Morphology of the Anterior Cingulate Gyrus in Patients With Schizophrenia: Relationship to Typical Neuroleptic Exposure
Abstract
OBJECTIVE: The anterior cingulate gyrus is a pivotal component of brain networks directing affective and cognitive functions, and abnormalities of the anterior cingulate gyrus may be involved in the pathophysiology of schizophrenia. However, magnetic resonance imaging studies of the morphology of this region have been few and the results inconsistent. Many of the studies have not accounted for confounding factors such as gender, handedness, or clinical variables such as neuroleptic exposure. METHOD: The morphology and clinical correlates of the anterior cingulate gyrus were evaluated in a group of 30 right-handed male subjects with schizophrenia and a comparison group matched for age, sex, and handedness. The patient group was specifically designed to provide measures of multiple phenomenological differences such as severity of illness, duration of illness, and exposure to typical neuroleptics. RESULTS: The patient group had a significantly larger left anterior cingulate gyrus volume relative to comparison subjects, whereas right anterior cingulate gyrus volume did not differ between groups. Depth of the left anterior cingulate gyrus was significantly correlated with neuroleptic exposure, indicating that greater exposure to neuroleptics was associated with greater cortical depth of this region. There was no significant relationship between anterior cingulate gyrus morphology and duration of illness or severity of symptoms. CONCLUSIONS: Anterior cingulate gyrus depth in subjects with schizophrenia is directly related to typical medication exposure (the greater the exposure, the thicker the anterior cingulate gyrus) but not to other clinical variables such as length of illness or symptom severity. Future research is needed to determine the functional consequences of these changes and relationship to atypical neuroleptic use.
The anterior cingulate gyrus is a pivotal component of brain networks directing affective and cognitive functions (1, 2). As part of the rostral limbic system, the anterior cingulate gyrus modulates internal emotional responses (2, 3). Cognitively, the anterior cingulate gyrus is considered to be an integral component of a variety of executive functions such as motivation, attention, working memory, learning (especially in novel situations and when overcoming habitual responses), decision making, and awareness and insight (1, 2, 4).
Structurally, the anterior cingulate gyrus is located bilaterally in the medial wall of the frontal lobes, following the curvature of the genu of the corpus callosum. The anterior cingulate gyrus is contiguous with the posterior cingulate; the two together form the cingulate gyrus. While the cingulate gyrus has the appearance of a single structural unit, functionally the anterior cingulate gyrus is quite different from the posterior cingulate gyrus, which is involved in visuospatial and memory function (2).
Within schizophrenia research, several lines of evidence suggest that structural and functional abnormalities of the anterior cingulate gyrus are involved in the pathophysiology of the illness. In functional neuroimaging research, positron emission tomography (PET) studies have shown differences in error monitoring (5), working memory control (6, 7), and selective attention (8, 9) and decreases in dopamine activation (10), glucose metabolism (11), regional cerebral metabolism (12), and regional cerebral blood flow (13). Electrophysiological studies have shown decreased anterior cingulate gyrus activation and slowed reaction times during information processing in subjects with schizophrenia (14, 15). Postmortem studies have shown key cytoarchitectural changes in layer II of the anterior cingulate gyrus and a decreased density of nonpyramidal neurons (16, 17) upon microscopic exam of people with schizophrenia versus normal subjects. However, gross examinations of postmortem brains have not shown observable changes (18, 19).
The literature on the structure of the anterior cingulate gyrus in schizophrenia as determined with magnetic resonance imaging (MRI) is small and highly inconsistent. Of the studies focusing on the overall anterior cingulate gyrus, findings have shown both a lack of significant differences in anterior cingulate gyrus volume in patients relative to comparison subjects (20, 21) as well as significant bilateral decreases in anterior cingulate gyrus volume in patients relative to comparison subjects (22, 23). Two studies have reported a significant asymmetry in anterior cingulate gyrus volume, with the left hemisphere anterior cingulate gyrus being smaller than the right (21, 24). Further studies have shown that the differences in the volume of the anterior cingulate gyrus are related to gender. Takahashi et al. (25) and Suzuki et al. (22) found a decrease in gray matter in the right anterior cingulate gyrus in female but not in male subjects. Szeszko et al. (26) found a correlation between decreased anterior cingulate gyrus volume and executive function in male but not female subjects with schizophrenia. Finally, Yucel et al. (27, 28) have taken a more qualitative approach by examining the sulcal-gyral morphology of this region, focusing on the presence or absence of a paracingulate sulcus. Studies using this method have documented significant gender effects in healthy subjects (27) as well as findings among male subjects with schizophrenia indicating abnormal cortical development in this region (28).
