Genotype-Phenotype Studies in Bipolar Disorder Showing Association Between the DAOA/G30 Locus and Persecutory Delusions: A First Step Toward a Molecular Genetic Classification of Psychiatric Phenotypes
Abstract
OBJECTIVE: The authors previously reported an association between the D-amino acid oxidase activator (DAOA)/G30 locus and both schizophrenia and bipolar affective disorder. Given the presumed role of DAOA/G30 in the neurochemistry of psychosis and its localization in a schizophrenia and bipolar affective disorder linkage region (13q34), it was hypothesized that the bipolar affective disorder finding would be mainly due to an association with psychotic features. METHOD: The marker/haplotype associations obtained in a subset of 173 bipolar affective disorder patients with psychotic features were similar to those in the overall patient group, suggesting that stratification on the basis of psychotic features in general might be too crude a procedure. The authors therefore tested whether confining caseness to specific psychotic features would improve detection of genotype-phenotype correlations. RESULTS: In a logistic regression, “persecutory delusions” were found to be the only significant explanatory variable for the DAOA/G30 risk genotype among 21 OPCRIT symptoms of psychosis. The authors therefore tested for association between DAOA/G30 and bipolar affective disorder in the 90 cases with a history of persecutory delusions. Whereas this subset showed strong association (odds ratio=1.83 for the best marker), the remaining larger sample of 165 patients with no such history did not differ from comparison subjects, suggesting that the association between DAOA/G30 and bipolar affective disorder is due to persecutory delusions. This was confirmed in an independent study of 294 bipolar affective disorder patients and 311 comparison subjects from Poland, in which an association between bipolar affective disorder and DAOA/G30 was only seen when case definition was restricted to cases with persecutory delusions. CONCLUSIONS: These data suggest that bipolar affective disorder with persecutory delusions constitutes a distinct subgroup of bipolar affective disorder that overlaps with schizophrenia.
The D-amino acid oxidase activator (DAOA)/G30 locus, formerly known as the G72/G30 locus, has been found to be associated with both schizophrenia and bipolar affective disorder (1, 2). We were able to replicate these findings for both disorders in a study of 299 patients with schizophrenia, 300 individuals with bipolar affective disorder (all bipolar type I), and 300 comparison subjects: a four-marker haplotype comprising markers M12 (rs1341402), M15 (rs2391191), M23 (rs3918342), and M24 (rs1421292) showed association with schizophrenia and bipolar affective disorder (3).
DAOA/G30, located on chromosome 13q34, was initially found through systematic fine-mapping (1) in a schizophrenia linkage region (4). This locus also shows linkage evidence for bipolar affective disorder (5). It is of interest that Potash et al. (6) found increased evidence of linkage with bipolar affective disorder in a neighboring region (13q31) in a conditional linkage analysis of bipolar affective disorder that used a subset of families enriched with psychotic symptoms. Despite this region being 28 cM centromeric to the DAOA/G30 locus, this linkage peak may still represent the effect conferred by DAOA/G30, given the low resolution of linkage findings (7–9). Furthermore, proneness to psychotic symptoms appears to be an inherited predisposition common to both schizophrenia and bipolar affective disorder (10). Finally, previous findings have suggested an interaction between DAOA/G30 and the gene D-amino-acid oxidase (DAAO), which is involved in the glutamatergic signaling pathway (1) and has in turn been implicated in the etiology of affective disorders and psychotic symptoms (11).
The separation of psychotic syndromes into etiologically homogeneous subtypes has been controversial among psychiatrists since the first attempts to classify psychiatric disorders (12–19). Although bipolar affective disorder and schizophrenia are defined as distinct and exclusive diagnostic entities, they show great overlap of symptoms, particularly psychotic features. The diagnostic boundaries are justified mainly by clinical convenience; their biological validity is still limited. The recent detection of disease-associated risk haplotypes in several susceptibility genes for psychiatric disorders offers the chance to test whether such an association is stronger between the haplotype and the diagnostic entity or between the haplotype and specific symptoms.
On the basis of the clinical, genetic, and neurochemical findings, we hypothesized that the association between bipolar affective disorder and the schizophrenia-associated DAOA/G30 alleles/haplotypes could be explained by the presence of psychotic symptoms in bipolar affective disorder rather than with the diagnostic entity “bipolar affective disorder” alone.
We tested this hypothesis in our group of German patients in which an overall association between DAOA/G30 and bipolar affective disorder had been detected (3). A replication study was performed in an independent group of patients from Poland that had been gathered using the same phenotype characterization procedures.
