Is Impaired Set-Shifting an Endophenotype of Anorexia Nervosa?
Abstract
OBJECTIVE: Set-shifting difficulties have been reported in subjects with anorexia nervosa and appear to persist after recovery; therefore, they may be endophenotypic traits. The goals of this study were to investigate whether set-shifting difficulties are familial by examining discordant sister-pairs in comparison with healthy unrelated women and to replicate, with a broader battery, the lack of influence of an acute illness state on neuropsychological performance. METHOD: Forty-seven pairs of sisters discordant for anorexia nervosa and 47 healthy unrelated women who were comparable in age and IQ completed neuropsychological tasks selected to assess set-shifting ability. Analyses of variance with standard errors that are robust against correlations within family clusters were used to compare the groups. Results were adjusted for obsessive-compulsive, anxiety, and depression symptoms. Subjects with acute (N=24) and fully remitted (N=23) anorexia nervosa were compared to assess state versus trait effects. RESULTS: Sisters with and without anorexia nervosa took significantly longer than unrelated healthy women to shift their cognitive set (CatBat task) and demonstrated greater perceptual rigidity (Haptic Illusion task) but did not differ significantly from each other. Women with anorexia nervosa were slower than other groups on Trail Making tasks. Women who had fully recovered from anorexia nervosa made significantly fewer errors than those with acute anorexia nervosa on the Trail Making alphabet task, but these subgroups did not differ on other measures. CONCLUSIONS: Both affected and unaffected sisters had more set-shifting difficulties than unrelated healthy women. This finding, together with the replicated finding that set-shifting difficulties persist after recovery, suggests that set-shifting difficulties are trait characteristics and may inform the search for the endophenotype in anorexia nervosa.
The classification of psychiatric disorders on the basis of overt clinical phenotypes might not be optimal in the search for vulnerability genes and other etiological factors because the genotype-phenotype relationship in complex disorders is indirect. For this reason, there has been renewed interest in the search for “endophenotypes,” measurable disease-associated traits that have a simpler relationship with underlying genes than clinical measures (1). One possible endophenotype of complex psychiatric disorders is neuropsychological function.
Executive functioning includes the processes that supervise the operation of other cognitive processes, such as inhibiting actions, restraining and delaying responses, attending selectively, setting goals, planning, and organizing, as well as set-maintaining and set-shifting, located primarily in the prefrontal cortex. Studies by our group (2–4) examined executive functioning in eating disorders, focusing on set-shifting tasks. Set-shifting involves the ability to move back and forth between tasks, operations, or sets (5). Using selected perceptual and cognitive tasks, our group (3) found that individuals with anorexia nervosa took significantly longer to “shift set” than subjects with similar IQ who did not have anorexia nervosa. Some difficulty in set-shifting persisted in women who had recovered from anorexia nervosa (4), suggesting that set-shifting difficulties are not purely a function of the acute illness state. The hypothesized association between set-shifting difficulties and anorexia nervosa have face validity in that individuals with anorexia nervosa are often described as persistent, with rigid, conforming, or obsessional personalities (6, 7).
An endophenotype linked to heritable etiological factors must fulfill several criteria: it must be state-independent; it must associate with illness and co-segregate within families; and it must be found at a higher rate in nonaffected family members than in the general population (1). If a characteristic fulfills these criteria but is not proven to be heritable, it is termed a “biological marker.” Our previous work indicated that the set-shifting abnormalities in anorexia nervosa were independent of the stage of illness (4). Thus, the primary aim of this study was to examine whether unaffected first-degree relatives (sisters) shared this pattern of neuropsychological impairment. We used the same test battery as in the previously reported study, and our subjects were women with anorexia nervosa, their healthy sisters, and healthy comparison women of similar age and IQ. We tested whether healthy sisters demonstrated set-shifting characteristics at a higher rate than the healthy unrelated women, and we tried to replicate, with a broader battery, the finding that these abnormalities are independent of acute illness state.