Why are the findings among anterior cingulate gyrus structural studies so varied? Many studies have not accounted for confounding differences within study groups. For example, gender differences have not been accounted for between subjects. MRI studies in normal subjects demonstrate a marked asymmetry of the anterior cingulate gyrus such that the right is significantly larger than the left. This laterality is not consistent across gender lines. Typically, women show a greater degree of asymmetry than men (3, 25, 29). However, one study found the normal asymmetry greater in male than in female subjects (27). Often, studies have also not accounted for differences between right- and left-handed patients. Other factors that could potentially affect the morphology of this region include duration of illness or exposure to neuroleptic medications. No studies to date have accounted for these variables.
Furthermore, the anterior cingulate gyrus is a region with poorly defined boundaries. These boundaries have most often been identified structurally as opposed to functionally. Methods of first defining and then parcellating the anterior cingulate have varied widely between studies. Thus, the “anterior cingulate” has not been rigidly defined as a set region replicable between studies. One example of this inconsistency in definition is that some studies have included the paracingulate region as part of the anterior cingulate (21) while other studies have not (20).
In a previous study by our laboratory, neuroleptic-naive, first-episode, right-handed male patients were found to have no significant differences in anterior cingulate gyrus morphologic measures relative to healthy comparison subjects (30). This current study was designed to reproduce and extend these findings by using a reliable and replicable MRI-based method of cortical parcellation to evaluate anterior cingulate gyrus morphology in a group of 30 subjects with schizophrenia compared with a matched group of 30 healthy subjects. Since symmetry of the anterior cingulate gyrus is affected by gender and handedness, the study group was restricted to right-handed male subjects. The patient group was specifically designed to provide a high degree of variability in multiple phenomenological variables (e.g., severity of illness, duration of illness, and exposure to neuroleptics) in order to assess the relationship between these variables and structure of the anterior cingulate gyrus.
Method
Subjects
All patients were inpatient admissions to the Mental Health Clinical Research Center at the University of Iowa Hospitals and Clinics. Because atypical neuroleptics have been shown to have a different effect on the morphology of brain tissue compared with typical neuroleptics, subjects who had been treated only with typical neuroleptics were included (31). All subjects were right-handed. Subjects who had ever received an atypical neuroleptic were excluded from the study. Currently, the vast majority of patients with schizophrenia are treated with atypical neuroleptics. It is important to note that the patients in the current study were assessed in the early to mid-1990s when atypical neuroleptics were just being introduced. Therefore, at the time these patients were a representative sample of the patient population as a whole.
Of the 30 patients, six were “first episode” (defined as first psychiatric hospitalization), 11 were “recent onset” (within 5 years), and the remaining 13 were “chronic” (disease duration greater than 5 years). There is no patient group overlap between our previously reported study of regional frontal lobe morphology (30) and the current study.
The “dose year” formula (32) was used to measure neuroleptic exposure. This required conversion of neuroleptic medication to chlorpromazine equivalents (33). Exposure was calculated over time, weighted for dose using the formula (X mg × Y years/100). At the time of scanning, all subjects had been exposed to typical neuroleptics. Exposure ranged from 0.5 to 650 “dose years.”
All patients met DSM-III-R criteria for schizophrenia and were evaluated with the Comprehensive Assessment of Symptoms and History (34). Clinical symptoms were rated using the Scale for the Assessment of Negative Symptoms (SANS) (35) and the Scale for the Assessment of Positive Symptoms (SAPS) (36). Summary scores for three dimensions of symptoms (psychotic, negative, and disorganized) were calculated using sums of global scores from the SANS/SAPS. The psychotic symptom dimension was the sum of global scores for hallucinations and delusions. The negative symptom dimension score was the sum of global scores for alogia, affective flattening, avolition-apathy, and anhedonia-asociality. The disorganized symptom dimension comprised the global scores of positive formal thought disorder, disorganized/bizarre behavior, and inappropriate affect. A “global” index of severity was calculated by summing the scores of all three dimensions.
The comparison subjects were 30 healthy men individually matched with the patients for age and handedness. These subjects were recruited from the community through newspaper advertisements. They had no current or lifetime history of psychiatric, neurological, or general medical illness, including substance abuse, as determined with an abbreviated version of the Comprehensive Assessment of Symptoms and History.
All subjects gave written informed consent in accordance with the Human Subjects Institutional Review Board of the University of Iowa.
