002; 159:291–296Link, Google Scholar
18. Williamson DA, Womble LG, Smeets MAM, Netemeyer RG, Thaw JM, Kutlesic V, Gleaves DH: Latent structure of eating disorder symptoms: a factor analytic and taxometric investigation. Am J Psychiatry 2002; 159:412–418Link, Google Scholar
19. Grigorenko EL, Wood FB, Meyer MS, Hart LA, Speed WC, Shuster A, Pauls DL: Susceptibility loci for distinct components of developmental dyslexia on chromosomes 6 and 15. Am J Hum Genet 1997; 60:27–39Medline, Google Scholar
20. Foa EB, Huppert JD, Leiberg S, Langner R, Kichic R, Hajcak G, Salkovskis PM: The Obsessive-Compulsive Inventory: development and validation of a short version. Psychol Assess 2002; 14:485–496Crossref, Medline, Google Scholar
21. Baer L: Factor analysis of symptom subtypes of obsessive compulsive disorder and their relation to personality and tic disorders. J Clin Psychiatry 1994; 55:18–23Google Scholar
22. Leckman JF, Grice DE, Boardman J, Zhang H, Vitale A, Bondi C, Alsobrook J, Peterson BS, Cohen DJ, Rasmussen SA, Goodman WK, McDougle CJ, Pauls DL: Symptoms of obsessive-compulsive disorder. Am J Psychiatry 1997; 154:911–917Link, Google Scholar
23. Summerfeldt LJ, Richter MA, Anthony MM, Huta VM, Swinson RP: Symptom structure in OCD: factor analytic evidence for subgroups, in 1997 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 1997, number 480Google Scholar
24. Summerfeldt LJ, Richter MA, Antony MM, Swinson RP: Symptom structure in obsessive-compulsive disorder: a confirmatory factor-analytic study. Behav Res Ther 1999; 37:297–311Crossref, Medline, Google Scholar
25. Mataix-Cols D, Rauch SL, Manzo PA, Jenike MA, Baer L: Use of factor-analyzed symptom dimensions to predict outcome with serotonin reuptake inhibitors and placebo in the treatment of obsessive-compulsive disorder. Am J Psychiatry 1999; 156:1409–1416Abstract, Google Scholar
26. Tek C, Ulug B: Religiosity and religious obsessions in obsessive-compulsive disorder. Psychiatry Res 2001; 104:99–108Crossref, Medline, Google Scholar
27. Cavallini MC, Di Bella D, Siliprandi F, Malchiodi F, Bellodi L: Exploratory factor analysis of obsessive-compulsive patients and association with 5-HTTLPR polymorphism. Am J Med Genet Neuropsychiatr Genet 2002; 114:347–353Crossref, Medline, Google Scholar
28. Mataix-Cols D, Marks IM, Greist JH, Kobak KA, Baer L: Obsessive-compulsive symptom dimensions as predictors of compliance with and response to behaviour therapy: results from a controlled trial. Psychother Psychosom 2002; 71:255–262Crossref, Medline, Google Scholar
29. Feinstein SB, Fallon BA, Petkova E, Liebowitz MR: Item-by-item factor analysis of the Yale-Brown Obsessive Compulsive Scale symptom checklist. J Neuropsychiatry Clin Neurosci 2003; 15:187–193Crossref, Medline, Google Scholar
30. Leckman JF, Pauls DL, Zhang H, Rosario-Campos MC, Katsovich L, Kidd KK, Pakstis AJ, Alsobrook JP, Robertson MM, Walkup JT, van de Wetering BJM, McMahon WM, King RA, Cohen DJ (Tourette Syndrome Association International Consortium for Genetics): Obsessive-compulsive symptom dimensions in affected sibling pairs diagnosed with Gilles de la Tourette syndrome. Am J Med Genet Neuropsychiatr Genet 2003; 116:60–68Crossref, Google Scholar
31. Holzer JC, Goodman WK, McDougle CJ, Baer L, Boyarsky BK, Leckman JF, Price LH: Obsessive-compulsive disorder with and without a chronic tic disorder: a comparison of symptoms in 70 patients. Br J Psychiatry 1994; 164:469–473Crossref, Medline, Google Scholar
