The Utility of Mandatory Depression Screening of Dementia Patients in Nursing Homes
Abstract
OBJECTIVE: Current methods for enhancing the recognition and treatment of depression in nursing home patients have been unsuccessful. This study examines the process, outcome, and impact of instituting a mandatory depression screening program for depressed dementia patients in nursing homes. METHOD: The experimental and comparison groups each consisted of two nursing homes of 519 and 363 patients, respectively. Two of the experimental group and one of the comparison group homes were more traditionally staffed facilities; one of the comparison group homes had an enriched staff of psychologists. The Cornell Scale for Depression in Dementia was administered to the residents with dementia. In the experimental group, the patients who scored ≥5 were referred for psychiatric assessment. RESULTS: In the experimental group, 100% of the referred dementia patients who met screening criteria for depression were seen by a psychiatrist. This resulted in a significant increase in the percentage of individuals given antidepressants. This was greater than the percentage of patients receiving antidepressants in the “typical” comparison group home but not the “staff-enriched” comparison group home. White patients were significantly more likely to receive antidepressants; however, screening significantly increased the proportion of depressed nonwhites receiving antidepressants. At the 12-week follow-up, there was a significant difference in scores between patients receiving antidepressants in each group. CONCLUSIONS: Mandatory depression screening can significantly increase the proportion of depressed dementia patients receiving antidepressants, lead to dose adjustments, diminish potential ethnic biases in treatment, and affect the depressive symptoms of treated individuals.
Depression in nursing home patients has been associated with subnutrition, behavioral problems, noncompliance with treatment, increased nursing staff time, and excessive mortality rates, especially among those with dementia (1). Estimates of the prevalence of major and minor depression among nursing home residents range from 6% to 24% and 30% to 50%, respectively (1). Among individuals with dementia, rates of all types of depression range from 14% to 39% (2, 3).
Various studies conducted in the 1990s found that fewer than one-fourth of depressed patients were identified and treated by nursing home physicians (1–4). More recent studies suggest that treatment rates may be increasing, but patients often receive suboptimal levels of antidepressant medications (5). Given the evidence that depression is frequently overlooked and undertreated, it is desirable to develop strategies for its recognition and treatment among dementia patients. One study found that a weekly educational course produced only a small increase in knowledge about depression, but the authors concluded that the identification and treatment of depression had not been altered appreciably (6). Moreover, weekly staff educational meetings can pose financial and logistical difficulties for many nursing homes.
Alternatively, on the basis of a small pilot project, we hypothesized that mandatory depression screening might be an effective and easily implemented method for augmenting the recognition and treatment of depression among nursing home patients with dementia. In this approach, once patients exceeded a cutoff score on a screening instrument, they would be referred for a psychiatric evaluation. In theory, the Minimum Data Set, a quarterly assessment of residents that is mandated by government regulations, should serve this screening function. However, we found previously that the Minimum Data Set missed 75% of major depressive disorders and 87% of all types of depression (7, 8), and among those identified by the Minimum Data Set as depressed, only 33% received an antidepressant medication.
This study addresses the following questions with respect to the process, outcome, and impact of instituting a mandatory depression screening program in two nursing homes:
| 1. | Are nursing home staff able to successfully use a standardized depression scale to screen all new admissions and all current dementia patients who are due for their 3-month Minimum Data Set evaluation? | ||||
| 2. | Among dementia patients who are rated as depressed by the screening instrument, what percentage is referred for further assessment? | ||||
| 3. | Are depressed dementia patients in the experimental nursing homes significantly more likely to receive antidepressant treatment after screening? | ||||
| 4. | After screening, do significantly more depressed dementia patients receive antidepressant treatment in the experimental nursing homes than the depressed dementia patients in the comparison nursing homes? | ||||
| 5. | What variables are associated with receiving antidepressant medication among depressed dementia patients in both experimental and comparison nursing homes? | ||||
| 6. | At the 12-week follow-up, what is the response of subjects to antidepressant medication, are subjects receiving adequate doses, and is there any difference in response between subjects who were receiving antidepressant medications in the experimental and comparison nursing homes? | ||||
Method
Procedures
The study was conducted in 1998–1999 using four racially diverse nursing homes in New York City. Two of the homes—199-bed and 320-bed institutions—served as the experimental group and two homes—123-bed and 240-bed facilities—served as the comparison group. The two experimental group homes and one of the comparison group homes were more traditionally staffed nursing homes with no psychologists and one or two consulting psychiatrists, whereas one of the comparison group homes (the “enriched” staff home) had three consulting psychologists and three psychiatrists who spent considerable time with patients as well as providing staff education.