Table 1 summarizes the demographic characteristics of the two groups. There were no significant differences between groups in age, height, and socioeconomic status of their parents. As expected, there was a significant difference in education between groups (t=2.52, df=58, p<0.02).
MRI Acquisition
All multimodal MRI scans were obtained at the University of Iowa Hospitals and Clinics using a 1.5-T General Electric SIGNA System (GE Medical Systems, Milwaukee). Three-dimensional T1-weighted images, using a spoiled grass sequence, were acquired in the coronal plane with the following parameters: echo time (TE)=5 msec, repetition time (TR)=24 msec, number of excitations=2, rotation angle=45°, field of view=26×24×18.8 cm, and a matrix of 256×192×124. Two-dimensional proton density and T2 sequences were acquired as follows: coronal slices (thickness: 3.0 mm or 4.0 mm), TR=3000 msec, TE=36 msec (for proton density) and 96 msec (for T2), number of excitations=1, field of view=26×26 cm, matrix=256×192.
Image Processing
MR data were processed on Silicon Graphics workstations by using locally developed software (BRAINS2 [37]). The T1-weighted images were spatially normalized so that the anterior-posterior axis of the brain was realigned parallel to the anterior-posterior commissure line, and the interhemispheric fissure was aligned on the other two axes. A 6-point transformation was used to place images into the standard Talairach orientation (38), and the images were resampled to 1.0156-mm cubic voxels. This process allows for the mapping of Talairach coordinates onto the image using a piecewise linear interpolation to account for the sizes of the individual brains. However, the individual’s image is not warped or scaled in any way.
The T2- and proton density-weighted images were aligned to the spatially normalized T1-weighted image. The data sets were then segmented using the multispectral data and a discriminate analysis method based on automated training class selection (39). Segmented images are processed by using the BRAINS2 program, which generates a visual map and quantitative measures of brain surface anatomy. The segmented image is used to extract a triangle-based polygonal model of an iso-surface, representing the parametric center of the gray matter tissue class. This triangulated surface was used as the basis for our calculations of cortical area, depth, and volume (40).
Region of Interest Definition and Reliability
Quantitative measures of the asymmetry coefficient were obtained using a previously developed and validated cortical parcellation atlas of the frontal lobe (41). Details of the region of interest are subsequently described, but in general the region of interest consisted of the anterior section of the cingulate corresponding to Brodmann’s area 25 and excluding the paracingulate gyrus.
Boundaries
The macroscopic limit between the anterior and posterior cingulate has not been clearly established in the literature, leading to the need for an arbitrary but consistent landmark to differentiate between the two parts of the cingulate gyrus. Plane B, an imaginary vertical line passing through the most anterior tip of the inner surface of the genu of the corpus callosum, was the boundary between anterior and posterior cingulate (41). The cingulate sulcus represented the outer boundary of the anterior cingulate gyrus; the callosal sulcus represented the inner boundary. The anterior cingulate gyrus presented as a double structure that extended above and below the corpus callosum.
Tracing
The anterior cingulate gyrus was traced on alternating serial coronal slices (every 1.0 mm). However, sagittal and transaxial slices were also examined to obtain an overall view of the anterior cingulate. The deepest point of the callosal sulcus and the most medial point of the dorsal bank of the cingulate sulcus constituted the inner and the outer boundary of the anterior cingulate in each coronal slice respectively. Tracing began at the Plane B and continued rostrally until the most anterior coronal slice containing anterior cingulate tissue. The precingulate gyrus was not included in the area of the anterior cingulate gyrus.
Reliability
The reliability study for the anterior cingulate was then performed by one of the authors (A.K.) on a set of 10 “gold standard” brains, separate from the data set. High intraclass correlation coefficients were seen for the right hemisphere (r=0.991) and left hemisphere (r=0.848) anterior cingulate gyrus.
Morphologic measures
From the anterior cingulate gyrus tracings, measures of cortical volume (cm3), surface area (mm2) and cortical depth (mm) were obtained. In addition, an asymmetry coefficient, a measure of laterality, for the anterior cingulate gray matter volume was calculated using the following formula: (right – left)/(right + left) × 100. A positive value indicates a larger right-sided structure.
Statistical Analyses
All morphologic measures of the anterior cingulate gyrus were subjected to analysis of covariance, with diagnosis as the between-group factor and age and the total intracranial volume as the covariates. A t test was used to compare the asymmetry coefficient between groups.