Method
Subjects
Exploratory study (German group)
This group comprised 300 patients (138 men and 162 women; mean age=42.3 years [SD=13.2]) with DSM-IV bipolar affective disorder (all bipolar type I) and 300 comparison subjects (121 men and 179 women; mean age=47.1 years [SD=15.2]) for whom we had genotype data available (3) (Table 1). Patients in this study were recruited from the same geographical area and were all of German descent. Patients were systematically recruited at the University of Bonn Department of Psychiatry. Subjects in the comparison group were anonymous blood donors of German descent recruited from the same geographical area as the patients. Written informed consent was obtained from all patients and comparison subjects. Of the 300 patients, 225 had been assessed by experienced psychiatrists with the Structured Clinical Interview for DSM-IV Disorders (SCID) (20), and 75 patients had been assessed with the Schedule for Affective Disorders and Schizophrenia—Lifetime Version (21). Lifetime “best estimate” diagnoses according to DSM-IV criteria were based on multiple sources of information including the structured interviews, medical records, and family history method. Consensus diagnoses were performed by two psychiatrists, and additional psychiatrists were included in the decision process whenever necessary (22). Furthermore, for the detailed polydiagnostic documentation of symptoms, we used the OPCRIT system (23). OPCRIT allows for the assessment and documentation of lifetime psychopathology without any preference for a particular classification system by decomposing diagnostic criteria into their component items (24).
Replication study (Polish group)
This group consisted of 294 bipolar affective disorder patients (type I: N=259, type II: N=35; 128 men and 166 women; mean age=46.9 years [SD=13.7]) and 311 comparison subjects (115 men and 196 women; mean age=43.7 years [SD=9.5]) from the Wielkopolska region (Western Poland) (Table 1). All individuals were of Polish descent. Patients were systematically recruited at two psychiatric hospitals, the Department of Psychiatry of the Poznan University of Medical Sciences and the psychiatric hospital in Koscian. The same diagnostic procedures were applied; all patients were interviewed with the SCID (20). Comparison subjects were drawn from anonymous blood donors, hospital staff, and medical students. Written informed consent was obtained from all patients and comparison subjects.
Assessment of Lifetime Psychotic Symptoms
We defined lifetime psychotic symptoms as the occurrence of delusional or hallucinatory symptoms. Patients with bipolar affective disorder were classified as having a positive history of psychosis if a “yes” rating was given on at least one of the 21 OPCRIT symptoms of delusions or hallucinations (Table 2). Of the 300 patients in the exploratory study, we identified 173 individuals with a definite positive history and 83 with a definite negative history. The latter assignment was only made when all 21 OPCRIT symptoms of psychosis were rated negative with no missing values. Thus, for 44 patients, no clear assignment could be made. Of the 294 patients in the replication study, 121 patients with a definite positive history of psychosis and 163 patients with a definite negative history were identified (10 patients could not be classified).
Genotyping
The DAOA/G30 single nucleotide polymorphisms (SNPs) genotyped in the exploratory study were determined by Masscode Technology (QIAGEN Genomics). The DAOA/G30 SNPs analyzed in the replication study were genotyped using the MassARRAY system (Sequenom, San Diego) according to Ding and Cantor (25) with minor modifications. The genotyping quality between both techniques is comparable: two SNPs (rs7320 and rs2185740) were genotyped using both techniques, the Masscode and the Mass ARRAY technology, and no discrepancies in genotypes were observed. Genotype frequencies were in Hardy-Weinberg equilibrium for cases and comparison subjects in both study groups.
Genetic Analyses
We performed both single-marker and haplotype analyses with the program COCAPHASE 2.40 (http://www.mrc-bsu.cam.ac. uk/personal/frank/software/unphased/) (26). Using a standard unconditional logistic regression, this software package performs likelihood ratio tests under a log-linear model of the probability that an allele or a haplotype belongs to a case rather than a noncase; the expectation-maximization algorithm is used to resolve uncertain haplotypes and provide maximum-likelihood estimates of frequencies.
For the single-marker analyses, a permutation procedure was used to estimate the significance of the best results, correcting for all loci tested. For haplotype analyses, the global null hypotheses that all odds ratios are equal were tested by permutation, owing to the fact that estimated haplotype frequencies cannot be treated as observed data. The permutation method implemented in COCAPHASE randomly reassigns the “patient” and “comparison” labels in the actual data. Ten thousand permutations were performed.
