The Schizophrenia Phenotype in 22q11 Deletion Syndrome
Abstract
OBJECTIVE: This study investigated the schizophrenia phenotype in 24 subjects with 22q11 deletion syndrome (22qDS) and schizophrenia (22qDS-schizophrenia), a rare but relatively homogenous genetic subtype of schizophrenia associated with a microdeletion on chromosome 22. Individuals with 22qDS are at genetically high risk for schizophrenia. METHOD: Standard measures of signs, symptoms, and course of schizophrenia were assessed in 16 adults with 22qDS-schizophrenia who did not meet criteria for mental retardation and in 46 adults with schizophrenia without evidence of 22qDS from a community familial sample. RESULTS: There were no significant differences in age at onset, lifetime or cross-sectional core positive and negative schizophrenic symptoms, or global functioning between the two groups of patients with schizophrenia. Patients with 22qDS-schizophrenia had higher excitement subscale scores and less lifetime substance use than the comparison patients with schizophrenia, but no significant differences in anxiety-depression symptom severity were found between the groups. CONCLUSIONS: These findings indicate that the core clinical schizophrenia phenotype would not distinguish individuals with a 22qDS subtype from those with schizophrenia who did not have the 22qDS subtype. The results provide further support for the utility of 22qDS-schizophrenia as a neurodevelopmental model of schizophrenia as well as support for prospective studies of individuals with 22qDS to help identify precursors of schizophrenia.
The pathogenesis of schizophrenia is hypothesized to involve abnormal neurodevelopment (1, 2). A subtype of schizophrenia has been identified that has a relatively homogeneous genetic etiology associated with a microdeletion on chromosome 22q11.2 (3). The genetic syndrome associated with this deletion, 22q11 deletion syndrome (22qDS), has a variable physical and neurobehavioral phenotype that includes schizophrenia (3, 4). High rates of congenital dysmorphic features (5, 6), developmental structural brain abnormalities (7), and cognitive dysfunction (8) in 22qDS are consistent with this subtype of schizophrenia, representing an especially neurodevelopmental form of the illness (2, 3). The genetic risk for schizophrenia is related to the deletion, which usually occurs as a spontaneous (de novo) mutation and in the absence of a family history of psychotic illness (9, 10). Individuals with 22qDS represent a particularly high-risk group (9): 25% or more are estimated to develop schizophrenia (4). The only groups at higher risk are individuals with two parents with schizophrenia or monozygotic co-twins of individuals with schizophrenia. A 22qDS subtype of schizophrenia may be present in up to one in 50 patients with schizophrenia (11). Higher rates of 22qDS may be present in subsets of schizophrenia with childhood onset or dual diagnosis schizophrenia and mental retardation (9). Individuals with 22qDS would therefore represent a relatively prevalent (≥1/4000 of the general population [12]) and identifiable (3) high-risk group for schizophrenia, well suited for studies of predictive features.
For a 22qDS subtype of schizophrenia to be accepted as a useful model for schizophrenia in general, researchers must be assured that the schizophrenia phenotype is similar to that in other forms of schizophrenia. Clinicians may also wonder if there are specific features of the schizophrenia phenotype that could be used to help identify patients with a 22qDS subtype of the illness (22qDS-schizophrenia). For example, there is some evidence that impulsivity may be a component of the adult neurobehavioral phenotype of 22qDS (5), but it is unclear if there are differences in core schizophrenic symptoms in 22qDS-schizophrenia. There are few studies detailing the clinical phenotype in adults with 22qDS-schizophrenia, however. Sample sizes have been small, particularly if one considers individuals without mental retardation (six or fewer subjects) (4, 5, 13). Differences in ascertainment between these small samples and inclusion of subjects with mental retardation may explain why different studies have proposed age at onset to be younger (5, 13) and older (4) in 22qDS-schizophrenia than in schizophrenia without 22qDS.
We investigated the largest group of subjects with 22qDS-schizophrenia yet reported to our knowledge (N=24). Our goal was to determine the similarities and differences of the schizophrenia phenotype from other, more typical groups of patients with schizophrenia. We studied a subgroup of individuals with 22qDS-schizophrenia who did not have mental retardation to eliminate potential confounders due to mental retardation and make our study group more comparable to subjects with schizophrenia usually studied. We hypothesized that age at onset and core signs and symptoms of 22qDS-schizophrenia would be similar to those of patients with schizophrenia without 22qDS. We further hypothesized that the 22qDS-schizophrenia group would have greater severity of the excitement symptom grouping, which includes an item on impulse control.