To evaluate the relationship between anterior cingulate morphology and the clinical correlates of symptom severity, illness duration, and cumulative neuroleptic exposure, Pearson r partial correlation coefficients were calculated that controlled for the effects of age and intracranial volume. In order to prevent a type I error, only those morphologic measures that significantly differed between groups were used in the correlation analyses.
Results
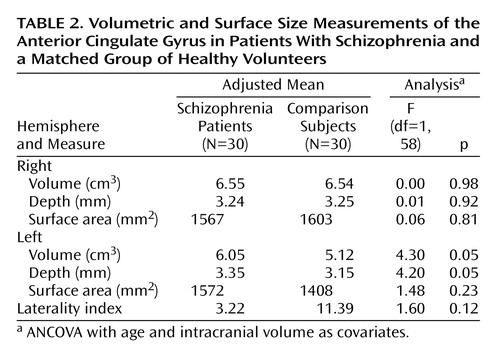
Volumetric and Surface Size Measurements
Comparisons of mean cortical volumes between subjects and healthy volunteers, adjusted for intracranial volume and age, are shown in Table 2. Patients with schizophrenia showed a significant enlargement of the left anterior cingulate gyrus relative to comparison subjects. This enlargement appeared to be due to a significant increase in cortical depth, since surface area was not significantly different between the two groups. No significant differences between the two groups were found for right hemisphere volume, depth, or surface area. The laterality index is a measure of asymmetry between right and left hemispheres, with a positive value indicating right larger than left. Healthy subjects showed a laterality index favoring the right hemisphere, replicating results from other studies of normal subjects (3, 27). Patients with schizophrenia showed a noticeable but nonsignificant reduction of asymmetry relative to comparison subjects.
Correlations Between Morphology and Clinical Variables
Within the patient group, correlations between left hemisphere anterior cingulate gyrus morphology and clinical variables are shown in Table 3. Cortical depth of the anterior cingulate gyrus on the left was significantly correlated with dose years: the greater the dose years, the greater the depth of the left anterior cingulate gyrus. There was no significant correlation between left hemisphere anterior cingulate gyrus volume or depth and duration of illness. Since dose years, calculated by measuring the amount of drug used over time, were highly correlated with duration of illness (r=0.64, df=28, p=0.0002), it was necessary to isolate the effects of neuroleptic exposure on anterior cingulate gyrus morphology. This was done by recalculating the correlations between dose years and anterior cingulate gyrus morphology after controlling for duration of illness. The results remained similar but were now more robust (dose years and volume: r=0.37 [p<0.08]); dose years and cortical depth: r=0.61 [p=0.001]).
Table 3 also shows the results of correlation analyses between left hemisphere anterior cingulate gyrus morphology and symptom severity. No significant correlations were found between either volume or depth and any of the three symptom domains.
Discussion
The literature to date on quantitative measures of the anterior cingulate gyrus in subjects with schizophrenia is variable and inconsistent, which may be in part due to lack of controlling for factors that may influence anterior cingulate gyrus volume such as sex, handedness, or even medication exposure. The current study was designed to minimize these factors by including in the study male subjects exposed to only one class of medications and matched to healthy subjects by sex, age, and handedness. In our previous study of neuroleptic-naive male subjects, we found no morphologic abnormalities of the anterior cingulate gyrus. In this study of a more heterogeneous group of patients, we found the structure of the anterior cingulate gyrus to be significantly different between groups, with patients having enlarged volumes of the left anterior cingulate gyrus relative to comparison subjects. This increase in volume was accounted for by an increase in cortical depth without any difference in surface area of the left anterior cingulate gyrus between groups. Moreover, the increase in the depth of the left anterior cingulate gyrus in patients was positively correlated with neuroleptic exposure, such that the greater the time or dose of medication, the thicker the left anterior cingulate gyrus cortex. This suggests that the morphologic differences between the two groups are an effect of medication exposure.
Although several previous studies have documented volume increase in the basal ganglia after exposure to typical neuroleptics (31, 42–47), few studies have shown structural changes in the cortex in response to medication. In fact, to our knowledge, the only other studies to document enlargement of the cortex in response to typical neuroleptics are previous reports from our group. Using the current data set of 30 male patients exposed to typical medications only, we have evaluated the morphology of the temporal plane (planum polare, planum temporale, and Heschl’s gyrus) (48), the ventral frontal cortex (49), and the insula (50). In these studies, exposure to typical neuroleptics was significantly correlated with greater volumes of the planum polare and the insula, but there was no relationship with the planum temporale, Heschl’s gyrus, or the ventral frontal cortex. This suggests that the effect of neuroleptics on the structure of the cerebral cortex may be regionally specific and not a global cortical phenomenon.