Figure 1 outlines the eligibility criteria and the selection of the patient groups in the experimental and comparison nursing homes. Based on our earlier work, we had established a cutoff score of 5 on the Cornell Scale for Depression in Dementia (9) as indicating possible clinical depression (minor or major) (2, 3) and scores of ≥8 as suggesting “significant depressive symptoms”(7). Patients in the experimental group scoring ≥5 were referred to the nursing home psychiatrist, who was responsible for confirming a diagnosis of depression and developing atreatment plan. In the experimental homes, all eligible patients were evaluated by a member of the social work department. One of the authors (K.H.) initially trained the social workers to use the Cornell scale, and they then conducted five interviews under the supervision of our research staff. They attained good interrater reliability with our research staff. In the comparison homes, all patients were evaluated by our research staff.
Instruments
The patients were evaluated with the Cornell scale (9), the Mini-Mental State Examination (MMSE) (8), the Global Deterioration Scale (10), the Alzheimer’s Disease Assessment Scale’s (11) neuropsychiatric items (delusions, hallucinations, pacing, and increased motor activity), the Minimum Data Set’s 8-item Activities of Daily Living Scale, a physical health score based on the total of 22 items on the Minimum Data Set, and the Core Research Center’s Modified Schedule for Affective Disorders and Schizophrenia (SADS) (12), which was used to confirm depression in the comparison subjects. Internal consistencies (Cronbach’s alphas) for the Cornell Scale, the MMSE, the Alzheimer’s Disease Assessment Scale, and the Activities of Daily Living Scale were 0.75, 0.76, 0.69, and 0.92, respectively. Alphas above 0.60 were considered acceptable (13).
The research staff consisted of graduate students in clinical psychology. Intraclass correlation coefficients (ICC) indicated that interrater reliability was high, ranging from 0.85 to 0.97 on the various instruments. The ICC for the Cornell scale, which included scores of participating nursing home staff members as well as our research staff, was 0.92.
Data Analysis
All bivariate comparisons were made using t tests and chi-square tests. For the former, we used a separate variance formula when Levene’s test for equality of variances was significant. We used Yates’s correction for all two-by-two chi-square analyses. We used McNemar’s test for longitudinal analyses of categorical variables. A repeated-measures analysis of variance (ANOVA) was used to contrast longitudinal changes in Cornell scale scores of the experimental group and comparison group patients receiving antidepressant medications. For this analysis, the scores met assumptions of normality, equality of variance, equality of covariance matrices (Box’s M), and compound symmetry. All statistical analyses used two-tailed tests.
Results
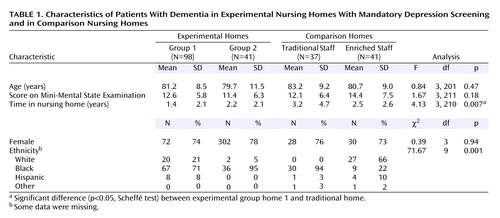
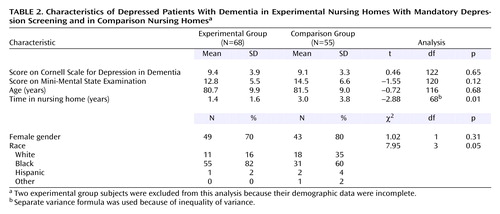
As shown in Table 1, there were no significant differences in age, MMSE score, and gender among the eligible subjects in the experimental group and the comparison group. However, the eligible subjects in the experimental group had shorter lengths of stays; the difference between experimental group home 1 and the “typical” comparison group home was statistically significant. There were also significant differences in ethnic distribution; the “enriched” comparison group home had appreciably more Caucasian residents than the other homes. As noted in Table 2, among the eligible subjects who were found to be depressed based on the Cornell scale screening (and confirmation with the SADS in the comparison group), there were no significant differences in Cornell scale scores, MMSE score, age, and gender; however, the residents in the experimental group had significantly shorter lengths of stay and were proportionately more nonwhite. Thus, the differences between nursing home groups in the depressed state were similar to the group differences in the eligible pool of dementia patients.
