Antidepressant Treatment in Bipolar Versus Unipolar Depression
Abstract
OBJECTIVE: Antidepressant responses were compared in DSM-IV bipolar and unipolar depression. METHOD: The authors analyzed clinical records for outcomes of antidepressant trials for 41 patients with bipolar depression and 37 with unipolar depression, similar in age and sex distribution. RESULTS: Short-term nonresponse was more frequent in bipolar (51.3%) than unipolar (31.6%) depression. Manic switching occurred only in bipolar depression but happened less in patients taking mood stabilizers (31.6% versus 84.2%). Cycle acceleration occurred only in bipolar depression (25.6%), with new rapid cycling in 32.1%. Late response loss (tolerance) was 3.4 times as frequent, and withdrawal relapse into depression was 4.7 times less frequent, in bipolar as in unipolar depression. Mood stabilizers did not prevent cycle acceleration, rapid cycling, or response loss. Modern antidepressants, in general, did not have lower rates of negative outcomes than tricyclic antidepressants. CONCLUSIONS: The findings suggest an unfavorable cost/benefit ratio for antidepressant treatment of bipolar depression.
Antidepressant effectiveness and safety in bipolar depression, especially in the long term, are far less established than in unipolar major depression, despite widespread antidepressant treatment for both groups (1, 2). Clinical experience suggests that antidepressants are effective in the short term for bipolar depression, but the few randomized, long-term trials of antidepressants (mainly tricyclics) failed to show superior protection against recurrence over lithium alone (2), and trials of modern antidepressants are rare (2–5). Manic switching and cycle acceleration appear to be common, may be somewhat less likely with some modern antidepressants, and may be ameliorated by cotreatment with some mood stabilizers (1, 2, 5–7). Given such limited information, we compared effects of modern and older antidepressants in patients with bipolar and unipolar depression.
Method
We identified 78 outpatients with DSM-IV major affective disorders exposed to 228 antidepressant trials (mean=74.0 weeks/trial, SD=75.9, median=53.4, range=2–416). Of these 78, 41 had bipolar disorder and 37 had unipolar major depression; ages and sex distributions were similar. Of the bipolar patients, 26 (63.4%) had bipolar I disorder, 10 (24.4%) had bipolar II disorder, and five (12.2%) had bipolar disorder not otherwise specified. They included 15 men and 26 women, and their mean age was 38.3 years (SD=12.0). The patients with unipolar depression included 19 men and 18 women, and their mean age was 37.8 years (SD=1.9). Chart reviews and clinical interviews by the treating psychiatrists (C.F.B., S.N.G., N.J.K.) based on systematic assessment of the DSM-IV criteria for major depressive and manic/hypomanic episodes supported consensus-based outcome assessments. These clinical, nonresearch assessments represent the clinical standard of care in our clinics. Our institutional review boards waived patient-specific informed consent for this confidential chart review and anonymous reporting of aggregate data.
Most of the antidepressants administered were selective serotonin-reuptake inhibitors (SSRIs; venlafaxine was used in 56 trials), bupropion (23 trials), or tricyclics (11 trials). Other “miscellaneous agents” were used in 12 trials and included the monoamine oxidase inhibitors phenelzine (two trials), isocarboxazid (one trial), and tranylcypromine (one trial), a stimulant (d-amphetamine, one trial), and the atypical agents nefazodone (three trials), mirtazapine (two trials), and St. John’s wort (two trials). SSRIs, bupropion, mirtazapine, and nefazodone were classified as “modern” antidepressants. Mood stabilizers included carbamazepine, divalproex, and lithium.
Nonresponse was a lack of recovery by 4 weeks at dose equivalents (1) of ≥20 mg/day of fluoxetine or ≥150 mg/day of imipramine. Loss of response was defined as the reemergence of a DSM-IV major depressive episode after 1 or more months of recovery. Relapse was a new depression less than 8 weeks after discontinuation of antidepressant treatment. Manic switching was defined as a new DSM-IV manic, hypomanic, or mixed episode less than 8 weeks after initiation of antidepressant treatment. Acceleration was the occurrence of an additional two or more DSM-IV affective episodes beyond the number experienced during similar exposure times immediately before antidepressant treatment. Rapid cycling was four or more new mood episodes per year of continuous antidepressant treatment.
The outcome rates are numbers of events per patient except for specific drug trials (events per trial). Statistical analyses used contingency tables (chi-square analysis or Fisher’s exact test if the number of subjects was less than 10). Risk ratios are provided with 95% confidence intervals (CIs). In the results that follow, the numbers of subjects do not always sum to the total number of bipolar patients (N=41), either because of how each outcome is defined or because of inconclusive data.