Possible Mechanisms of Anterior Cingulate Gyrus Structural Change in Response to Typical Neuroleptics
Theories devised to explain structural changes in the basal ganglia in response to neuroleptic exposure are appropriate to consider as explanation for the effects on the cortex as well. One theory is that tonic inhibition of dopamine receptors by typical neuroleptics may lead to compensatory upregulation by other dopamine receptors manifesting as an increase in regional volumes (10, 51). Dopamine receptors are found throughout the brain. Rat studies have shown D1 receptors in the anterior cingulate gyrus or increased binding of D2 receptors in the frontal cortex (52). However, it is generally thought that dopamine receptor density is not as great in the frontal cortex as it is in other brain regions such as the basal ganglia (53). An alternate theory is that upstream changes might lead to downstream effects via neural circuitry, such that the changes seen in the anterior cingulate gyrus might be caused by direct typical neuroleptic effects elsewhere in the brain. This theory is less accepted as a primary cause of changes in the anterior cingulate gyrus (5).
One difficulty that our study encountered, as have all previous MRI morphology studies of this region, is that of defining a structurally and functionally unique unit. Our study was able to trace a reliable and replicable area that encompasses the anterior cingulate. However, at best, MRI of brain morphology is a “proxy” measure that must ultimately be integrated with functional imaging to best delineate a true anterior cingulate. Because the anterior cingulate gyrus is a region of intricate functions and connectivity, it will take further functional MRI and PET studies to define these changes in relation to both schizophrenia and typical neuroleptic exposure.
Correlations Between Anterior Cingulate Morphology and Clinical Variables
Severity of positive, negative, and disorganized symptoms, as assessed by the SANS/SAPS, were not correlated with anterior cingulate gyrus morphology. Of these three symptom domains, positive symptoms alone have previously been reported to be related to the structure or function of the anterior cingulate gyrus. PET (53, 54) and single photon emission computed tomography (55) studies have suggested that psychotic symptoms, especially hallucinations, are associated with the entire cingulate region (anterior and posterior regions were not separately examined in these studies). A decrease in D2 receptors in the anterior cingulate gyrus in neuroleptic-naive people with schizophrenia as compared with healthy subjects has been posited as a mechanism for positive symptoms (53). There has been only one structural study to date associating a bilateral decrease in gray matter in the cingulate gyrus (anterior and posterior) with psychosis. However, this was not a direct correlation, and the study was not limited to people with schizophrenia, making it difficult to draw associations solely to schizophrenia (56). In the current study, the correlation between psychosis and left anterior cingulate gyrus depth was inverse, indicating the same direction of relationship (less cortical depth associated with greater severity of symptoms), although not statistically significant (r=–0.22, p=0.28). Finally, the anterior cingulate gyrus has been more commonly associated with executive function, selective attention, error detection, and self-monitoring (57). It has been suggested that these latter roles contribute to conceptual disorganization and hallucinatory behavior (53). Future studies evaluating the relationship between cognitive functioning and the structure of the anterior cingulate gyrus in subjects with schizophrenia may help define its functional role more clearly.
Specificity to Schizophrenia
Morphologic abnormalities of the anterior cingulate gyrus are most likely not unique to schizophrenia. However, there may be regional specificity with regard to different illnesses. For instance, a section of the cingulate gyrus located adjacent (posterior) to the currently defined anterior cingulate gyrus is a region variably defined as the subgenual cingulate. This region has been documented to be structurally abnormal in subjects with depression (58–60). Yet in contrast, this region has been reported to be structurally normal in subjects with schizophrenia (60), indicating that the morphology of the subgenual cingulate is unique to subjects with depression. Further studies will need to verify the regional and diagnostic specificity of structural abnormalities in this brain region.
Future Directions
The current study supports the notion that typical neuroleptics change the structure of the anterior cingulate gyrus. This study does not address, however, the clinical implications of these changes. We are currently evaluating patients in a prospective, longitudinal study to address the issue of whether these changes in morphology over time are related in any way to changes in symptom severity.