Method
Participants
Forty-seven women with anorexia nervosa and one healthy sister of each were invited to participate in this study as part of a collaborative, multicenter study conducted across Europe to investigate risk factors for eating disorders. Individuals with anorexia nervosa were recruited from a clinical population of individuals receiving treatment for eating disorders within the South London and Maudsley National Health Service Trust (N=18 [38%]) and from a register of 500 individuals with past or current eating disorder (N=29 [62%]). Individuals on the register were ascertained from clinical populations and service user groups within the United Kingdom. Forty-four of the participants with anorexia nervosa (94%) were newly recruited for this study and have not been reported on previously. Three of the individuals with anorexia nervosa completed the neuropsychological battery for a previous paper published by our group (3). Twenty-eight (60%) of the individuals with anorexia nervosa reported a lifetime history of anorexia nervosa, binge-purge subtype, and the remaining 19 individuals (40%) were diagnosed as having anorexia nervosa, restrictive subtype.
Forty-seven comparison women of normal weight with no history of eating disorders were recruited by public advertisement in the local community. The comparison group consisted of the 35 women previously reported on by our group (3) and 12 additional participants recruited for this study.
All participants were native English speakers, and 135 (96%) of the women were of white Caucasian origin. Ethical approval for the study was obtained from the South London and Maudsley Trust Research Ethics Committee. After complete description of the study to the subjects, written informed consent was obtained from all participants.
Individuals were included in the sister-pair group if they had a DSM-IV lifetime diagnosis of anorexia nervosa and a healthy sister who did not have any form of eating disorder (anorexia nervosa, bulimia nervosa, or eating disorder not otherwise specified). Women with anorexia nervosa approached about this study were asked if they had a sister who might be willing to participate. With their permission, the sister was contacted and invited to take part. Sisters had to be less than 10 years apart in age and to have lived in the same family as the patient for a minimum of 8 years. If the patient had more than one sister, the sister closest in age was approached. Each sister was interviewed independently.
Of the 52 sister-pairs originally recruited for this study, three were excluded because the sister met criteria for anorexia nervosa, bulimia nervosa, or eating disorder not otherwise specified. Individuals with neurological illness, head injury, comorbid psychotic disorder, or learning difficulties were also excluded (N=2). Of the women with a lifetime diagnosis of anorexia nervosa, 23 (49%) met the criteria for full recovery (normal weight for at least a year and regular menses), 21 (45%) had acute anorexia nervosa, and three (6%) had recently recovered normal weight (body mass index >17.5). Women at different stages of recovery were included in this study because our primary aim was to examine neuropsychological performance in individuals with anorexia nervosa, their unaffected sisters, and healthy unrelated women. In the subsidiary analysis comparing acutely ill and recovered individuals, the three women who had recently recovered normal weight were included in the acutely ill group because they were currently inpatients receiving treatment for anorexia nervosa and continued to fulfill all the DSM-IV criteria with the exception of body mass index. A previous study by our group (4) examining the effect of short-term weight gain on neuropsychological performance showed no significant improvement across any of the tasks.
None of the healthy unrelated women were taking psychoactive medication. Within the sister-pair group, one of the healthy sisters reported taking antidepressant medication (a selective serotonin reuptake inhibitor [SSRI]) at the time of the study. Seventeen (36%) of the women with anorexia nervosa were currently taking psychoactive medication: one reported taking a tricyclic antidepressant (imipramine), one reported taking mirtazapine, and the rest were taking SSRIs. The proportion of individuals taking psychoactive medication was similar among those who were acutely ill and those with remitted anorexia (nine [38%] of 24 and eight [35%] of 23, respectively).
Measures
Eating disorder diagnoses were made according to DSM-IV criteria on the basis of a clinical interview (a European adaptation of the Longitudinal Interval Follow-Up Evaluation [8]) and the Eating Disorder Examination (9). The Eating Disorder Examination has demonstrated good interrater reliability in terms of diagnoses (kappa=0.82–1.0) and illness history (kappa=0.80–0.99) variables. Diagnostic validity (compared with clinical notes) yielded kappas between 0.77 and 1.0 for sequential diagnoses (Anderluh et al., personal communication).