Method
Subjects
We investigated adult subjects who were diagnosed with 22qDS and confirmed to have a chromosome 22q11.2 deletion by standard cytogenetic studies that used fluorescence in situ hybridization and a probe from the commonly deleted 22q11.2 region (14). There were 24 subjects in the 22qDS-schizophrenia group (10 men, 14 women) ascertained from three sources: patients with schizophrenia who met clinical screening criteria (3) (N=18), individuals previously diagnosed with 22qDS (N=4), and individuals newly diagnosed with schizophrenia (N=2) from a cohort of nonpsychotic 22qDS subjects. All 22qDS patients had physical congenital anomalies commonly associated with the syndrome (3, 6, 15). A subgroup of 16 individuals without mental retardation (IQ≥70) was used for the main analyses; six of these patients were reported in detail previously (5).
The comparison schizophrenia group (N=46) was drawn from a community study of familial schizophrenia reported previously (16, 17). We excluded comparison patients older than 50 years of age (N=20) and one with childhood-onset schizophrenia from an initial group of 67 patients to make the group more comparable to the 22qDS group and to groups of patients with schizophrenia generally reported in the literature. For both groups we included only patients clinically stable at assessment for whom complete data were available.
Assessments
Patients were assessed for lifetime psychiatric diagnoses by research psychiatrists (A.S.B., E.W.C.C.) using a modified Structured Clinical Interview for DSM-III-R (18) or DSM-IV (19) Axis I Disorders (SCID-I), with direct interview, collateral information from family members, and data from medical records. The SCID-I provided comprehensive data allowing both DSM-III-R and DSM-IV criteria to be used. For consistency, all diagnoses reported are DSM-IV diagnoses. Lifetime diagnoses of substance use disorders (abuse or dependence) were also available from the SCID-I assessments. Age at onset of psychosis was defined as age when patients had their first documented psychotic symptoms. Information on family history of psychotic illnesses in first-degree relatives was obtained from direct interview of patients and their relatives and from medical records. In the 22qDS-schizophrenia group, determination of mild mental retardation was made from full-scale IQ determined by using the WAIS-R (N=16), IQ estimated by using WAIS-R vocabulary and block design scores (N=7) (20), and/or premorbid history (N=1).
Cross-sectional assessments performed by research psychiatrists (A.S.B., E.W.C.C.) included the 30-item Positive and Negative Syndrome Scale (21), Mini-Mental State Examination (MMSE) (22), and Global Assessment of Functioning Scale (GAF) (DSM-III-R, p. 12). Positive and Negative Syndrome Scale items in two core and three auxiliary symptom groupings were used (23): 1) positive (delusions, hallucinations, grandiosity, unusual thought content, stereotyped thinking), 2) negative (blunted affect, lack of spontaneous conversation, emotional withdrawal, passive social withdrawal, active social avoidance, poor rapport) symptoms, 3) cognitive (conceptual disorganization, difficulty in abstract thinking, poor attention, disorientation, mannerisms), 4) excitement (excitement, hostility, poor impulse control, uncooperativeness), and 5) anxiety-depression (anxiety, tension, depression, guilt feelings). Medication use and demographic characteristics at the time of assessment were also recorded; the designation “married” included common-law relationships of 1 year or more.
Study of the two groups was approved by the research ethics boards of the University of Toronto and Centre for Addiction and Mental Health, and written informed consent was obtained after complete description of the study to the patients.
Analysis
Statistical analyses involved comparisons between the patients with and without the 22qDS subtype of schizophrenia. Chi-square or Fisher’s exact tests were used to compare categorical variables, and two-tailed Student’s t tests were used for continuous variables. All outcome variables (Positive and Negative Syndrome Scale, GAF, and MMSE scores) were treated as continuous. To assess for the effects of demographic variables on outcome variables, regression analyses using general linear modelling techniques were carried out with sex, duration of illness, years of education, lifetime substance use disorder, antipsychotic medication use, and antiparkinsonian medication use as potential covariates. Duration of illness was selected over age at assessment because we thought that it was more pertinent to the assessment scores.