Another important question is whether or not there is differential effect on structure on the basis of antipsychotic class. While typical neuroleptic exposure has been shown to increase basal ganglia volumes, atypical neuroleptic exposure has been reported to decrease the volume of many of these same regions (42–44). Further studies are needed to see what, if any, effects atypical neuroleptics have on anterior cingulate gyrus morphology.
 |
 |
 |
Received April 9, 2004; revisions received June 16 and Oct. 13, 2004; accepted Oct. 29, 2004. From the Mental Health Clinical Research Center, Department of Psychiatry, College of Medicine, University of Iowa Hospitals and Clinics, Iowa City; and The MIND Institute and the University of New Mexico, Albuquerque. Address correspondence and reprint requests to Dr. Nopoulos, University of Iowa Health Care, Department of Psychiatry, 1-180 MEB, 500 Newton Rd., Iowa City, IA 52242–1057; [email protected] (e-mail). Supported by NIMH grants MH-31593, MH-40856, MH-19113, and MHCRC43271.
1. Vogt BA, Finch DM, Olson CR: Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 1992; 2:435–443Medline, Google Scholar
2. Devinsky O, Morrell MJ, Vogt BA: Contributions of anterior cingulate cortex to behaviour. Brain 1995; 118(part 1):279–306Google Scholar
3. Pujol J, Lopez A, Deus J, Cardoner N, Vallejo J, Capdevila A, Paus T: Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. Neuroimage 2002; 15:847–855Crossref, Medline, Google Scholar
4. Badgaiyan RD, Posner MI: Mapping the cingulate cortex in response selection and monitoring. Neuroimage 1998; 7:255–260Crossref, Medline, Google Scholar
5. Carter CS, MacDonald AW III, Ross LL, Stenger VA: Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry 2001; 158:1423–1428Link, Google Scholar
6. Artiges E, Salamé P, Recasens C, Poline J-B, Attar-Levy D, De la Raillère A, Paillère-Martinot ML, Danion J-M, Martinot J-L: Working memory control in patients with schizophrenia: a PET study during a random number generation task. Am J Psychiatry 2000; 157:1517–1519Link, Google Scholar
7. Meyer-Lindenberg A, Poline J-B, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF: Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry 2001; 158:1809–1817Link, Google Scholar
8. Carter CS, Mintun M, Nichols T, Cohen JD: Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry 1997; 154:1670–1675Link, Google Scholar
9. Yücel M, Pantelis C, Stuart GW, Wood SJ, Maruff P, Velakoulis D, Pipingas A, Crowe SF, Tochon-Danguy HJ, Egan GF: Anterior cingulate activation during Stroop task performance: a PET to MRI coregistration study of individual patients with schizophrenia. Am J Psychiatry 2002; 159:251–254Link, Google Scholar
10. Dolan RJ, Fletcher P, Frith CD, Friston KJ, Frackowiak RS, Grasby PM: Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature 1995; 378:180–182Crossref, Medline, Google Scholar
11. Haznedar MM, Buchsbaum MS, Luu C, Hazlett EA, Siegel BV Jr, Lohr J, Wu J, Haier RJ, Bunney WE Jr: Decreased anterior cingulate gyrus metabolic rate in schizophrenia. Am J Psychiatry 1997; 154:682–684Link, Google Scholar
12. Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, Carpenter WT: Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry 1992; 49:522–530Crossref, Medline, Google Scholar
13. Liddle PF, Friston KJ, Frith CD, Frackowiak RS: Cerebral blood flow and mental processes in schizophrenia. J R Soc Med 1992; 85:224–227Medline, Google Scholar
14. Alain C, McNeely HE, He Y, Christensen BK, West R: Neurophysiological evidence of error-monitoring deficits in patients with schizophrenia. Cereb Cortex 2002; 12:840–846Crossref, Medline, Google Scholar
15. Mulert C, Gallinat J, Pascual-Marqui R, Dorn H, Frick K, Schlattmann P, Mientus S, Herrmann WM, Winterer G: Reduced event-related current density in the anterior cingulate cortex in schizophrenia. Neuroimage 2001; 13:589–600Crossref, Medline, Google Scholar
16. Benes FM, Vincent SL, Todtenkopf M: The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry 2001; 50:395–406Crossref, Medline, Google Scholar
17. Benes FM: Neurobiological investigations in cingulate cortex of schizophrenic brain. Schizophr Bull 1993; 19:537–549Crossref, Medline, Google Scholar
18. Albanese AM, Merlo AB, Mascitti TA, Tornese EB, Gomez EE, Konopka V, Albanese EF: Inversion of the hemispheric laterality of the anterior cingulate gyrus in schizophrenics. Biol Psychiatry 1995; 38:13–21Crossref, Medline, Google Scholar