32. Petter T, Richter MA, Sandor P: Clinical features distinguishing patients with Tourette’s syndrome and obsessive-compulsive disorder from patients with obsessive-compulsive disorder without tics. J Clin Psychiatry 1998; 59:456–459Crossref, Medline, Google Scholar
33. Mataix-Cols D, Baer L, Rauch SL, Jenike MA: Relation of factor-analyzed symptom dimensions of obsessive-compulsive disorder to personality disorders. Acta Psychiatr Scand 2000; 102:199–202Crossref, Medline, Google Scholar
34. Frost RO, Steketee G, Williams LF, Warren R: Mood, personality disorder symptoms and disability in obsessive-compulsive hoarders: a comparison with clinical and nonclinical controls. Behav Res Ther 2000; 38:1071–1081Crossref, Medline, Google Scholar
35. Samuels J, Bienvenu OL III, Riddle MA, Cullen BA, Grados MA, Liang KY, Hoehn-Saric R, Nestadt G: Hoarding in obsessive-compulsive disorder: results from a case-control study. Behav Res Ther 2002; 40:517–528Crossref, Medline, Google Scholar
36. Saxena S, Maidment KM, Vapnik T, Golden G, Rishwain T, Rosen RM, Tarlow G, Bystritsky A: Obsessive-compulsive hoarding: symptom severity and response to multimodal treatment. J Clin Psychiatry 2002; 63:21–27Crossref, Medline, Google Scholar
37. Rettew DC, Swedo SE, Leonard HL, Lenane MC, Rapoport JL: Obsessions and compulsions across time in 79 children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 1992; 31:1050–1056Crossref, Medline, Google Scholar
38. Mataix-Cols D, Rauch SL, Baer L, Eisen JL, Shera DM, Goodman WK, Rasmussen SA, Jenike MA: Symptom stability in adult obsessive-compulsive disorder: data from a naturalistic two-year follow-up study. Am J Psychiatry 2002; 159:263–268Link, Google Scholar
39. Cavallini MC, Pasquale L, Bellodi L, Smeraldi E: Complex segregation analysis for obsessive compulsive disorder and related disorders. Am J Med Genet 1999; 88:38–43Crossref, Medline, Google Scholar
40. Nestadt G, Samuels J, Riddle M, Bienvenu OJ III, Liang KY, LaBuda M, Walkup J, Grados M, Hoehn-Saric R: A family study of obsessive-compulsive disorder. Arch Gen Psychiatry 2000; 57:358–363Crossref, Medline, Google Scholar
41. Alsobrook II JP, Leckman JF, Goodman WK, Rasmussen SA, Pauls DL: Segregation analysis of obsessive-compulsive disorder using symptom-based factor scores. Am J Med Genet 1999; 88:669–675Crossref, Medline, Google Scholar
42. Zhang H, Leckman JF, Tsai C-P, Kidd KK, Rosario Campos MC (Tourette Syndrome Association International Consortium for Genetics): Genome wide scan of hoarding in sibling pairs both diagnosed with Gilles de la Tourette syndrome. Am J Hum Genet 2002; 70:896–904Crossref, Medline, Google Scholar
43. Tourette Syndrome International Consortium for Genetics: A complete genome screen in sib-pairs affected with Gilles de la Tourette syndrome. Am J Hum Genet 1999; 65:1428–1436Crossref, Medline, Google Scholar
44. Saxena S, Bota RG, Brody AL: Brain-behaviour relationships in obsessive-compulsive disorder. Semin Clin Neuropsychiatry 2001; 6:82–101Crossref, Medline, Google Scholar
45. Rauch SL, Dougherty DD, Shin LM, Alpert NM, Manzo P, Leahy L, Fischman AJ, Jenike MA, Baer L: Neural correlates of factor-analyzed OCD symptom dimensions: a PET study. CNS Spectr 1998; 3:37–43Crossref, Google Scholar
46. Phillips ML, Marks IM, Senior C, Lythgoe D, O’Dwyer A-M, Meehan O, Williams SCR, Brammer MJ, Bullmore ET, McGuire PK: A differential neural response in obsessive-compulsive patients with washing compared with checking symptoms to disgust. Psychol Med 2000; 30:1037–1050Crossref, Medline, Google Scholar