Comparisons were performed across clinical subgroups by stratifying the patients and comparing allele and haplotype frequencies against the same comparison population.
Phenotype-Genotype Association Analysis
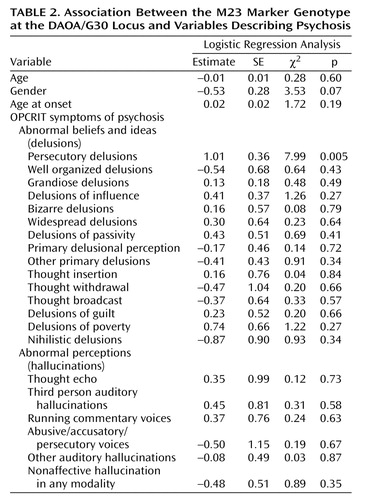
Statistical genotype-phenotype association analysis was performed by using logistic regression analysis as implemented in the SAS procedure PROC LOGISTIC. For this analysis, we chose the genotype of the DAOA/G30 marker M23, which had yielded the most marked result in our previous analysis (3), as the dependent variable with three categories (i.e., the genotypes TT, TC, CC). The 21 OPCRIT symptoms of psychosis (Table 2) were entered as explanatory variables in the model, as well as the variables age, gender, and age at onset. The logistic regression analysis was performed on a complete case basis including only those patients for whom information on all items was available (N=225). The logistic model was used in its generalized version allowing for dependent variables that take their values in a set of more than two ordered categories. This approach is based on the proportional odds assumption according to which the odds ratios related to all possible dichotomized versions of the dependent variable, except for the intercept terms, are the same function of the covariables for any choice of the cutoff. Generalized logistic regression was carried out for the 21 OPCRIT symptoms of psychosis both as a full set and in a stepwise manner selecting the symptoms that showed a significant contribution to the goodness of fit of the model.
Empirical Significance for Clinically Defined Subgroups
It has been suggested that, given a population with significant association, an improvement in p value may exist in random subsets of the population (27). Therefore, we established the empirical significance of any asymptomatically significant finding by simulation. In 10,000 replicates, random subsets of the size of the actual, clinically defined subset were drawn from the overall group and analyzed.
Power Calculations (Polish group)
Power calculations were performed using the Genetic Power Calculator (http://statgen.iop.kcl.ac.uk/gpc/cc2.html) (28) and were based on the odds ratio of 1.33 for the best associated marker (M23) in the German sample. We used both a recessive and a dominant model, assuming equal allele frequencies for the disease and marker alleles, and a D′ of 0.8 between disease and marker locus.
Results
Genetic Analysis of Bipolar Affective Disorder Patients With Psychosis
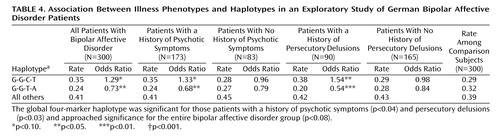
In the German group of 300 patients and 300 comparison subjects, the association between bipolar affective disorder and markers and haplotypes at the DAOA/G30 locus yielded odds ratios of around 1.3 (Table 3 and Table 4). We investigated whether this finding was due to an association with the overall group or with the subset of patients displaying psychotic features.
For the analysis of the subgroup of patients with psychotic bipolar disorder, we chose the four markers that made up the risk haplotype for both schizophrenia and bipolar affective disorder in the entire group of patients with bipolar affective disorder, i.e., M12, M15, M23, and M24.
In the single marker analysis, only M23 showed a significant association (p=0.005) in bipolar disorder patients with psychotic symptoms, as was the case for the entire group of patients with bipolar affective disorder (p<0.02). The robust permutation procedure in COCAPHASE, which we applied to correct for the four markers tested, yielded a p value of 0.012. By simulation analysis, a p value of 0.005 or smaller was observed 1,162 times out of 10,000 replicates, suggesting that the observed change in p value obtained through phenotype refinement bears a 12% probability of being a chance finding (empirical p value=0.12).
For the haplotype analyses (Table 4), significant associations were seen for the global four-marker haplotype (p=0.04) and the individual haplotype G-G-T-A (p<0.03), with the G-G-C-T haplotype approaching significance (p<0.09), respectively.
The 83 patients with bipolar affective disorder and no lifetime history of delusional or hallucinatory symptoms showed no association, either in the single marker or in the haplotype analyses (Table 3 and Table 4).