19. Highley JR, Walker MA, Esiri MM, McDonald B, Harrison PJ, Crow TJ: Schizophrenia and the frontal lobes: post-mortem stereological study of tissue volume. Br J Psychiatry 2001; 178:337–343Crossref, Medline, Google Scholar
20. Szeszko PR, Bilder RM, Lencz T, Pollack S, Alvir JM, Ashtari M, Wu H, Lieberman JA: Investigation of frontal lobe subregions in first-episode schizophrenia. Psychiatry Res 1999; 90:1–15Crossref, Medline, Google Scholar
21. Noga JT, Aylward E, Barta PE, Pearlson GD: Cingulate gyrus in schizophrenic patients and normal volunteers. Psychiatry Res 1995; 61:201–208Crossref, Medline, Google Scholar
22. Suzuki M, Nohara S, Hagino H, Kurokawa K, Yotsutsuji T, Kawasaki Y, Takahashi T, Matsui M, Watanabe N, Seto H, Kurachi M: Regional changes in brain gray and white matter in patients with schizophrenia demonstrated with voxel-based analysis of MRI. Schizophr Res 2002; 55:41–54Crossref, Medline, Google Scholar
23. Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, Tourville J, Caviness VS Jr, Faraone SV, Tsuang MT: Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry 1999; 56:537–547Crossref, Medline, Google Scholar
24. Ohnuma T, Kimura M, Takahashi T, Iwamoto N, Arai H: A magnetic resonance imaging study in first-episode disorganized-type patients with schizophrenia. Psychiatry Clin Neurosci 1997; 51:9–15Crossref, Medline, Google Scholar
25. Takahashi T, Suzuki M, Kawasaki Y, Kurokawa K, Hagino H, Yamashita I, Zhou SY, Nohara S, Nakamura K, Seto H, Kurachi M: Volumetric magnetic resonance imaging study of the anterior cingulate gyrus in schizotypal disorder. Eur Arch Psychiatry Clin Neurosci 2002; 252:268–277Crossref, Medline, Google Scholar
26. Szeszko PR, Bilder RM, Lencz T, Ashtari M, Goldman RS, Reiter G, Wu H, Lieberman JA: Reduced anterior cingulate gyrus volume correlates with executive dysfunction in men with first-episode schizophrenia. Schizophr Res 2000; 43:97–108Crossref, Medline, Google Scholar
27. Yucel M, Stuart GW, Maruff P, Velakoulis D, Crowe SF, Savage G, Pantelis C: Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: an MRI morphometric study. Cereb Cortex 2001; 11:17–25Crossref, Medline, Google Scholar
28. Yucel M, Stuart GW, Maruff P, Wood SJ, Savage GR, Smith DJ, Crowe SF, Copolov DL, Velakoulis D, Pantelis C: Paracingulate morphologic differences in males with established schizophrenia: a magnetic resonance imaging morphometric study. Biol Psychiatry 2002; 52:15–23Crossref, Medline, Google Scholar
29. Paus T, Otaky N, Caramanos Z, MacDonald D, Zijdenbos A, D’Avirro D, Gutmans D, Holmes C, Tomaiuolo F, Evans AC: In vivo morphometry of the intrasulcal gray matter in the human cingulate, paracingulate, and superior-rostral sulci: hemispheric asymmetries, gender differences and probability maps. J Comp Neurol 1996; 376:664–673Crossref, Medline, Google Scholar
30. Crespo-Facorro B, Kim J, Andreasen NC, O’Leary DS, Magnotta V: Regional frontal abnormalities in schizophrenia: a quantitative gray matter volume and cortical surface size study. Biol Psychiatry 2000; 48:110–119Crossref, Medline, Google Scholar
31. Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC: Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am J Psychiatry 1999; 156:1200–1204Abstract, Google Scholar
32. Miller DD, Flaum MA, Nopoulos P, Arndt S, Andreasen NC: The concept of dose years: a reliable method for calculating lifetime psychotropic drug exposure (abstract). Schizophr Res 1995; 15:159Crossref, Google Scholar
33. Davis JM: Dose equivalence of the antipsychotic drugs. J Psychiatr Res 1974; 11:65–69Crossref, Medline, Google Scholar
34. Andreasen NC, Flaum M, Arndt S: The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry 1992; 49:615–623Crossref, Medline, Google Scholar
35. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
36. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
37. Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D: Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph 2002; 26:251–264Crossref, Medline, Google Scholar
38. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
39. Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, Arndt S: Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Tomogr 1999; 23:144–154Crossref, Medline, Google Scholar