The neuropsychological battery consisted of several paradigms assessing both perceptual and cognitive set-shifting ability: the Haptic Illusion task (10, 11), Brixton Test (12), Trail Making task (13), CatBat task (4), and neurological test for dysdiadochokinesis (14). A full description of the tasks is provided in a previous publication (3) and is also available at http://www.eatingresearch.com.
Participants also completed the National Adult Reading Test, 2nd ed. (15), to provide an estimate of premorbid intellectual ability; the verbal fluency task (13) as a general measure of executive function; and the Hospital Anxiety and Depression Scale (16) and Maudsley Obsessive Compulsive Inventory (17) to assess current anxiety, depression, and obsessive-compulsive symptoms.
Data Analyses
The nominal significance level was chosen to be 1% to adjust for multiple neuropsychological tests. Women with anorexia nervosa, their healthy sisters, and healthy unrelated women were compared on demographic, clinical, and neuropsychological variables by using an analysis of variance (ANOVA) model. However, analysis of data from sibling pairs needs to account for the fact that measures from siblings are not independent. Standard methods for group comparison such as ANOVA assume that observations are independent. In contrast, because members of the same family share a number of characteristics, observations from the same family pair or cluster might be positively correlated. We dealt with this by using ANOVA with standard errors that are robust against correlations within family clusters (18). When a significant group difference was detected, post hoc t tests, again based on robust standard errors, were used to carry out three groupwise comparisons at a Bonferroni-adjusted significance level of 0.33%.
Box plots were used to check that the remaining assumptions of the model were fulfilled. The distribution of the clinical and neuropsychological variables appeared normal apart from depression scores on the Hospital Anxiety and Depression Scale, which were positively skewed in the healthy unrelated group and the unaffected sister group. Transformation of this variable (by square root) produced a normal distribution; therefore, the transformed values were used in the analysis. The Mann-Whitney U test and the Wilcoxon signed ranks test were used to carry out pairwise comparisons of the low error frequencies on the neuropsychological tasks. In a subsidiary analysis, to assess the possible influence of acute illness state, independent t tests and Mann-Whitney U tests were used as appropriate to compare women who were fully recovered from anorexia nervosa and those with acute anorexia nervosa.
Since anxiety and depression scores on the Hospital Anxiety and Depression Scale and scores on the Maudsley Obsessive Compulsive Inventory differed between the groups (see Results section) and might potentially influence neuropsychological performance, Spearman’s correlation coefficient was used to determine any relationship between these variables and neuropsychological test scores. Although these features may be considered part of the clinical phenotype of anorexia nervosa, when a relationship was observed, anxiety, depression, and/or Maudsley Obsessive Compulsive Inventory scores were included as covariates in the analysis to control group comparisons for these potential confounders. Both unadjusted and adjusted results are presented. Given that antidepressant (particularly SSRI) medication may also affect performance on the neuropsychological battery, medicated (N=17) and nonmedicated (N=30) subgroups within the anorexia nervosa group were compared to test this possibility. Two-tailed tests were used throughout. All analyses were carried out using Stata (19).
Results
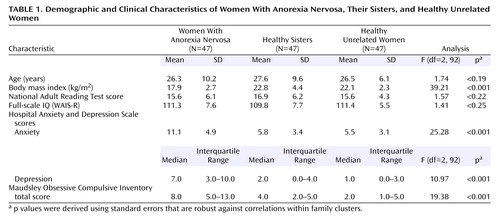
The women with anorexia nervosa, their healthy sisters, and the healthy unrelated women were well balanced in age and IQ (Table 1). The women with anorexia nervosa had a wide range of illness duration (median=6.0 years, interquartile range=3.0–9.0). The median age at onset for anorexia nervosa was 16.0 years (interquartile range=13.0–19.0). The median lowest reported body mass index was 13.5 (interquartile range=11.9–14.9), indicating that the majority of the women had experienced a severe form of anorexia nervosa. The women with anorexia nervosa obtained significantly higher depression, anxiety, and obsessive-compulsive symptom scores than their healthy sisters and the healthy unrelated women (Table 1).