Results
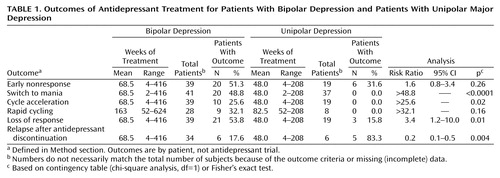
Short-term antidepressant nonresponse (Table 1) was 1.6 times (risk ratio) as common in bipolar as in unipolar depression and was similar in patients who were (55.0%, 11 of 20) and were not (47.4%, nine of 19) taking mood stabilizers. Among patients with bipolar depression, nonresponse to antidepressants varied nonsignificantly by drug type: 66.7% (eight of 12 trials) for miscellaneous agents, 43.5% (10 of 23 trials) for bupropion, 36.4% (four of 11 trials) for tricyclic antidepressants, and 32.1% (18 of 56 trials) for SSRIs (χ2=5.18, df=3, p=0.16). The rates were similar in trials of modern agents (36.9%, 31 of 84 trials) and tricyclic antidepressants (36.4%, four of 11 trials) (risk ratio=1.0, 95% CI=0.4–2.3, Fisher’s exact p>0.99).
Antidepressant-associated manic switching occurred in 48.8% of the patients with bipolar depression and none of those with unipolar depression (Table 1). Of the 20 patients who experienced an antidepressant-induced manic switch, an assessment of the potential preventive effect of mood stabilizer cotreatment was possible in 16 subjects (excluding three who experienced antidepressant-induced mania both with and without mood stabilizers and one patient with inconclusive data). Of these 16 remaining patients, antidepressant-induced mania was 4.3 times as common without (81.3%, 13 of 16) as with (18.8%, three of 16) mood stabilizers (risk ratio=4.3, 95% CI 1.5–12.3, Fisher’s exact p=0.001). Mania occurred similarly among antidepressant types: 22.0% (18 of 82) of SSRI trials, 20.7% (six of 29) of bupropion trials, 13.0% (three of 23) of tricyclic trials, and 9.5% (two of 21) of trials with other agents (χ2=2.29, df=3, p=0.51).
Antidepressant-associated cycle acceleration occurred in 25.6% of the bipolar patients and none of those with unipolar depression (Table 1) and somewhat more often among patients with bipolar I disorder (33.3%, eight of 24) than among patients with bipolar II disorder (20.0%, two of 10) or bipolar disorder not otherwise specified (none of five) (χ2=1.03, df=2, p=0.31). Mood stabilizers did not prevent cycle acceleration in the 10 patients who experienced this outcome, as the rate for patients taking them (55.6%, five of nine) did not differ significantly from the rate for patients not taking them (44.4%, four of nine) (Fisher’s exact p>0.99; data missing on one patient). Cycle acceleration risk varied little by drug type: 14.3% (eight of 56) for SSRI trials, 9.1% (one of 11) for tricyclic trials, 4.3% (one of 23) for bupropion trials, and none of the 12 trials of miscellaneous agents (χ2=3.36, df=3, p=0.34). Antidepressant-associated rapid cycling occurred in 32.1% of the bipolar patients exposed to antidepressants for 52 or more weeks but none of the eight similarly exposed patients with unipolar depression (Table 1). The risk of rapid cycling was somewhat (nonsignificantly) higher among patients with bipolar I disorder (43.8%, seven of 16) than among bipolar II patients (22.2%, two of nine) (risk ratio=2.0, 95% CI=0.5–7.5). In the nine patients who experienced rapid cycling, this outcome was not decreased by mood stabilizers (50.0% [four of eight] for the groups with and without mood stabilizers; data missing on one patient). The risk of rapid cycling did not differ significantly among antidepressants; 15.0% (three of 20) of SSRI trials, 11.1% (one of nine) of bupropion trials, 0% (zero of five) of tricyclic trials, and 0% (zero of two) of the trials with other agents (χ2=1.18, df=3, p=0.76).
Loss of response during treatment occurred 3.4 times as often among patients with bipolar depression as among unipolar subjects (Table 1), and the rates were similar among patients with bipolar I disorder (50.0%, 12 of 24), bipolar II disorder (70.0%, seven of 10), and bipolar disorder not otherwise specified (40.0%, two of five) (χ2=0.71, df=2, p=0.70). The rates of response loss were similar among antidepressant types: 36.4% (four of 11) of tricyclic trials, 30.4% (17 of 56) of SSRI trials, 21.7% (five of 23) of bupropion trials, and 8.3% (one of 12) of the trials with other agents (χ2=3.28, df=3, p=0.35). In the 21 patients who experienced loss of response, this outcome was not significantly less common with (42.9%, nine of 21) than without (57.1%, 12 of 21) mood stabilizer cotreatment (χ2=0.38, df=1, p=0.54). Relapsing after antidepressant discontinuation was 4.7 times as frequent in unipolar depression as in bipolar depression (Table 1) but did not differ, in the six bipolar patients who experienced this outcome, by mood stabilizer treatment (both 50.0%, three of six patients) or by antidepressant type (data not shown).