40. Magnotta VA, Andreasen NC, Schultz SK, Harris G, Cizadlo T, Heckel D, Nopoulos P, Flaum M: Quantitative in vivo measurement of gyrification in the human brain: changes associated with aging. Cereb Cortex 1999; 9:151–160Crossref, Medline, Google Scholar
41. Crespo-Facorro B, Kim JJ, Andreasen NC, O’Leary DS, Wiser AK, Bailey JM, Harris G, Magnotta VA: Human frontal cortex: an MRI-based parcellation method. Neuroimage 1999; 10:500–519Crossref, Medline, Google Scholar
42. Scheepers FE, Gispen de Wied CC, Hulshoff Pol HE, Kahn RS: Effect of clozapine on caudate nucleus volume in relation to symptoms of schizophrenia. Am J Psychiatry 2001; 158:644–646Link, Google Scholar
43. Scheepers FE, de Wied CC, Hulshoff Pol HE, van de Flier W, van der Linden JA, Kahn RS: The effect of clozapine on caudate nucleus volume in schizophrenic patients previously treated with typical antipsychotics. Neuropsychopharmacology 2001; 24:47–54Crossref, Medline, Google Scholar
44. Lang DJ, Kopala LC, Vandorpe RA, Rui Q, Smith GN, Goghari VM, Honer WG: An MRI study of basal ganglia volumes in first-episode schizophrenia patients treated with risperidone. Am J Psychiatry 2001; 158:625–631Link, Google Scholar
45. Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M: Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 1994; 151:1430–1436Link, Google Scholar
46. Chakos MH, Lieberman JA, Alvir J, Bilder R, Ashtari M: Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine (letter). Lancet 1995; 345:456–457Crossref, Medline, Google Scholar
47. Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW: Changes in caudate volume with neuroleptic treatment (letter). Lancet 1994; 344:1434Crossref, Medline, Google Scholar
48. Crespo-Facorro B, Kim JJ, Chemerinski E, Magnotta V, Andreasen NC, Nopoulos P: Morphometry of the superior temporal plane in schizophrenia: relationship to clinical correlates. J Neuropsychiatry Clin Neurosci 2004; 16:284–294Crossref, Medline, Google Scholar
49. Chemerinski E, Nopoulos PC, Crespo-Facorro B, Andreasen NC, Magnotta V: Morphology of the ventral frontal cortex in schizophrenia: relationship with social dysfunction. Biol Psychiatry 2002; 52:1–8Crossref, Medline, Google Scholar
50. Pressler M, Nopoulos P, Ho BC, Andreasen NC: Insular cortex abnormalities in schizophrenia: relationship to symptom and typical neuroleptic exposure. Biol Psychiatry 2005; 57:394–398Crossref, Medline, Google Scholar
51. Robertson GS, Jian M: D1 and D2 dopamine receptors differentially increase Fos-like immunoreactivity in accumbal projections to the ventral pallidum and midbrain. Neuroscience 1995; 64:1019–1034Crossref, Medline, Google Scholar
52. Ichikawa J, Meltzer HY: The effect of ipsapirone and S(-)-pindolol on dopamine release in rat striatum and nucleus accumbens. Brain Res 1999; 842:445–451Crossref, Medline, Google Scholar
53. Suhara T, Okubo Y, Yasuno F, Sudo Y, Inoue M, Ichimiya T, Nakashima Y, Nakayama K, Tanada S, Suzuki K, Halldin C, Farde L: Decreased dopamine D2 receptor binding in the anterior cingulate cortex in schizophrenia. Arch Gen Psychiatry 2002; 59:25–30Crossref, Medline, Google Scholar
54. Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L, et al: A functional neuroanatomy of hallucinations in schizophrenia. Nature 1995; 378:176–179Crossref, Medline, Google Scholar
55. Sabri O, Erkwoh R, Schreckenberger M, Owega A, Sass H, Buell U: Correlation of positive symptoms exclusively to hyperperfusion or hypoperfusion of cerebral cortex in never-treated schizophrenics. Lancet 1997; 349:1735–1739Crossref, Medline, Google Scholar
56. Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK: Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet 2003; 361:281–288Crossref, Medline, Google Scholar
57. Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD: Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry 1998; 155:1285–1287Link, Google Scholar
58. Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, Raichle ME: Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997; 386:824–827Crossref, Medline, Google Scholar
59. Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD: Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry 2002; 51:342–344Crossref, Medline, Google Scholar
60. Hirayasu Y, Shenton ME, Salisbury DF, Kwon JS, Wible CG, Fischer IA, Yurgelun-Todd D, Zarate C, Kikinis R, Jolesz FA, McCarley RW: Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry 1999; 156:1091–1093Abstract, Google Scholar