Mandatory screening had a significant effect on the two experimental group homes. We found that before screening, only 16% of the patients with Cornell scale scores ≥5 were given prescriptions for antidepressant medication, whereas after the screening procedures were introduced, 36% of these patients were given prescriptions for antidepressants (χ2=20.29, df=1, p<0.001, McNemar test). Similarly, for patients with more severe depression (Cornell scale score ≥8), 20% and 44% were prescribed medication pre- and postscreening, respectively (χ2=11.39, df=1, p<0.001, McNemar test).
There were no differences in the number of patients given prescriptions for antidepressants in the experimental group postintervention (36%) and the comparison group (37%) (χ2=0.00, df=1, p=1.00). However, on more detailed analysis, we found that in the “traditional” comparison group, only 19% of all patients with Cornell scale scores ≥5 received antidepressants, although this was not significantly different from the experimental group postintervention (χ2=1.07, df=1, p=0.30). Among the more severely depressed patients (Cornell scale score ≥8) in the “traditional” comparison group, 23% were receiving antidepressants, which was nearly one-half the level of the experimental group postintervention, although this difference was also not significant (χ2=1.14, df=1, p=0.29). On the other hand, in the “enriched” comparison group, 52% of the individuals with Cornell scale scores of ≥5 were given antidepressants, and 65% of the patients with Cornell scale scores of ≥8 were given antidepressants. Neither of these percentages were statistically different from the experimental group (for Cornell scale scores ≥5, χ2=1.49, df=1, p=0.22; for Cornell scale scores ≥8: χ2=1.59, df=1, p=0.21).
In looking at both groups postintervention, the use of antidepressant medication was significantly associated with scores on the Cornell scale (r=0.32, p=<0.001), being Caucasian (r=0.31, p=<0.001), and higher MMSE scores (r=0.24, p=<0.01), whereas health, score on the Activities of Daily Living Scale, score on the Alzheimer’s Disease Assessment Scale for neuropsychiatric symptoms, gender, and time in the nursing home were not significantly associated with receiving antidepressant treatment. After adding control for Cornell scale and MMSE scores, being Caucasian was still significantly correlated with receiving treatment (r=0.25, p<0.01); this association was significant (r=0.40, p<0.001) even when the analysis was restricted to the two experimental group homes and the one comparison group home that had predominantly non-Caucasian residents. Of note, in the experimental group, after we instituted mandatory screening, there was a significant increase in the proportion of depressed nonwhites receiving antidepressants (9% versus 27%) (χ2=11.19, df=1, p=0.002, McNemar test).
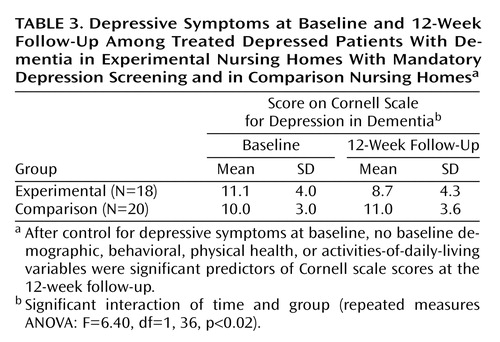
Using a repeated-measures ANOVA, we contrasted depressed patients in the experimental group and the comparison group who were receiving antidepressant medication. At the 12-week follow-up, there were significant differences between the two groups; the experimental group showed a 22% decline and the comparison group a 9% increase in Cornell scale scores (Table 3). Among the more severely depressed patients (Cornell scale score ≥8), the experimental group likewise had a significantly better outcome than the comparison group (a 24% decline versus 4% increase) (F=5.44, df=1, 29, p=0.03). Within the experimental group, there was no significant difference in the reduction in Cornell scale scores between the patients already taking antidepressants and those given antidepressants after mandatory screening (F=0.25, df=1, 16, p=0.63). Finally there was a significant difference in the percentage of patients in the experimental group (28%) and those in the comparison group (0%), who showed at least a 50% decline in depressive symptoms (χ2=4.20, df=1, p=0.04).