Discussion
Our main findings, shown in Table 1, were 1) short-term antidepressant nonresponse was 1.6 times (risk ratio) as common in bipolar as in unipolar depression; 2) antidepressant-associated manic switching was found only in patients with bipolar disorder (48.8%), at about 10 times typically reported spontaneous rates (1, 8), and was only partly prevented by mood stabilizers; 3) cycle acceleration occurred only in bipolar patients (25.6%) and involved rapid cycling in 32.1% of the patients given antidepressants for 1 year or more; 4) late loss of antidepressant response was 3.4 times as common in bipolar as in unipolar depression; but 5) postdiscontinuation relapse was 4.7 times as common in unipolar as in bipolar depression. Effects 3–5 were largely independent of antidepressant type and were little modified by inclusion of a mood stabilizer.
The limitations of this study include possibly unrepresentative subjects from tertiary care settings, incomplete matching of bipolar and unipolar subjects, nonrandomized treatment with unmatched agents, doses, and times, unknown treatment adherence, and nonblinded retrospective outcome assessments. Nevertheless, the findings may reflect real-world interactions of clinically selected antidepressant treatments and illness natural history. They support our impression that antidepressants have lesser benefits and greater psychiatric risks among bipolar than unipolar depressed patients (1, 2). They also strongly suggest a need for randomized long-term trials of treatments for bipolar depression, currently the dominant morbidity in treated bipolar disorder patients (9).
 |
Received July 30, 2002; revisions received March 19 and June 18, 2003; accepted June 20, 2003. From the Department of Psychiatry and the Bipolar Disorder Research Program, Cambridge Hospital; the Department of Psychiatry, Harvard Medical School, Boston; the Bipolar Outpatient Program, Department of Psychiatry, University of Pennsylvania, Philadelphia; and the Mailman Research Center, McLean Division of Massachusetts General Hospital, Belmont, Mass. Address reprint requests to Dr. Ghaemi, Bipolar Disorder Research Program, Cambridge Hospital, 1493 Cambridge St., Cambridge, MA 02139; [email protected] (e-mail). Supported by NIMH Research Career Award MH-64189 to Dr. Ghaemi, by an award from the Bruce J. Anderson Foundation, and by a grant from the McLean Private Donors Neuropsychopharmacology Research Fund to Dr. Baldessarini.
1. Baldessarini RJ: Drugs and the treatment of psychiatric disorders: depression and anxiety disorders, in Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 10th ed. Edited by Hardman JG, Limbird LE, Gilman AG. New York, McGraw-Hill, 2001, pp 447–483Google Scholar
2. Ghaemi SN, Lenox MS, Baldessarini RJ: Effectiveness and safety of long-term antidepressant treatment in bipolar disorder. J Clin Psychiatry 2001; 62:565–569Crossref, Medline, Google Scholar
3. Amsterdam J, Garcia-Espana F, Fawcett J, Quitkin F, Reimherr F, Rosenbaum J, Schweizer E, Beasley C: Efficacy and safety of fluoxetine in treating bipolar II major depressive episode. J Clin Psychopharmacol 1998; 18:435–440Crossref, Medline, Google Scholar
4. Altshuler LL, Kiriakos L, Calcagno J, Goodman R, Gitlin M, Frye M, Mintz J: The impact of antidepressant discontinuation vs antidepressant continuation on 1-year risk for relapse of bipolar depression. J Clin Psychiatry 2001; 62:612–616Crossref, Medline, Google Scholar
5. Nemeroff CB, Evans DL, Gyulai L, Sachs GS, Bowden CL, Gergel IP, Oakes R, Pitts CD: Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry 2001; 158:906–912Link, Google Scholar
6. Altshuler LL, Post RM, Leverich GS, Mikalauskas K, Rosoff A, Ackerman L: Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry 1995; 152:1130–1138Link, Google Scholar
7. Sharma V: Loss of response to antidepressants and subsequent refractoriness. J Affect Disord 2001; 64:99–106Crossref, Medline, Google Scholar
8. Calabrese JR, Bowden CL, Sachs GS, Ascher JA, Monaghan E, Rudd GD: A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry 1999; 60:79–88Crossref, Medline, Google Scholar
9. Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, Leon AC, Rice JA, Keller MB: The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry 2002; 59:530–537Crossref, Medline, Google Scholar