Results
Demographic and Basic Clinical Features
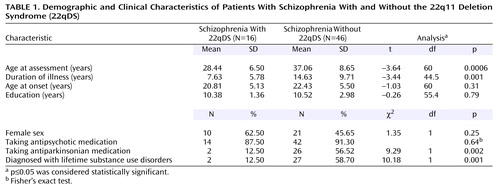
The demographic and basic clinical features of the two groups are shown in Table 1. There were no significant differences between the two groups in sex or educational level. Further details available only for the 22qDS-schizophrenia group showed that these patients had a mean full-scale IQ of 75.5 (SD=5.4); 15 patients were in the borderline range, and one was in the average range of IQ.
The 22qDS-schizophrenia group was significantly younger at assessment than the schizophrenia comparison group (Table 1). This and ascertainment differences may partially account for differences between the groups in marital and employment status and living situation. Significantly fewer of the 22qDS-schizophrenia patients (18.8% [N=3]) than comparison patients (52.2% [N=24]) had ever married or had children (χ2=5.39, df=1, p=0.02) or had ever been employed (56.3% [N=9] versus 95.7% [N=44], respectively) (p=0.0006, Fisher’s exact test). No 22qDS-schizophrenia patients but 19 comparison patients (41.3%) lived with a spouse. Eight 22qDS-schizophrenia patients (50.0%) lived with parents and five (31.3%) were in mental health boarding homes at the time of assessment, compared with nine (19.6%) of the comparison patients in each of these living situations. About the same proportion of each group lived alone: three (18.8%) of the 22qDS-schizophrenia patients and nine (19.6%) of the comparison patients.
There were expected differences in physical features and family history, given the ascertainment differences, between the two groups. With respect to major birth defects requiring surgery, five (31.3%) 22qDS-schizophrenia patients had congenital cardiac defects, and seven (43.8%) had palatal defects. Five (31.3%) 22qDS-schizophrenia patients and all of the comparison patients had neither of these major birth defects. Only one patient (6.3%) in the 22qDS-schizophrenia group but 38 (82.6%) in the comparison group had a family history of psychotic illness.
There were no significant differences between the two groups in the proportion of patients with schizoaffective disorder: three (18.8%) of the 22qDS-schizophrenia patients and 12 (26.1%) of the comparison patients (p=0.74, Fisher’s exact test), or of patients prescribed any antipsychotic treatment (Table 1). Patients who were not receiving antipsychotic medications were either receiving ECT (one 22qDS-schizophrenia patient) or no psychiatric treatment (one 22qDS-schizophrenia patient and four comparison patients). A nonsignificantly higher proportion of 22qDS-schizophrenia patients (25.00% [N=4]) than comparison patients (6.52% [N=3]) were prescribed an anticonvulsant medication (p=0.07, Fisher’s exact test). These prescriptions followed a medication-related seizure, except in the case of one comparison patient who had an abnormal EEG. Antiparkinsonian use was significantly lower in the 22qDS-schizophrenia group (Table 1), probably because more second-generation antipsychotic medications were used in this group. A lifetime history of substance use disorder (abuse or dependence) was significantly less common in the 22qDS-schizophrenia group (Table 1).
Course of Schizophrenic Illness and Functioning
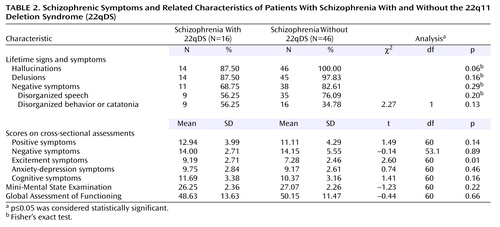
Consistent with our hypothesis, the mean age at onset of psychosis was similar in the 22qDS-schizophrenia and comparison groups (Table 1), ranging from 15 to 30 years in the 22qDS-schizophrenia group and 14 to 34 years in the comparison group. There were no significant differences in age at onset between men and women in either group: in the 22qDS-schizophrenia group, mean=19.17 years (SD=5.11) for men and mean=21.80 (SD=5.14) for women (t=–0.99, df=14, p=0.34); in the comparison group: mean=22.76 years (SD=5.46) for men and mean=22.05 years (SD=5.65) for women (t=0.43, df=44, p=0.67). Duration of illness was significantly longer in the comparison group, attributable to the older age at assessment in this group (Table 1). GAF scores indicated similar global functioning in both groups (Table 2).