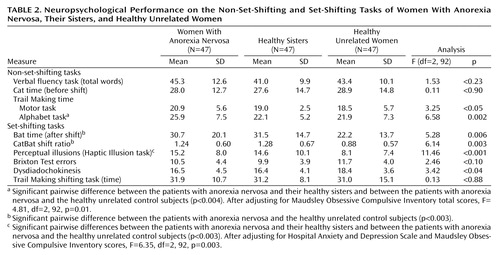
Anxiety and depression scores on the Hospital Anxiety and Depression Scale and scores on the Maudsley Obsessive Compulsive Inventory did not correlate with scores on the CatBat task, the Brixton Test, the neurological test for dysdiadochokinesis, or the Trail Making shifting task (all Pearson correlation coefficients below 0.20 in absolute value); therefore, unadjusted results are presented for these variables (Table 2). The number of perceptual illusions was positively correlated with depression score on the Hospital Anxiety and Depression Scale (r=0.27, N=141, p=0.001) and total score on the Maudsley Obsessive Compulsive Inventory (r=0.27, N=141, p=0.002). Total score on the Maudsley Obsessive Compulsive Inventory was also correlated with response time on the Trail Making alphabet task (r=0.26, N=141, p=0.004). Maudsley Obsessive Compulsive Inventory scores and Hospital Anxiety and Depression Scale depression scores, therefore, were considered covariates, as appropriate, for the number of perceptual illusions and response time on the Trail Making alphabet task.
The three groups were equivalent in verbal fluency and did not differ on the nonshift component of the CatBat task. Compared with their healthy sisters, the women with anorexia nervosa took significantly longer to complete the alphabet component of the Trail Making task (estimated difference=3.8 seconds, 99% CI=1.0 to 6.6) (t=3.55, df=92, p=0.001 [unadjusted]; t=3.17, df=92, p=0.002 [adjusted]) and showed a similar but statistically nonsignificant delay on the motor component (estimated difference=2.0 seconds, 99% CI=–0.1 to 4.1) (t=2.5, df=92, p<0.02). The same directional effect was observed in a comparison of the women with anorexia nervosa and the healthy unrelated women, but these differences did not reach significance when the Bonferroni-adjusted significance level of 0.003 was applied (motor component: 2.4 seconds, 99% CI=–0.7 to 5.5, t=2.01, df=92, p<0.05; alphabet component: 4.0 seconds, 99% CI=–0.9 to 8.1, t=2.57, df=92, p<0.02 [unadjusted]; t=1.95, df=92, p<0.06 [adjusted]). Healthy sisters did not differ from healthy unrelated women on any of the general cognitive tasks.
On the set-shifting tasks, sisters with no eating disorders took significantly longer than healthy unrelated women on the shift component of the CatBat task, equating to a mean difference of 9.2 seconds (99% CI=1.5 to 17.0) (t=3.14, df=92, p=0.002), and had a higher ratio of Cat time to Bat time of 0.40 (99% CI=0.06 to 0.74) (t=3.12, df=92, p=0.002). A similar but nonsignificant effect was observed when women with anorexia nervosa were compared with healthy unrelated women for Cat time (estimated difference=8.4 seconds, 99% CI=–0.9 to 17.8) (t=2.37, df=92, p=0.02) and the CatBat ratio (increase of 0.36, 99% CI=0.04 to 0.68) (t=2.96, df=92, p=0.004). The women with anorexia nervosa and their unaffected sisters took significantly longer than healthy unrelated women to adjust to the change in ball size in the Haptic Illusion task, with differences of 7.1 illusions (99% CI=3.0 to 11.4) (t=4.48, df=92, p<0.001) and 6.5 illusions (99% CI=1.7 to 11.3) (t=3.54, df=92, p=0.001), respectively, from the healthy unrelated women. After adjustment for depression and obsessive-compulsive symptoms, this group difference remained significant in unaffected sisters (t=3.52, df=92, p=0.001) but fell below the significance level in the women with anorexia nervosa (t=1.78, df=92, p<0.08).