Discussion
The findings have important implications for clinical care and public policy. First, with respect to process evaluation, we demonstrated that clinical staff can easily use the Cornell scale and that referrals based on a cutoff score of ≥5 did not place an undue burden on staff psychiatrists (i.e., 100% of patients who scored ≥5 on the Cornell scale were evaluated by psychiatrists).
Second, with respect to outcome evaluation, we showed that depression screening resulted in significantly more depressed dementia patients receiving antidepressants in the experimental nursing homes, although it was somewhat disappointing that only 44% of the more severely depressed patients were given antidepressants. This may in part reflect the fact that depression was sometimes accompanied by agitation and other neuropsychiatric symptoms, thereby resulting in the prescription of other classes of psychotropic medications.
Our choice of using one comparison home that was more staff enriched than the experimental homes or the other comparison home attenuated the overall differences between the experimental and comparison groups. Nevertheless, it afforded an opportunity to contrast our intervention with different types of settings and staffing. The percentage of patients receiving antidepressants in the experimental group (postintervention) exceeded that of the “traditional” comparison group, whereas the percentage of patients receiving antidepressants in the “staff-enriched” comparison group was greater than that of the experimental group, although none of these differences were statistically significant. While the current study group had sufficient power to detect “medium” size effects between groups (14), a larger group would have been able to detect smaller effects that might have revealed significant group differences. Of importance, our data indicated that a psychologically enriched staff who are sensitized to recognize and treat depression, as well as being able to communicate the importance of the recognition of depression to nonpsychologically-trained staff, can generate a high level of treatment. Unfortunately, most nursing homes cannot afford the breadth of staff or do not have the psychological ethos that characterized the enriched home.
With respect to impact, experimental group patients, both newly treated and those who had been receiving antidepressants, improved significantly on 12-week follow-up versus the patients in the comparison group who had been receiving antidepressants. The fact that there were no longitudinal changes in depression among the treated patients in the comparison group suggested that a screening intervention program in these facilities might have led clinicians to adjust the dose of medication for some of those who had been receiving antidepressants. Indeed, based on the recommendations of expert consensus on pharmacotherapy in older depressed patients (15), 30% of the patients in all the nursing homes who had been receiving antidepressants before the commencement of our study were at “below average” (“starting”) doses, 55% were receiving “average” 6-week target doses, and 11% were receiving antidepressants that experts “would not generally use”; only one subject (4%) was receiving the “usual highest” dose. As Nelson (16) pointed out, while it is important to “start low and go slow,” it is essential to “keep going,” since older patients often require “usual” final doses. In the experimental homes, one-third of the patients who had been receiving antidepressants had their dose increased after screening. Of note, the modest response (28% improvement on the Cornell scale score) in the experimental group may have reflected the fact that after 12 weeks of treatment, 72% of the patients were still receiving starting doses. A limitation of our study was that we did not examine whether any nonpharmacological strategies were instituted for depressed persons in the experimental homes after their screening that may have contributed to their improvement.
Finally, while mandatory screening complements the Minimum Data Set and serves to facilitate its goal of triggering treatment, it is possible that Medicare may not reimburse psychiatrists if referrals emanate from screening instruments per se. Medicare will reimburse psychiatrists if patients are referred by licensed social workers or primary care physicians. Thus, screening can serve as the impetus for social workers and physicians to refer patients to psychiatrists for further evaluation and treatment.
Although the results of this study must be considered provisional because it was limited to four nursing homes—three of which had a large minority residential population—within one geographical area, our favorable findings with respect to process, outcome, and impact indicate that mandatory depression screening can provide a solution for enhancing the treatment of depression among dementia patients in nursing homes. Moreover, it can ameliorate some of the undertreatment of minorities that has been reported for depression in general (17). Future research should examine whether the coupling of mandatory depression screening with staff education and nonpharmacological behavioral approaches can further augment treatment recognition and response.
 |
 |
 |
Received May 30, 2001; revisions received May 20, July 24, and Oct. 29, 2002; accepted May 8, 2003. From the Division of Geriatric Psychiatry, State University of New York Downstate Medical Center; Hillside Hospital, Glen Oaks, NY; and Long Island University, Brooklyn, NY. Address request reprints to Dr. Cohen, Division of Geriatric Psychiatry, State University of New York Downstate Medical Center, 450 Clarkson Ave., Box 1203, Brooklyn, NY, 11203; cohen_c@hscbklyn. edu (e-mail). Supported in part by the New York State Department of Health, Bureau of Long-Term Care Services. The authors thank New York Congregational, Concord, and Cabrini Nursing Homes, the Center for Nursing and Rehabilitation and Community Research Applications, and Jeanne Teresi, Ph.D., Mary Devlin, Hulda Trotman, Joseph Wallach, and Barbara Singh for their assistance.