Schizophrenic Symptom Profile
There were no significant differences between the two groups in the presence of lifetime signs and symptoms of schizophrenia (DSM-IV A criteria items) (Table 2). Consistent with our hypothesis, we found no significant differences in the severity of core positive or negative schizophrenic symptom scores between the 22qDS-schizophrenia and comparison groups (Table 2). The results remained unchanged after we adjusted for each of the six covariates in the regression analyses. With respect to auxiliary features of schizophrenia, there were no significant group differences in the severity of anxiety-depression or cognitive symptom scores or in MMSE scores (Table 2).
Consistent with our prediction, however, excitement factor scores were higher in the 22qDS-schizophrenia group than the comparison group (Table 2). Poor impulse control (t=3.79, df=60, p=0.0003), uncooperativeness (t=3.29, df=60, p=0.002), and hostility (t=2.70, df=60, p=0.009) were the individual excitement grouping items that showed significantly greater severity in the 22qDS-schizophrenia group. However, group differences accounted for only 10% of the variation in excitement symptoms. Mean values in both groups for this symptom grouping and its individual component items were comparable to those previously reported for other patients with schizophrenia (23, 24). These findings did not appear to be related to manic-type symptoms; Young Mania Rating Scale scores (25) available only for the 22qDS-schizophrenia patients (mean=8.5, SD=4.9) were relatively low for a group of psychotic subjects (26, 27).
22qDS-Schizophrenia Patients With Mild Mental Retardation
Several authors (28–30) have made a case for considering subjects with mild mental retardation and schizophrenia to have a neurodevelopmental form of schizophrenia with similar symptom profiles and courses as schizophrenia in the general population. We therefore compared the symptom severity scale scores and age at onset of the eight 22qDS-schizophrenia patients with mild mental retardation and the 16 22qDS-schizophrenia patients without mental retardation. The results showed that the only significant differences between these two groups were, as would be expected, that mean MMSE scores were lower (21.63 versus 26.25) (t=3.53, df=22, p=0.002) and mean Positive and Negative Syndrome Scale cognitive symptom scores higher (16.38 versus 11.69) (t=–3.38, df=22, p=0.003) in the mild mental retardation subgroup.
Discussion
The results of this study indicate that the schizophrenia phenotype of a 22qDS etiologic subtype of the illness is largely indistinguishable from other forms of schizophrenia. The longitudinal and cross-sectional features of schizophrenia in patients with 22qDS-schizophrenia are similar to those reported for other groups of patients with schizophrenia (DSM-IV) (23, 24). Any differences appear to be in auxiliary features of the illness rather than in the major phenotypic features of schizophrenia, such as onset, course, and core symptoms. These results concur with previous reports indicating that the schizophrenia in 22qDS appears relatively typical in terms of symptoms and age at onset (5, 13, 31). They differ from one study’s conclusions that there is a clinical subtype of schizophrenia associated with 22qDS, including later age at onset and fewer negative symptoms (4).
Ascertainment of subjects has undoubtedly played a role in differences between studies. When patients with 22qDS-schizophrenia are recruited from groups of patients with schizophrenia, as in the current study and others (13, 31), they are likely to appear more similar to the general population of individuals with schizophrenia than if they were recruited as parents transmitting a 22q11.2 deletion to affected offspring (4). Inclusion in previous studies of substantial proportions of patients with mental retardation (4) may also have played a role, although we found no significant differences in the schizophrenia phenotype between patients with and without mental retardation in our study.
Our results also support previous work (4) indicating that mood disturbances (in the form of major mood episodes as part of schizoaffective disorder) or symptoms (as indicated by the anxiety-depression Positive and Negative Syndrome Scale symptom grouping) do not play a major role in the majority of individuals with 22qDS-schizophrenia. The more severe excitement/impulsivity features in 22qDS-schizophrenia may be auxiliary neurobehavioral characteristics of 22qDS, possibly associated with temper or emotional outbursts in the syndrome (5). Low lifetime comorbidity with substance use disorders and low marital and employment rates in 22qDS-schizophrenia may be related in part to the presence of congenital cardiac or other physical or cognitive features of the syndrome, younger age, and/or lifestyle differences between individuals with 22qDS-schizophrenia and other forms of schizophrenia.