The performance of affected and unaffected sisters did not differ significantly on any set-shifting task. There were no differences in error rate between groups on the Trail Making tasks (data not shown but available on request). A comparison of individuals with anorexia nervosa who were or were not receiving current psychoactive medication revealed no significant group differences across all neuropsychology tasks (p>0.2).
In a subsidiary analysis to address the influence of illness state on performance in the women with anorexia nervosa, fully recovered women (N=23) were compared with those who were acutely ill and those who had recently recovered normal weight (N=24). The recovered group made significantly fewer errors than the acutely ill on the alphabet component of the Trail Making task (p=0.003, Mann-Whitney U test). Four (17%) of the fully recovered women made one or more errors on the alphabet, compared with 15 (63%) of those with acute anorexia nervosa. Error rates on these tasks were not correlated with anxiety or depression scores on the Hospital Anxiety and Depression Scale or scores on the Maudsley Obsessive Compulsive Inventory; therefore, there was no need for adjustment. Recovered women did not differ significantly from those with acute anorexia nervosa on any of the other neuropsychological tasks (all p>0.1).
A secondary analysis excluding the three women who had recently achieved normal weight from the acute group produced similar, nonsignificant findings (all p values p>0.1). Furthermore, within the anorexia nervosa group, body mass index did not correlate significantly with performance on any neuropsychological task (all Pearson correlation coefficients were below 0.20 in absolute value).
Discussion
This study set out to examine the possibility that set-shifting difficulties, thought to be trait markers for anorexia nervosa, could be classified as endophenotypes on the basis of findings in first-degree relatives. We found that the set-shifting difficulties evident in women with anorexia nervosa were shared by their healthy sisters. The most striking results were apparent from the CatBat cognitive set-shifting task and the Haptic Illusion task, which assess perceptual rigidity. Both affected and unaffected sisters took significantly longer to shift set on these tasks. There was also evidence of slowed alternation on the test for dysdiadochokinesis in women with anorexia nervosa and their unaffected sisters. None of these tests differed between women with acute anorexia nervosa and those who had fully recovered, suggesting that they are trait and not state effects. However, slower performance on some general cognitive tasks was specific to the anorexia nervosa group. The women with anorexia nervosa were slower than their healthy sisters and healthy unrelated subjects to complete nonshift components of the Trail Making task. The women who were fully recovered from anorexia nervosa were equally slow to complete these components, although they made significantly fewer errors on one of the tasks, suggestive of a different cognitive style.
This is the first study, to our knowledge, to investigate neuropsychological functioning in the unaffected siblings of individuals with an eating disorder. The finding that women with anorexia nervosa and their unaffected sisters exhibit similar difficulties in some set-shifting tasks has several implications. It suggests that reduced cognitive and perceptual flexibility may constitute a familial trait associated with a greater risk of developing anorexia nervosa rather than a consequence or scar of the illness. One possibility is that mental rigidity is linked to persistent abnormalities in the serotonergic system seen in anorexia nervosa, including elevated serotonin 5-HT metabolites in CSF and alteration in 5-HT2A receptor binding (20, 21). The serotonergic system has been strongly implicated in the regulation of impulsivity and cognitive inflexibility (22–25).
The results of this study are in agreement with the hypothesis that the pattern of neuropsychological functioning observed in previous studies may constitute a biological marker or heritable endophenotype in anorexia nervosa; unaffected sisters carry copies of illness susceptibility genes that are nonpenetrant for illness but manifest as the endophenotype. Indeed, it is now well accepted that human prefrontal function, which includes factors such as information processing speed and specific cognitive abilities, is under substantial genetic control (26).
Evidence of shared neuropsychological traits in unaffected relatives parallels the results of studies in other complex disorders, such as schizophrenia (27–29), where illness-associated neuropsychological traits are evident in unaffected first-degree relatives when compared with unrelated subjects. A perhaps surprising finding is that unaffected sisters in this study had scores very similar to those of their affected sisters on the tasks of interest. One would expect the sisters’ scores to be intermediate because, on average, 50% of genes are shared. However, other studies of cognitive functioning in discordant siblings (e.g., for schizophrenia) have also reported similar impairments in both affected and unaffected siblings relative to unrelated comparison subjects (29).