Further Phenotype-Genotype Analyses
Given that the aforementioned stratification on psychotic features might still be too crude a procedure for refining phenotypes, we were interested in whether the association between bipolar affective disorder and DAOA/G30 was explained instead by an even more specific subset, defined by specific psychotic features. We performed a logistic regression in a model that included age, gender, age at onset, and the 21 OPCRIT symptoms of psychosis (Table 2). This analysis identified the variable “lifetime history of persecutory delusions” as the only significant (p<0.005) explanatory variable for the M23 genotype. Individuals with a history of persecutory delusions were more likely to have genotypes containing the C allele than those with no such history. Generalized logistic regression was carried with the 21 OPCRIT symptoms of psychosis both as a full set and in a stepwise manner. Since both approaches led to the same qualitative conclusions, only the results of analyzing the full multivariate model are presented.
We thus divided our group of bipolar affective disorder patients for whom detailed information regarding the OPCRIT item “persecutory delusion” was available into those with (N=90) and those without (N=165) a history of persecutory delusions in order to perform two separate comparisons with the comparison group. When comparing the subset of 90 bipolar disorder patients with persecutory delusions versus comparison subjects, markers M12 and M15 did not yield significant results. As seen in Table 3, we observed significant association for markers M23 (p<0.0004) and M24 (p<0.007).
In a simulation analysis, p values <0.0004 and <0.007 were observed for, respectively, 24 and 67 out of 10,000 random subsets of 90 individuals, indicating that the association findings at these significance levels are very unlikely to occur in equally sized random subsets (p=0.0024 and p=0.0067).
In the haplotype analysis (Table 4), significant associations were seen for the global four-marker haplotype (p<0.03) and the individual haplotypes G-G-T-A (odds ratio=0.54, p<0.006) and G-G-C-T (odds ratio=1.54, p<0.04). We also evaluated the empirical significance of these haplotype-based results: a p value smaller or equal to 0.006 would be seen in 1,360 out of 10,000 random subsets of 90 individuals (p=0.136). Comparing the larger subsample of patients with bipolar disorder and no history of persecutory delusions (N=165) with the comparison group (N=300) yielded no association (odds ratios ~1) (Table 3, Table 4). We also compared the comparison subjects with those 83 patients that had a history of psychotic symptoms but not of persecutory delusions (i.e., the 173 patients with psychosis minus the 90 patients with a history of persecutory delusions [see Table 1]). For all four markers, again, there was no association. Allele and haplotype frequencies were similar between this particular subset of cases and comparison subjects (odds ratios ranged from 0.86 to 1.14, p values ranged from 0.50 to 0.97 [data not shown]).
The subsample of patients with bipolar disorder with persecutory delusions did not differ from the entire sample of patients with bipolar disorder regarding gender (χ2=0.37, p=0.54), age (t=0.26, p=0.79), or age at onset (t=0.62, p=0.536).
Given that only markers M23 and M24 showed association with bipolar affective disorder, we analyzed these in our replication study with the Polish group. As with the analysis of the German subjects, we performed single-marker and haplotype-based analyses, using three case definitions: all patients with bipolar affective disorder, a subgroup of those with a history of psychotic symptoms, and a subgroup of those with a history of persecutory delusions. In the single-marker analysis, neither M23 nor M24 showed significant association with bipolar affective disorder in any of the three sets (Table 5). However, for M23, we observed a nearly significant association (odds ratio=1.48, p<0.08) in the subset of bipolar disorder patients with persecutory delusions. Similar to the German group, the bipolar disorder patients with persecutory delusions were enriched with the C allele relative to the entire group of bipolar affective disorder patients (57% versus 49%). With regard to the haplotype analysis, no association was observed in the bipolar affective disorder patients and the bipolar affective disorder subgroup with a history of psychotic symptoms. When case definition was confined to those with persecutory delusions, however, we observed a significant association (Table 6): The C-T haplotype, which is part of the associated G-G-C-T haplotype in the German subjects, was similarly more frequent in patients than in comparison subjects (odds ratio=1.61, p<0.04). We also evaluated the empirical significance of this haplotype-based result: a p value ≤0.04 would be seen in 220 out of 10,000 random subsets of 55 individuals (p=0.022). Thus, while there was no association when using the total group of patients with bipolar affective disorder or the subgroup with a history of psychotic symptoms, there was a significant one when cases were restricted to patients with persecutory delusions. The “protective” haplotype T-A did not show a significant association. It is interesting to note, however, that it showed the same frequency in the total group of bipolar affective disorder patients, the subgroup of bipolar affective disorder patients with psychotic symptoms, and comparison subjects while being less frequent in bipolar disorder patients with persecutory delusions, as observed in the German subjects.