47. Shapira NA, Liu Y, He AG, Bradley MM, Lessig MC, James GA, Stein D, Lang PJ, Goodman WK: Brain activation by disgust-inducing pictures in obsessive-compulsive disorder. Biol Psychiatry 2003; 54:751–756Crossref, Medline, Google Scholar
48. Saxena S, Brody AL, Maidment KM, Smith EC, Zohrabi N, Katz E, Baker SK, Baxter LR Jr: Cerebral glucose metabolism in obsessive-compulsive hoarding. Am J Psychiatry 2004; 161:1038–1048Link, Google Scholar
49. Mataix-Cols D, Cullen S, Lange K, Zelaya F, Andrew C, Amaro E, Brammer MJ, Williams SCR, Speckens A, Phillips ML: Neural correlates of anxiety associated with obsessive-compulsive symptom dimensions in normal volunteers. Biol Psychiatry 2003; 53:482–493Crossref, Medline, Google Scholar
50. Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML: Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry 2004; 61:564–576Crossref, Medline, Google Scholar
51. Alexander GE, Crutcher MD: Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 1990; 13:266–271Crossref, Medline, Google Scholar
52. Khanna S, Vijaykumar DR: Neuropsychology of obsessive compulsive disorder (abstract). Biol Psychiatry 2000; 47:127SCrossref, Medline, Google Scholar
53. Deckersbach T, Savage CR, Mataix-Cols D, Wilhelm S, Baer L, Jenike MA: Verbal memory in OCD subtypes, in Proceedings of the 33rd Annual Convention of the Association for the Advancement of Behavior Therapy. New York, AABT, 1999Google Scholar
54. Black DW, Monahan P, Gable J, Blum N, Clancy G, Baker P: Hoarding and treatment response in 38 nondepressed subjects with obsessive-compulsive disorder. J Clin Psychiatry 1998; 59:420–425Crossref, Medline, Google Scholar
55. Winsberg ME, Cassic KS, Koran LM: Hoarding in obsessive-compulsive disorder: a report of 20 cases. J Clin Psychiatry 1999; 60:591–597Crossref, Medline, Google Scholar
56. Erzegovesi S, Cavallini MC, Cavedini P, Diaferia G, Locatelli M, Bellodi L: Clinical predictors of drug response in obsessive-compulsive disorder. J Clin Psychopharmacol 2001; 21:488–492Crossref, Medline, Google Scholar
57. Alonso P, Menchón JM, Pifarré J, Mataix-Cols D, Torres L, Salgado P, Vallejo J: Long-term follow-up and predictors of clinical outcome in obsessive-compulsive patients treated with serotonin reuptake inhibitors and behavioral therapy. J Clin Psychiatry 2001; 62:535–540Crossref, Medline, Google Scholar
58. Jenike MA, Baer L, Minichiello WE, Rauch SL, Buttolph ML: Placebo-controlled trial of fluoxetine and phenelzine for obsessive-compulsive disorder. Am J Psychiatry 1997; 154:1261–1264Link, Google Scholar
59. Baer L, Rauch SL, Ballantine HT, Martuza R, Cosgrove R, Cassem E, Giriunas I, Manzo PA, Dimino C, Jenike MA: Cingulotomy for intractable obsessive compulsive disorder: prospective long-term follow-up of 18 patients. Arch Gen Psychiatry 1995; 52:384–392Crossref, Medline, Google Scholar
60. Basoglu M, Lax T, Kasvikis Y, Marks IM: Predictors of improvement in obsessive-compulsive disorder. J Anxiety Disord 1988; 2:299–317Crossref, Google Scholar
61. Foa EB, Goldstein A: Continuous exposure and complete response prevention in the treatment of obsessive-compulsive neurosis. Behav Ther 1978; 9:821–829Crossref, Google Scholar