The set-shifting difficulties evident in this group of women with anorexia nervosa are largely consistent with previous reports from our group (2–4). Slowness on the dysdiadochokinesis test in anorexia nervosa, which did not reach statistical significance in our study, has also been reported by a different group (30, 31). Our previous study using the same test battery (3) found no effects of SSRI medication use on neuropsychological performance.
It is possible that the tasks included in our “set-shifting” battery are testing different aspects of information processing. The Haptic Illusion and CatBat tasks have an element of learning, i.e., they involve a period of training to establish the set (presentation of unequal-sized balls 15 times) or a narrative set where the letter c for cat is required (six times). When the set is changed, previous learning has to be extinguished and a new pattern put in place. Thus, these tasks may be measuring a failing in the process of extinction in individuals with anorexia nervosa. Strober (32) has developed a theory about the maintenance of anorexia nervosa in which he suggests that “genetically driven variations in mechanisms underlying fear conditioning are posited as the second-stage contributor to the morbid level of fear that quickly ensues, and its prolonged resistance to extinction.” Both the abnormalities detected and the evidence of trait effects are compatible with this theory, although they suggest that the failure to extinguish learned responses may not be limited to fear conditioning. This line of thought merits further investigation.
In contrast to the finding in the current study, in an earlier study (3) our group found that subjects with anorexia nervosa demonstrated difficulties on the Brixton Test and the Trail Making shift tasks. It is possible that these tasks are more sensitive to aspects of illness state than the CatBat and Haptic Illusion tasks. The women with anorexia nervosa in the current study had a higher current body mass index and a shorter illness duration than subjects with anorexia nervosa in our earlier study. Moreover, 37%, compared with 69% in the earlier study, were inpatients at the time of testing. Thus, the failure in replication could be ascribed to the differences in clinical state.
In common with our previous findings (3), the women with anorexia nervosa in the current study took significantly longer to complete the two nonshift components of the Trail Making task. Comparable response times between acute and recovered subjects suggest that longer response time on these tasks is not a function of acute state. However, the absence of this feature in the healthy sisters suggests that this is either an individual-specific trait or a residual scar effect of having had anorexia nervosa.
An intriguing finding was that recovered subjects made significantly fewer errors than all other groups on the Trail Making alphabet task. Combined with the greater response times in the anorexia nervosa group relative to comparison subjects, across several tasks, this may indicate a pattern of responding in the recovered state that is consistent with a more cautious cognitive style. This finding is in keeping with the slow, accurate response style reported previously in anorexia nervosa (33). The links between neuropsychological performance and aspects of personality in anorexia nervosa such as persistence and perfectionism, especially regarding concern over mistakes (34), would be an interesting avenue for future research.
The strengths of this study include the use of a discordant sibling-pair design, which is a powerful tool in investigating the extent to which illness-associated characteristics are unique or shared within families (35). Eating disorder diagnoses were based on a semistructured interview that uses a timeline approach to elicit lifetime eating disorder symptoms, circumventing some of the reliability questions raised by self-reported, questionnaire-based diagnoses. The neuropsychological battery used in this study was specifically selected to test the hypothesis that individuals with anorexia nervosa might have difficulty in set-shifting and was identical to that used in a number of previous studies, allowing comparisons to be made. We were able to consider the influence of depression, anxiety, obsessive-compulsive symptoms, and medication use in our analysis, all of which may be considered potential confounders when assessing neuropsychological performance.
This study has some limitations. Because women with anorexia nervosa were recruited at different stages of recovery and treatment for this study, we could not control for the effects of current nutritional state on neuropsychological performance. In addition, we cannot determine from these data whether the pattern of set-shifting difficulties reported in women with anorexia nervosa and their healthy sisters is specific to this disorder or is a feature of other axis I disorders. The finding that anxiety, depression, and obsessive-compulsive symptoms had minimal effects on performance, however, suggests some degree of specificity. This test battery has not been used in individuals with primary depression, anxiety, or obsessional disorders in the absence of an eating disorder; therefore, the use of this battery in these groups would represent a valuable future extension of the current research.