Discussion
We had previously reported an association between bipolar affective disorder and DAOA/G30 (3). Given that DAOA/G30 has also been shown to be associated with schizophrenia (1, 3), we were interested in whether bipolar affective disorder patients with a history of psychotic symptoms would differ from those with no such history. Restricting case definition to the subgroup of patients with a history of psychotic symptoms yielded no significant results. We were therefore interested in whether confining case definition to specific delusional or hallucinatory symptoms would be better at revealing a potential genotype-phenotype correlation between bipolar affective disorder and DAOA/G30.
Our analysis revealed that the association between bipolar affective disorder and the DAOA/G30 locus is an association involving those with a history of persecutory delusions rather than bipolar affective disorder in general. In fact, patients with no history of persecutory delusions did not differ at all from comparison subjects with regard to their distribution of DAOA/G30 marker alleles and haplotypes. These findings are supported by our analysis in an independent group of bipolar affective disorder patients and comparison subjects from Poland.
A general limitation of case/control analyses is the susceptibility to population stratification. The patients and comparison subjects investigated in the German and Polish samples were all of German and Polish descent, respectively, and were recruited from the same well circumscribed geographical areas (the cities of Bonn and Poznan and their vicinities). Preliminary results from large-scale genomic analyses in several population-based samples from different geographic German regions (National Genome Research Network [http://www.ngfn.de/]) suggest that population stratification is negligible in the German population (personal communication, M. Krawczak, June 2, 2004). Furthermore, an association between bipolar affective disorder and markers on or near to DAOA/G30 has now been shown in three independent samples from Germany (3) and the United States (2, 29), rendering the possibility of this being a mere artifact due to population stratification unlikely.
Our observations in the exploratory and replication studies suggest that the subgroup of patients with persecutory delusions represents a genetically more homogenous group than patients with bipolar affective disorder as a whole. The intuitive approach to divide bipolar affective disorder patients into those with versus those without psychotic features might not be sufficient for determining biologically meaningful subtypes for genetic studies. Psychosis is perhaps too broad a concept; well-established clinical subtypes of psychosis might be more amenable to genetic study. The gene mapping efforts in inflammatory bowel disease—a group of genetically heterogeneous, complex phenotypes (i.e., Crohn’s disease, ulcerative colitis)—may serve as an example. For this condition, detailed phenotypic characterization and the establishment of fine-scaled diagnostic categories (according to, for example, anatomic location of disease, extent of disease, and disease behavior) helped to detect the underlying genetic factors (30).
The importance of such detailed refinement of phenotype is illustrated in the Polish study. In the overall group, no association between DAOA/G30 markers and bipolar affective disorder could be detected. This could be due to the limited power (less than 20%) of the Polish study. However, we were able to detect an association in the Polish subjects when only patients with persecutory delusions were considered, despite the even lower a priori power of this subset of less than 10%. To our knowledge, this is the first time in psychiatric genetics that an association between a genotype and a specific clinical feature of a broader phenotype could be replicated in an independent sample. Given the inconsistencies of genetic findings in the field of psychiatric association studies, our approach may serve as an example of how to identify more homogeneous samples, thus enhancing the chances of success in replication attempts (31).
Formal genetic analyses (10, 32–35) have suggested that psychotic symptoms are an inherited predisposition common to both schizophrenia and bipolar affective disorder. The observation in our study that the risk-conferring DAOA/G30 haplotype is the same in both bipolar affective disorder and schizophrenia indicates that not only the same gene but the same (hitherto undetected) variant contributes to both disorders. The reported genotype-phenotype correlation further pinpoints the nature of the DAOA/G30-mediated genetic overlap between schizophrenia and bipolar affective disorder.
While our findings support the idea of a genetic overlap between schizophrenia and bipolar affective disorder, the question remains whether the symptom “persecutory delusion” per se is important or rather some other trait that correlates with it. What could that be? A patient’s idea of future harm triggering anxiety-associated processes has been suggested as a key feature of persecutory delusions (36). One could speculate that a delusional patient with a genetic proneness to anxiety may be more likely to have delusions of a persecutory nature than a patient without this predisposition. Put another way, delusions of reference or influence that are accompanied by fear are more likely to be labeled as persecutory delusions. Clinical experience has long taught us about a connection between anxiety and persecutory delusions in the context of toxic psychoses such as PCP and amphetamine intoxication, where extreme anxiety, panic, and persecutory delusions usually occur together (37–39).