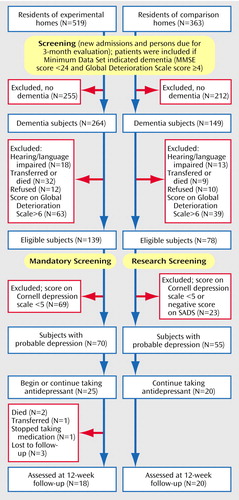
Figure 1. Design of a Study to Measure the Utility of Mandatory Depression Screening of Dementia Patients in Nursing Homes
1. Katz IR, Parmelee PA: Depression in elderly patients in residential care settings, in Diagnosis and Treatment of Depression in Late Life: Results of the NIH Consensus Development Conference. Edited by Schneider LS, Reynolds CF III. Washington, DC, American Psychiatric Press, 1994, pp 463–490Google Scholar
2. Cohen CI, Hyland K, Framowitz I: Depression Among Nursing Home Residents With Dementia: Final Report. Albany, New York State Department of Health, Bureau of Long-Term Care Services, 1995Google Scholar
3. Cohen CI, Hyland K, Magai C: Depression among African American nursing home patients with dementia. Am J Geriatr Psychiatry 1998; 6:162–175Crossref, Medline, Google Scholar
4. Samuels SC, Katz IB: Depression in the nursing home. Psychiatr Annals 1995; 25:419–424Crossref, Google Scholar
5. Datto CJ: Specificity of antidepressant prescribing in the nursing home setting, in Proceedings of the 15th Annual Meeting of the American Association of Geriatric Psychiatry. Bethesda, Md, AAGP, 2002Google Scholar
6. Teresi JA, Abrams R: Developing, testing, and packaging techniques for staff use in recognizing depression among nursing home residents, in Guidebook for Nursing Homes: Dementia Projects: Descriptions and Results. Albany, New York State Department of Health, Bureau of Long-Term Care Services, 1997Google Scholar
7. Alexopoulos GS, Abrams RC, Young RC, Shamoian CA: Cornell Scale for Depression in Dementia. Biol Psychiatry 1988; 23:271–284Crossref, Medline, Google Scholar
8. Burns A, Lawlor B, Craig S: Assessment Scales in Old Age Psychiatry. London, Martin Dunitz, 1999Google Scholar
9. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
10. Reisberg B, Ferris SH, De Leon MJ, Crook T: The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 1982; 139:1136–1139Link, Google Scholar
11. Rosen WG, Mohs RC, Davis KL: A new rating scale for Alzheimer’s disease. Am J Psychiatry 1984; 141:1356–1364Link, Google Scholar
12. Endicott J, Spitzer RL: A diagnostic interview: the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry 1978; 35:837–844Crossref, Medline, Google Scholar
13. Nunally JC: Psychometric Theory. New York, McGraw-Hill, 1967Google Scholar
14. Cohen J: Statistical Power Analysis for the Behavioral Sciences, revised ed. Orlando, Fla, Academic Press, 1977Google Scholar
15. Alexopoulos GS, Katz IR, Reynold CF, Carpenter D, Docherty JP: Pharmacotherapy of depressive disorders in older patients. Postgrad Med Special Report, Oct 2001, pp 1–88Google Scholar
16. Nelson JC: Diagnosing and treating depression in the elderly. J Clin Psychiatry 2001; 62(suppl 24):18–22Google Scholar
17. Lawson WB: Issues in pharmacotherapy for African Americans, in Ethnicity and Psychopharmacology. Edited by Ruiz P. Washington, DC, American Psychiatric Press, 2000, pp 37–53Google Scholar