To conclude, this study provides further support for the possibility that information processing (set-shifting or extinction) difficulties may be part of the endophenotype in anorexia nervosa. To our knowledge, this is the first study to investigate such traits in the relatives of individuals with anorexia nervosa. The extent to which altered performance on the neuropsychological battery reflects a susceptibility to anorexia nervosa or correlates of certain personality traits is an interesting question that merits further exploration. Future studies assessing the heritability and exact form of these traits would be required to accept or refute the endophenotype theory.
 |
 |
Received Feb. 27, 2004; revision received July 29, 2004; accepted Sept. 13, 2004. From the Division of Psychological Medicine, the Social, Genetic and Developmental Psychiatry Research Centre, and the Department of Biostatistics and Computing, Institute of Psychiatry, King’s College London (KCL); and the KCL Department of Academic Psychiatry, Guy’s, King’s and St. Thomas’s Medical School, London. Address correspondence and reprint requests to Dr. Holliday, Eating Disorders Unit, Box 059, Institute of Psychiatry, De Crespigny Park, London, SE5 8AF, UK; [email protected] (e-mail). Supported by grant 1206/87 from the Health Foundation (formerly the PPP Foundation) (Professor Treasure, Professor Collier, and Dr. Ulrike Schmidt), by grant QLK1-1999-916 from the European Commission Framework V program, and by Wellcome Trust International Fellowship number 059049 (Dr. Tchanturia).
1. Gottesman II, Gould TD: The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 2003; 160:636–645Link, Google Scholar
2. Tchanturia K, Serpell L, Troop N, Treasure J: Perceptual illusions in eating disorders: rigid and fluctuating styles. J Behav Ther Exp Psychiatry 2001; 32:107–115Crossref, Medline, Google Scholar
3. Tchanturia K, Anderluh MB, Morris RG, Rabe-Hesketh S, Collier DA, Sanchez P, Treasure JL: Cognitive flexibility in anorexia nervosa and bulimia nervosa. J Int Neuropsychol Soc 2004; 10:513–520Crossref, Medline, Google Scholar
4. Tchanturia K, Morris RG, Surguladze SA, Treasure JL: An examination of perceptual and cognitive set shifting tasks in acute anorexia nervosa and following recovery. Eat Weight Disord 2002; 7:312–315Crossref, Medline, Google Scholar
5. Miyake A, Friedman N, Emerson AH, Witzki AH, Howerter A, Wager TD: The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cognit Psychol 2000; 41:49–100Crossref, Medline, Google Scholar
6. Casper RC, Hedeker D, McClough JF: Personality dimensions in eating disorders and their relevance for subtyping. J Am Acad Child Adolesc Psychiatry 1992; 31:830–840Crossref, Medline, Google Scholar
7. Vitousek KB, Manke F: Personality variables and disorders in anorexia nervosa and bulimia nervosa. J Abnorm Psychol 1994; 103:137–147Crossref, Medline, Google Scholar
8. Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC: The Longitudinal Interval Follow-Up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry 1987; 44:540–548Crossref, Medline, Google Scholar
9. Fairburn CG, Cooper Z: The Eating Disorder Examination, 12th ed, in Binge Eating: Nature, Assessment and Treatment. Edited by Fairburn CG, Wilson GT. New York, Guilford, 1993, pp 317–360Google Scholar
10. Uznadze DN: The Psychology of Set. New York, Consultants’ Bureau, 1966Google Scholar
11. Kawaguchi I: [Investigation of figural after-effects from the standpoint of set-theory.] Z Psychol Z Angew Psychol 1980; 188:377–395 (German)Medline, Google Scholar
12. Burgess PW, Shallice T: The Hayling and Brixton Tests. Bury St Edmunds, UK, Thames Valley Test Co, 1997Google Scholar
13. Lezak MD: Neuropsychological Assessment. Oxford, UK, Oxford University Press, 1995Google Scholar