With this in mind, it is intriguing that we recently identified the same DAOA/G30 markers and haplotypes as potential risk factors for panic disorder (40).
The genetic overlap between schizophrenia and bipolar affective disorder hints at a weakness of specificity of the current classification systems, which are based merely on clinical symptoms. To our knowledge, our study is the first systematic genotype-phenotype analysis of a well-established susceptibility gene for schizophrenia and bipolar affective disorder and may be considered a first step toward a molecular genetic classification of psychiatric phenotypes.
 |
 |
 |
 |
 |
 |
Received July 26, 2004; revision received Oct. 30, 2004; accepted Dec. 3, 2004. From the Department of Psychiatry, Division of Genetic Epidemiology in Psychiatry, and Division of Biostatistics, Central Institute of Mental Health, University of Heidelberg; the University of Bonn (the Department of Psychiatry; Institute of Human Genetics; Institute for Medical Biometry, Informatics and Epidemiology; and Department of Genomics, Life & Brain Center), Bonn, Germany; the Genetics Unit, Mood and Anxiety Disorders Program, National Institute of Mental Health, Bethesda, Md.; the Department of Adult Psychiatry, Poznan University of Medical Sciences, Poznan, Poland; the Institute of Epidemiology and the Genome Analysis Center, GSF-National Research Center for Environment and Health, Neuherberg, Germany; and the University of Antwerp Department of Medical Genetics, Antwerp, Belgium. Address correspondence and reprint requests to Dr. Schulze, Division of Genetic Epidemiology in Psychiatry, Central Institute of Mental Health–J5, 68159 Mannheim, Germany; [email protected] (e-mail). The authors thank all individuals who cooperated in this study and acknowledge critical input received from Celia Greenwood and Wei Xu. Supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression (Dr. Schulze) and the 2004 Annual Stipend for Young Scientists from The Foundation for Polish Science (Dr. Czerski). Funding for this study was received from the National Genome Research Network of the German Ministry of Education and Research; the Deutsche Forschungsgemeinschaft (SFB 400 subprojects D1 and D3, FOR 423 subproject D1, Graduiertenkolleg GRK 246); the Fund for Scientific Research, Flanders (grants G.0425.02 and G.0438.03); and the Belgium Interuniversity Attraction Pole (“Molecular Genetics and Cell Biology”), a concerted research project by the University of Antwerp, the Alfried Krupp von Bohlen und Halbach-Stiftung, and the Polish State Committee for Scientific Research (grant 6P05B 05320).
1. Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, Bougueleret L, Barry C, Tanaka H, La Rosa P, Puech A, Tahri N, Cohen-Akenine A, Delabrosse S, Lissarrague S, Picard FP, Maurice K, Essioux L, Millasseau P, Grel P, Debailleul V, Simon AM, Caterina D, Dufaure I, Malekzadeh K, Belova M, Luan JJ, Bouillot M, Sambucy JL, Primas G, Saumier M, Boubkiri N, Martin-Saumier S, Nasroune M, Peixoto H, Delaye A, Pinchot V, Bastucci M, Guillou S, Chevillon M, Sainz-Fuertes R, Meguenni S, Aurich-Costa J, Cherif D, Gimalac A, Van Duijn C, Gauvreau D, Ouellette G, Fortier I, Raelson J, Sherbatich T, Riazanskaia N, Rogaev E, Raeymaekers P, Aerssens J, Konings F, Luyten W, Macciardi F, Sham PC, Straub RE, Weinberger DR, Cohen N, Cohen D: Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA 2002; 99:13675–13680; correction 99:17221Crossref, Medline, Google Scholar
2. Hattori E, Liu C, Badner JA, Bonner TI, Christian SL, Maheshwari M, Detera-Wadleigh SD, Gibbs RA, Gershon ES: Polymorphisms at the G72/G30 gene locus, on 13q33, are associated with bipolar disorder in two independent pedigree series. Am J Hum Genet 2003; 72:1131–1140Crossref, Medline, Google Scholar
3. Schumacher J, Jamra RA, Freudenberg J, Becker T, Ohlraun S, Otte AC, Tullius M, Kovalenko S, Bogaert AV, Maier W, Rietschel M, Propping P, Nöthen MM, Cichon S: Examination of G72 and D-amino-acid oxidase as genetic risk factors for schizophrenia and bipolar affective disorder. Mol Psychiatry 2004; 9:203–207Crossref, Medline, Google Scholar
4. Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV, Brzustowicz LM, Kaufmann CA, Garver DL, Gurling HM, Lindholm E, Coon H, Moises HW, Byerley W, Shaw SH, Mesen A, Sherrington R, O’Neill FA, Walsh D, Kendler KS, Ekelund J, Paunio T, Lonnqvist J, Peltonen L, O’Donovan MC, Owen MJ, Wildenauer DB, Maier W, Nestadt G, Blouin JL, Antonarakis SE, Mowry BJ, Silverman JM, Crowe RR, Cloninger CR, Tsuang MT, Malaspina D, Harkavy-Friedman JM, Svrakic DM, Bassett AS, Holcomb J, Kalsi G, McQuillin A, Brynjolfson J, Sigmundsson T, Petursson H, Jazin E, Zoega T, Helgason T: Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: schizophrenia. Am J Hum Genet 2003; 73:34–48Crossref, Medline, Google Scholar
5. Badner JA, Gershon ES: Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry 2002; 7:405–411Crossref, Medline, Google Scholar
6. Potash JB, Zandi PP, Willour VL, Lan T-H, Huo Y, Avramopoulos D, Shugart YY, MacKinnon DF, Simpson SG, McMahon FJ, DePaulo JR Jr, McInnis MG: Suggestive linkage to chromosomal regions 13q31 and 22q12 in families with psychotic bipolar disorder. Am J Psychiatry 2003; 160:680–686Link, Google Scholar
7. Terwilliger JD, Shannon WD, Lathrop GM, Nolan JP, Goldin LR, Chase GA, Weeks DE: True and false positive peaks in genomewide scans: applications of length-biased sampling to linkage mapping. Am J Hum Genet 1997; 61:430–438Crossref, Medline, Google Scholar
8. Roberts SB, MacLean CJ, Neale MC, Eaves LJ, Kendler KS: Replication of linkage studies of complex traits: an examination of variation in location estimates. Am J Hum Genet 1999; 65:876–884Crossref, Medline, Google Scholar
9. Gordon D, Hoh J, Finch SJ, Levenstien MA, Edington J, Li W, Majewski J, Ott J: Two approaches for consolidating results from genome scans of complex traits: selection methods and scan statistics. Genet Epidemiol 2001; 21(suppl 1):S396-S402Google Scholar
10. Schürhoff F, Szöke A, Méary A, Bellivier F, Rouillon F, Pauls D, Leboyer M: Familial aggregation of delusional proneness in schizophrenia and bipolar pedigrees. Am J Psychiatry 2003; 160:1313–1319Link, Google Scholar
11. Millan MJ: N–Methyl-d–aspartate receptor-coupled glycineB receptors in the pathogenesis and treatment of schizophrenia: a critical review. Curr Drug Target CNS Neurol Disord 2002; 1:191–213Crossref, Medline, Google Scholar
12. Guislain J: Leçons orales sur les phrénopathies ou traité théorique et pratique des maladies mentales. Ghent, Belgium, L Hebbelynck, 1852Google Scholar
13. Griesinger W: Die Pathologie und Therapie der psychischen Krankheiten: für Ärzte und Studierende. Stuttgart, Germany, Krabbe, 1861Google Scholar
14. Kraepelin E: Die Erscheinungsformen des Irreseins. Z Gesamte Neurol Psychiat 1920; 62:1–29Crossref, Google Scholar
15. Leonhard K: Aufteilung der endogenen Psychosen und ihre differenzierte Ätiologie. Berlin, Akademie Verlag, 1957Google Scholar
16. Crow TJ: The continuum of psychosis and its implication for the structure of the gene. Br J Psychiatry 1986; 149:419–429Crossref, Medline, Google Scholar
17. Maier W, Lichtermann D, Minges J, Hallmayer J, Heun R, Benkert O, Levinson DF: Continuity and discontinuity of affective disorders and schizophrenia: results of a controlled family study. Arch Gen Psychiatry 1993; 50:871–883Crossref, Medline, Google Scholar
18. Gershon ES, DeLisi LE, Hamovit J, Nurnberger JI Jr, Maxwell ME, Schreiber J, Dauphinais D, Dingman CW II, Guroff JJ: A controlled family study of chronic psychoses: schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 1988; 45:328–336Crossref, Medline, Google Scholar
19. Berrettini WH: Are schizophrenic and bipolar disorders related? a review of family and molecular studies. Biol Psychiatry 2000; 48:531–538Crossref, Medline, Google Scholar
20. Spitzer RL, Williams JBW, Gibbon M, First MB: The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Crossref, Medline, Google Scholar