Advantages and Limitations
The current study has several advantages over previous studies. To our knowledge, this is the largest group of patients with 22qDS-schizophrenia reported. There was no ascertainment bias with respect to recruiting transmitting parents, usually the largest group of adults with 22qDS in studies (4, 15), which potentially biases to health and reproductive fitness. However, there are also several potential limitations in the study. The comparison group used was a familial schizophrenia sample. Others have used such comparison groups (4), and we have previously demonstrated the similarity of our familial sample to other schizophrenia groups with respect to symptoms, functioning, and age at onset (16). The main difference with such a sample is that the sex ratio is closer to 50:50 and the age at onset more similar between the sexes than in other schizophrenia groups (16, 17).
The familial group we ascertained, however, is a community-based sample with a more representative range of IQ than many university-based research samples of schizophrenia. This likely contributed to the similarity in educational level and MMSE scores of the comparison and 22qDS-schizophrenia groups. IQ results were not available for the comparison group, and IQ testing of the 22qDS-schizophrenia patients was performed after onset of schizophrenia and may have been higher premorbidly, with more patients in the average range. The numbers of subjects in the subgroups with and without mental retardation in the 22qDS-schizophrenia group were small, and this may have limited the ability to detect statistically significant differences between these two subgroups. A schizophrenia comparison group where one-third of the subjects had a dual diagnosis of mental retardation and schizophrenia would have allowed for inclusion of both group and mental retardation in regression analyses using all 24 22qDS-schizophrenia patients. However, comparisons between the eight 22qDS-schizophrenia patients with and the 16 without mental retardation suggested that differences would be in variables related to intellect (e.g., cognitive symptoms), not schizophrenia.
The comparison subjects were not tested for a 22q11.2 deletion; however, there was no evidence of linkage to the 22q11.2 region in this group (17), and most 22q11.2 deletions result from spontaneous mutations (9, 10). Therefore, familial schizophrenia samples are less likely than other schizophrenia samples to have 22q11.2 deletions. Neither treatment response nor the length of the chromosome 22q11.2 deletion would appear likely to have affected results of the current study; these important variables will be reported separately.
22qDS-Schizophrenia as an Etiologic Subtype of Schizophrenia
This study and others (4, 13, 31) support 22qDS as a true etiologic subtype of schizophrenia, identifiable by its associated physical and cognitive features and chromosomal abnormality. On the other hand, 22qDS-schizophrenia without mental retardation does not appear to represent a clinical subtype of schizophrenia in terms of the core clinical schizophrenia phenotype. In etiologic subtypes of an illness the basic condition is the same, although clinical nuances may be discernible. Subtypes based on etiology can facilitate identification of contributory genes and improve understanding of the pathophysiology of the condition as a whole. For example, a parallel may be drawn between psychotic disorders, the most common of which is schizophrenia, and dementias, the most common of which is Alzheimer’s disease. There are several etiologic subtypes of Alzheimer’s disease (32). One of these is associated with mutations of the beta amyloid precursor protein gene (βAPP) on chromosome 21 (33). Alzheimer’s disease found in Down’s syndrome (trisomy chromosome 21), a possible familial association between Alzheimer’s disease and Down’s syndrome (34), and the localization of the βAPP gene near the key region of chromosome 21 associated with the Down’s phenotype (35) preceded the determination that βAPP plays a role in some forms of Alzheimer’s disease. In Alzheimer’s disease as a whole, the Alzheimer’s disease associated with Down’s syndrome is rare, and βAPP mutations are a rare cause of familial Alzheimer’s disease. However, the related molecular discoveries have contributed to a greater understanding of the pathophysiology of Alzheimer’s disease in general (32). There are no comparable mutations yet identified for schizophrenia. However, establishing 22qDS as an identifiable subtype of schizophrenia may help lead to corresponding molecular genetic discoveries and subsequent neurobiological insights.
Etiologic categorizing differs from the clinical subtyping of schizophrenia familiar to psychiatric clinicians (e.g., paranoid and disorganized subtypes). It also differs from that found in Davison and Bagley’s review of schizophrenia-like psychoses (36). This classic review included conditions in which psychosis dominated the clinical picture, as well as those with more complete schizophrenia-like syndromes, and conceptualized these as “phenocopies” associated with a variety of causes. In contrast, our conceptualization of a 22qDS subtype of schizophrenia is that, similar to other genetic forms of schizophrenia (17), the 22q11.2 deletion is likely to be one of multiple possible genetic predispositions to the neurodevelopmental changes that could lead to expression of schizophrenia and related disorders (2). We have found similar structural brain abnormalities in 22qDS-schizophrenia as those commonly seen in schizophrenia without 22qDS (37), supporting the likelihood that a 22qDS subtype of schizophrenia may share some aspects of a general pathogenetic pathway for schizophrenia.