62. Christensen H, Hadzai-Pavlovic D, Andrews G, Mattick R: Behavior therapy and tricyclic medication in the treatment of obsessive-compulsive disorder: a quantitative review. J Consult Clin Psychol 1987; 55:701–711Crossref, Medline, Google Scholar
63. Ball SG, Baer L, Otto MW: Symptom subtypes of obsessive-compulsive disorder in behavioural treatment studies: a quantitative review. Behav Res Ther 1996; 34:47–51Crossref, Medline, Google Scholar
64. Freeston MH, Ladouceur R, Rheaume J: Cognitive behavioural treatment of obsessive thoughts. J Consult Clin Psychol 1997; 65:405–413Crossref, Medline, Google Scholar
65. Frost RO, Steketee G: Hoarding: clinical aspects and treatment strategies, in Obsessive Compulsive Disorder: Practical Management, 3rd ed. Edited by Jenike MA, Baer L, Minichiello WE. St Louis, Mosby, 1998, pp 533–554Google Scholar
66. Steketee G, Frost RO, Wincze J, Greene KAI, Douglass H: Group and individual treatment of compulsive hoarding: a pilot study. Behav Cognit Psychother 2000; 28:259–268Crossref, Google Scholar
67. Krueger RF, Piasecki TM: Toward a dimensional and psychometrically-informed approach to conceptualizing psychopathology. Behav Res Ther 2002; 40:485–499Crossref, Medline, Google Scholar
68. Maser JD, Patterson T: Spectrum and nosology: implications for DSM-V. Psychiatr Clin North Am 2002; 25:855–885Crossref, Medline, Google Scholar
69. Rachman S, de Silva P: Abnormal and normal obsessions. Behav Res Ther 1978; 16:233–248Crossref, Medline, Google Scholar
70. Black DW, Noyes R, Goldstein RB, Blum N: A family study of obsessive-compulsive disorder. Arch Gen Psychiatry 1992; 49:362–368Crossref, Medline, Google Scholar
71. Pauls DL, Alsobrook JP II, Goodman W, Rasmussen S, Leckman JF: A family study of obsessive-compulsive disorder. Am J Psychiatry 1995; 152:76–84Link, Google Scholar
72. Leckman JF, Mayes LC, Feldman R, Evans D, King RA, Cohen DJ: Early parental preoccupations and behaviors and their possible relationship to the symptoms of obsessive-compulsive disorder. Acta Psychiatr Scand 1999; 100:1–26Crossref, Medline, Google Scholar
73. Pryce CR: Determinants of motherhood in human and nonhuman primates, in Motherhood in Human and Nonhuman Primates: Biological and Social Determinants. Edited by Price CR, Martin RD. Basel, Karger, 1995, pp 1–15Google Scholar
74. Abramowitz J, Moore K, Carmin C, Wiegartz PS, Purdon C: Acute onset of obsessive-compulsive disorder in males following childbirth. Psychosomatics 2001; 42:429–431Crossref, Medline, Google Scholar
75. Sichel DA, Cohen LS, Dimmock JA, Rosenbaum JF: Postpartum obsessive compulsive disorder: a case series. J Clin Psychiatry 1993; 54:156–159Medline, Google Scholar
76. Leckman JF, Herman AE: Maternal behavior and developmental psychopathology. Biol Psychiatry 2002; 51:27–43Crossref, Medline, Google Scholar
77. Gesell A, Ilg FL: Infant and Child in the Culture of Today. New York, Harper & Brothers, 1943Google Scholar
78. Evans DW, Leckman JF, Carter A, Reznick JS, Henshaw D, Pauls DL: Ritual, habit, and perfectionism: the prevalence and development of compulsive like behavior in normal young children. Child Dev 1997; 68:58–68Crossref, Medline, Google Scholar
79. Zohar AH, Feliz L: Ritualistic behavior in young children. J Abnorm Child Psychol 2001; 29:121–128Crossref, Medline, Google Scholar
80. Evans DW, Gray FL, Leckman JF: The rituals, fears and phobias of young children: insights from development, psychopathology and neurobiology. Child Psychiatry Hum Dev 1999; 29:261–276Crossref, Medline, Google Scholar