21. Endicott J, Spitzer RL: A diagnostic interview: the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry 1978; 35:837–844Crossref, Medline, Google Scholar
22. Fangerau H, Ohlraun S, Granath RO, Nöthen MM, Rietschel M, Schulze TG: Computer-assisted phenotype characterization for genetic research in psychiatry. Hum Hered 2004; 58:122–130Crossref, Medline, Google Scholar
23. McGuffin P, Farmer A, Harvey I: A polydiagnostic application of operational criteria in studies of psychotic illness: development and reliability of the OPCRIT system. Arch Gen Psychiatry 1991; 48:764–770Crossref, Medline, Google Scholar
24. McGuffin P, Farmer A: Polydiagnostic approaches to measuring and classifying psychopathology. Am J Med Genet 2001; 105:39–41Crossref, Medline, Google Scholar
25. Ding C, Cantor CR: A high-throughput gene expression analysis technique using competitive PCR and matrix-assisted laser desorption ionization time-of-flight MS. Proc Natl Acad Sci USA 2003; 100:3059–3064Crossref, Medline, Google Scholar
26. Dudbridge F: Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol 2003; 25:115–121Crossref, Medline, Google Scholar
27. Johnson GC, Koeleman BP, Todd JA: Limitations of stratifying sib-pair data in common disease linkage studies: an example using chromosome 10p14–10q11 in type 1 diabetes. Am J Med Genet 2002; 113:158–166Crossref, Medline, Google Scholar
28. Purcell S, Cherny SS, Sham PC: Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics 2003; 19:149–150Crossref, Medline, Google Scholar
29. Chen YS, Akula N, Detera-Wadleigh SD, Schulze TG, Thomas J, Potash JB, DePaulo JR, McInnis MG, Cox NJ, McMahon FJ: Findings in an independent sample support an association between bipolar affective disorder and the G72/G30 locus on chromosome 13q33. Mol Psychiatry 2004; 9:87–92Crossref, Medline, Google Scholar
30. Duerr RH: Update on the genetics of inflammatory bowel disease. J Clin Gastroenterol 2004; 37:358–367Crossref, Google Scholar
31. Schulze TG, Hardy J, McMahon FJ: Inconsistent designs of association studies: a missed opportunity. Mol Psychiatry 2003; 8:770–772Crossref, Medline, Google Scholar
32. Kendler KS, Karkowski LM, Walsh D: The structure of psychosis: latent class analysis of probands from the Roscommon Family Study. Arch Gen Psychiatry 1998; 55:492–499Crossref, Medline, Google Scholar
33. Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D: The Roscommon Family Study, IV: affective illness, anxiety disorders, and alcoholism in relatives. Arch Gen Psychiatry 1993; 50:952–960Crossref, Medline, Google Scholar
34. Maier W, Lichtermann D, Franke P, Heun R, Falkai P, Rietschel M: The dichotomy of schizophrenia and affective disorders in extended pedigrees. Schizophr Res 2002; 57:259–266Crossref, Medline, Google Scholar
35. Cardno AG, Rijsdijk FV, Sham PC, Murray RM, McGuffin P: A twin study of genetic relationships between psychotic symptoms. Am J Psychiatry 2002; 159:539–545Link, Google Scholar
36. Freeman D, Garety PA: Comments on the content of persecutory delusions: does the definition need clarification? Br J Clin Psychol 2000; 39:407–414Crossref, Medline, Google Scholar
37. Snyder SH: Amphetamine psychosis: a “model” schizophrenia mediated by catecholamines. Am J Psychiatry 1973; 130:61–67Link, Google Scholar
38. Bell DS: Comparison of amphetamine psychosis and schizophrenia. Br J Psychiatry 1965; 111:701–707Crossref, Medline, Google Scholar
39. Gorelick DA, Balster RL: Phencyclidine (PCP), in Psychopharmacology: The Fourth Generation of Progress. Edited by Bloom FE, Kupfer DJ. New York, NY, Raven Press, 1995, pp 1767–1776Google Scholar
40. Schumacher J, Abou Jamra R, Becker T, Klopp N, Franke P, Jacob C, Sand P, Fritze J, Ohlraun S, Schulze TG, Rietschel M, Illig T, Propping P, Cichon S, Deckert J, Nöthen MM: Investigation of the DAOA/G30 locus in panic disorder. Mol Psychiatry 2005; 10:428–429Crossref, Medline, Google Scholar