Neurobehavioral Phenotype of 22qDS
The focus in this study is 22qDS-schizophrenia in adults, but there is a complex neurobehavioral phenotype of 22qDS that is likely to be as variable as the physical phenotype. Cognitive abnormalities are the most prevalent aspect of the neurobehavioral phenotype, ranging from average intellect with minimal learning difficulties to severe deficits (8). Children and adults with 22qDS may have psychiatric disorders other than schizophrenia that meet standardized diagnostic criteria, such as anxiety disorders, mood disorders, and attention deficit disorder. There is no evidence as yet that rates of these other disorders are higher than expected in the general population for a given intellectual level (38) or for individuals at elevated genetic risk for schizophrenia. It is likely, however, that there are other aspects of neurobehavior in 22qDS that do not fit a diagnostic category (38), such as impulsivity and emotional outbursts (5, 39). Results of the current study suggest that these other neurobehavioral features may color the presentation of major illnesses without necessarily affecting the core diagnostic phenotype.
22qDS in Research on Schizophrenia
Results from this study indicate that physical and intellectual phenotypic features (3) will remain the primary means of identifying a 22qDS subtype of schizophrenia. However, it is apparent from this study and others (4, 15, 31) that many individuals with 22qDS-schizophrenia will not have obvious congenital anomalies and that the majority of such individuals will not have mental retardation, although learning difficulties may be prevalent. This is consistent with the fact that individuals with 22qDS-schizophrenia have been recruited into research samples despite intensive prescreening, as illustrated by the National Institute of Mental Health study of childhood-onset schizophrenia (31). Lower rates of comorbid substance use disorders may further facilitate the potential inclusion of subjects with 22qDS-schizophrenia in research studies of schizophrenia. A high index of suspicion, careful medical history taking, and assessment for the more subtle physical and cognitive features of the syndrome will often be necessary to identify subjects with 22qDS (3, 6, 9). Separate study of these individuals will be important to determine which features distinguish this etiologic subtype from other forms of schizophrenia.
Summary
The results of this study support the potential for 22qDS-schizophrenia as an appropriate model for schizophrenia and as an etiologic subtype of the illness. The greater homogeneity of groups of subjects with 22qDS-schizophrenia compared with general population groups of subjects with schizophrenia should be helpful in learning more about the neurodevelopmental pathogenesis of schizophrenia, particularly with respect to the genetic and nongenetic factors contributing to expression of the illness (2). There do not appear to be major clinical differences in the core schizophrenia phenotype, and this provides further support for prospective studies of young individuals with 22qDS for onset of schizophrenia.
 |
 |
Received Oct. 15, 2002; revision received Jan. 22, 2003; accepted Feb. 6, 2003. From the Clinical Genetics Research Program, Centre for Addiction and Mental Health; the Department of Psychiatry, University of Toronto; the Department of Health Studies and Gerontology, University of Waterloo, Waterloo, Ont., Canada; and the Department of Pediatrics and the Department of Genetics, Hospital for Sick Children, University of Toronto. Address reprint requests to Dr. Bassett, Centre for Addiction and Mental Health, 1001 Queen St. West, Toronto, Ont., M6J 1H4 Canada; [email protected] (e-mail). Supported by Medical Research Council of Canada grants MT-12155 and MOP-38099 (Dr. Bassett), by the Bill Jeffries Schizophrenia Endowment Fund, the Ontario Mental Health Foundation, the Scottish Rite Schizophrenia Program, and an Independent Investigator Award from the National Alliance for Research on Schizophrenia and Depression (Dr. Bassett). The authors thank the subjects and their families for their participation; genetic counselors Heather Dorman, Linda Chiu, Sheri O’Neill, and Jillian Murphy; Drs. Nighat Parveen and Oana Caluseriu; and Jackie MacAlduff and Laura Scutt, who assisted in the collection of data for the study.