14. Gillberg IC: Children with minor neurodevelopmental disorders, III: neurological and neurodevelopmental problems at age 13. Dev Med Child Neurol 1985; 27:3–16Crossref, Medline, Google Scholar
15. Nelson HE, Willison J: National Adult Reading Test (NART). Windsor, UK, National Foundation for Educational Research-Nelson, 1991Google Scholar
16. Zigmond AS, Snaith RP: The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67:361–370Crossref, Medline, Google Scholar
17. Hodgson RJ, Rachman S: Obsessional-compulsive complaints. Behav Res Ther 1977; 15:389–395Crossref, Medline, Google Scholar
18. Binder DA: On the variances of asymptotically normal estimators from complex surveys. Int Stat Rev 1983; 51:279–292Crossref, Google Scholar
19. Stata Reference Manual: Release 7.0. College Station, Tex, Stata Corp, 2001Google Scholar
20. Frank GK, Kaye WH, Meltzer CC, Price JC, Greer P, McConaha C, Skovira K: Reduced 5-HT2A receptor binding after recovery from anorexia nervosa. Biol Psychiatry 2002; 52:896–906Crossref, Medline, Google Scholar
21. Kaye WH, Klump KL, Frank GK, Strober M: Anorexia and bulimia nervosa. Annu Rev Med 2000; 51:299–313Crossref, Medline, Google Scholar
22. Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC: Cognitive inflexibility after prefrontal serotonin depletion. Science 2004; 304:878–880Crossref, Medline, Google Scholar
23. Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK: Low cerebrospinal-fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci 1983; 33:2609–2614Crossref, Medline, Google Scholar
24. Soubrié P: Reconciling the role of central serotonin neurons in human and animal behavior. Behav Brain Sci 1986; 9:319–364Crossref, Google Scholar
25. Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW: 5-HT(2A) and 5-HT(2C) receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 2004; 176:376–385Crossref, Medline, Google Scholar
26. Winterer G, Goldman D: Genetics of human prefrontal function. Brain Res Brain Res Rev 2003; 43:134–163Crossref, Medline, Google Scholar
27. Egan MF, Goldberg TE, Gscheidle T, Weirich M, Rawlings R, Hyde TM: Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry 2001; 50:98–107Crossref, Medline, Google Scholar
28. Cannon TD, Zorrilla LE, Shtasel D, Gur RE, Gur RC, Marco EJ, Moberg P, Price RA: Neuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers. Arch Gen Psychiatry 1994; 51:651–661Crossref, Medline, Google Scholar
29. Cannon TD, Bearden CE, Hollister JM, Rosso IM, Sanchez LE, Hadley T: Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull 2000; 26:379–393Crossref, Medline, Google Scholar
30. Gillberg IC, Gillberg C, Rastam M, Johansson M: The cognitive profile of anorexia nervosa: a comparative study including a community-based sample. Compr Psychiatry 1996; 37:23–30Crossref, Medline, Google Scholar
31. Wentz E, Gillberg IC, Gillberg C, Rastam M: Ten-year follow-up of adolescent-onset anorexia nervosa: physical health and neurodevelopment. Dev Med Child Neurol 2000; 42:328–333Crossref, Medline, Google Scholar
32. Strober M: Pathological fear conditioning and anorexia nervosa: on the search for novel paradigms. Int J Eat Disord 2004; 35:504–508Crossref, Medline, Google Scholar
33. Kaye WH, Bastiani AM, Moss H: Cognitive style of patients with anorexia nervosa and bulimia nervosa. Int J Eat Disord 1995; 18:287–290Crossref, Medline, Google Scholar
34. Bulik CM, Tozzi F, Anderson C, Mazzeo SE, Aggen S, Sullivan PF: The relation between eating disorders and components of perfectionism. Am J Psychiatry 2003; 160:366–368Link, Google Scholar
35. Dick DM, Johnson JK, Viken RJ, Rose RJ: Testing between-family associations in within-family comparisons. Psychol Sci 2000; 11:409–413Crossref, Medline, Google Scholar