1. Weinberger DR: Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
2. Bassett AS, Chow EWC, O’Neill S, Brzustowicz LM: Genetic insights into the neurodevelopmental hypothesis of schizophrenia. Schizophr Bull 2001; 27:417–430Crossref, Medline, Google Scholar
3. Bassett AS, Chow EWC: 22q11 deletion syndrome: a genetic subtype of schizophrenia. Biol Psychiatry 1999; 46:882–891Crossref, Medline, Google Scholar
4. Murphy KC, Jones LA, Owen MJ: High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry 1999; 56:940–945Crossref, Medline, Google Scholar
5. Bassett AS, Hodgkinson K, Chow EWC, Correia S, Scutt LE, Weksberg R: 22q11 deletion syndrome in adults with schizophrenia. Am J Med Genet Neuropsychiatr Genet 1998; 81:328–337Crossref, Medline, Google Scholar
6. Scutt L, Chow EWC, Weksberg R, Honer WG, Bassett AS: Patterns of dysmorphic features in schizophrenia. Am J Med Genet Neuropsychiatr Genet 2001; 105:713–723Crossref, Medline, Google Scholar
7. Chow EWC, Mikulis DJ, Zipursky RB, Scutt LE, Weksberg R, Bassett AS: Qualitative MRI findings in adults with 22q11 deletion syndrome and schizophrenia. Biol Psychiatry 1999; 46:1436–1442Crossref, Medline, Google Scholar
8. Swillen A, Vogels A, Devriendt K, Fryns JP: Chromosome 22q11 deletion syndrome: update and review of the clinical features, cognitive-behavioral spectrum, and psychiatric complications. Am J Med Genet 2000; 97:128–135Crossref, Medline, Google Scholar
9. Bassett AS, Chow EWC, Weksberg R: Chromosomal abnormalities and schizophrenia. Am J Med Genet Semin Med Genet 2000; 97:45–51Crossref, Medline, Google Scholar
10. Carlson C, Papolos D, Pandita RK, Faedda GL, Veit S, Goldberg R, Shprintzen R, Kucherlapati R, Morrow B: Molecular analysis of velo-cardio-facial syndrome patients with psychiatric disorders. Am J Hum Genet 1997; 60:851–859Medline, Google Scholar
11. Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, Gos A, Nestadt G, Wolyniec PS, Lasseter VK, Eisen H, Childs B, Kazazian HH, Kucherlapati R, Antonarakis SE, Pulver AE, Housman DE: Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci USA 1995; 92:7612–7616Crossref, Medline, Google Scholar
12. Goodship J, Cross I, LiLing J, Wren C: A population study of chromosome 22q11 deletions in infancy. Arch Dis Child 1998; 79:348–351Crossref, Medline, Google Scholar
13. Gothelf D, Frisch A, Munitz H, Rockah R, Laufer N, Mozes T, Hermesh H, Weizman A, Frydman M: Clinical characteristics of schizophrenia associated with velo-cardio-facial syndrome. Schizophr Res 1999; 35:105–112Crossref, Medline, Google Scholar
14. Driscoll DA, Salvin J, Sellinger B, Budarf ML, McDonald-McGinn DM, Zackai EH, Emanuel BS: Prevalence of 22q11 microdeletions in DiGeorge and velocardiofacial syndromes: implications for genetic counselling and prenatal diagnosis. J Med Genet 1993; 30:813–817Crossref, Medline, Google Scholar
15. Cohen E, Chow EWC, Weksberg R, Bassett AS: Phenotype of adults with the 22q11 deletion syndrome: a review. Am J Med Genet 1999; 86:359–365Crossref, Medline, Google Scholar
16. Bassett AS, Collins EJ, Nuttall SE, Honer WG: Positive and negative symptoms in families with schizophrenia. Schizophr Res 1993; 11:9–19Crossref, Medline, Google Scholar
17. Brzustowicz LM, Hodgkinson KA, Chow EWC, Honer WG, Bassett AS: Location of a major susceptibility locus for familial schizophrenia on chromosome 1q21-q22. Science 2000; 288:678–682Crossref, Medline, Google Scholar
18. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Non-Patient Edition (SCID-NP, Version 1.0). Washington, DC, American Psychiatric Press, 1990Google Scholar
19. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Administration Booklet. Washington, DC, American Psychiatric Press, 1997Google Scholar
20. Silverstein AB: Two- and four-subtest short forms of the Wechsler Adult Intelligence Scale—Revised. J Consult Clin Psychol 1982; 50:415–418Crossref, Google Scholar
21. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
22. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
23. Lindenmayer J-P, Bernstein-Hyman R, Grochowski S, Bark N: Psychopathology of schizophrenia: initial validation of a 5-factor model. Psychopathology 1995; 28:22–31Crossref, Medline, Google Scholar
24. Citrome L, Volavka J, Czobor P, Sheitman B, Lindenmayer J-P, McEvoy J, Cooper TB, Chakos M, Lieberman JA: Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatr Serv 2001; 52:1510–1514Link, Google Scholar
25. Young RC, Biggs JT, Ziegler VE, Meyer DA: A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
26. Fleck DE, Sax KW, Strakowski SM: Reaction time measures of sustained attention differentiate bipolar disorder from schizophrenia. Schizophr Res 2001; 52:251–259Crossref, Medline, Google Scholar
27. Green AI, Tohen M, Patel JK, Banov M, DuRand C, Berman I, Chang H, Zarate C Jr, Posener J, Lee H, Dawson R, Richards C, Cole JO, Schatzberg AF: Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry 2000; 157:982–986Link, Google Scholar
28. Simonoff E, Bolton P, Rutter M: Mental retardation: genetic findings, clinical implications and research agenda. J Child Psychol Psychiatry 1996; 37:259–280Crossref, Medline, Google Scholar
29. Reiss AL, Eliez S, Schmitt JE, Patwardhan A, Haberecht M: Brain imaging in neurogenetic conditions: realizing the potential of behavioral neurogenetics research. Ment Retard Dev Disabil Res 2000; 6:186–197Crossref, Medline, Google Scholar
30. Doody GA, Johnstone EC, Sanderson TL, Cunningham Owens DG, Muir WJ: “Pfropfschizophrenie” revisited: schizophrenia in people with mild learning disability. Br J Psychiatry 1998; 173:145–153Crossref, Medline, Google Scholar
31. Nicolson R, Giedd JN, Lenane M, Hamburger S, Singaracharlu S, Bedwell J, Fernandez T, Thaker GK, Malaspina D, Rapoport JL: Clinical and neurobiological correlates of cytogenetic abnormalities in childhood-onset schizophrenia. Am J Psychiatry 1999; 156:1575–1579Link, Google Scholar
32. St. George-Hyslop PH: Molecular genetics of Alzheimer’s disease. Biol Psychiatry 2000; 47:183–199Crossref, Medline, Google Scholar
33. Goate A, Chartier-Harlin M-C, Mullan M, Brown J, Crawford F, Fidani L, Guiffra L, Haynes A, Irving N, James L, Mant R, Newton P, Rooke K, Roques P, Talbot C, Pericak-Vance M, Roses A, Williamson R, Rossor M, Owen M, Hardy J: Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 1991; 349:704–706Crossref, Medline, Google Scholar
34. Heston L, Mastri A: The genetics of Alzheimer’s disease: associations with hematologic malignancy and Down’s syndrome. Arch Gen Psychiatry 1977; 34:976–981Crossref, Medline, Google Scholar
35. Robakis N, Wisniewski H, Jenkins E, Devine-Gage E, Houck G, Yao X-L, Ramakrishna N, Wolfe G, Silverman W, Brown W: Chromosome 21q21 sublocalisation of gene encoding beta-amyloid peptide in cerebral vessels and neuritic (senile) plaques of people with Alzheimer disease and Down syndrome. Lancet 1987; 1:384–385Crossref, Medline, Google Scholar
36. Davison K, Bagley CR: Schizophrenia-like psychoses associated with organic disorders of the central nervous system: a review of the literature, in Current Problems in Neuropsychiatry: Schizophrenia, Epilepsy, the Temporal Lobe. Edited by Herrington RN. London, Headley, 1969, pp 113–184Google Scholar
37. Chow EWC, Zipursky RB, Mikulis DJ, Bassett AS: Structural brain abnormalities in patients with schizophrenia and 22q11 deletion syndrome. Biol Psychiatry 2002; 51:208–215Crossref, Medline, Google Scholar
38. Feinstein C, Eliez S, Blasey C, Reiss AL: Psychiatric disorders and behavioral problems in children with velocardiofacial syndrome: usefulness as phenotypic indicators of schizophrenia risk. Biol Psychiatry 2002; 51:312–318Crossref, Medline, Google Scholar
39. Graf WD, Unis AS, Yates CM, Sulzbacher S, Dinulos MB, Jack RM, Dugaw KA, Paddock MN, Parson WW: Catecholamines in patients with 22q11.2 deletion syndrome and the low-activity COMT polymorphism. Neurology 2001; 57:410–416Crossref, Medline, Google Scholar



