Decision Making in Adolescents With Behavior Disorders and Adults With Substance Abuse
Abstract
OBJECTIVE: The study assessed the validity of the Gambling Task as a test of decision-making ability in adolescents and examined whether adolescents with behavior disorders, who are at risk for substance abuse, have deficits in decision making similar to those exhibited by adults with substance abuse. METHOD: Performance on the Gambling Task in two testing sessions separated by 1 week was assessed in 64 12–14-year-old adolescents (31 healthy, 33 with externalizing behavior disorders) and 52 adults (22 healthy, 30 with substance abuse). RESULTS: The healthy adolescents and the healthy adults had similar performance on the Gambling Task. Adolescents with behavior disorders performed more poorly than healthy adolescents, but only in the second testing session. In adults, overall Gambling Task performance did not differ between the healthy and substance abuse groups at either testing session, indicating no difference in learning of decision-making strategies between groups. However, adults with substance abuse performed more poorly than healthy adults during an early stage of the task, when participants presumably begin to understand the rewards and penalties involved in the task but are not yet sure of the actual risk of incurring penalities. CONCLUSIONS: The Gambling Task can be used with adolescents. Testing with the Gambling Task revealed a deficit in decision making in adolescents with behavior disorders, who are at risk for substance abuse. This deficit may represent a vulnerability factor for the development of substance abuse.
Research focused on elucidating the neural processes underlying decision making has included adults only (1–4). The extension of such studies to adolescents requires the assessment and validation of cognitive tasks in adolescents.
This study evaluated the Gambling Task as a test of decision making in adolescents. In adults, this task is sensitive to lesions of the frontal cortex (5, 6), and it reveals impairments in subjects who have maladaptive behaviors, such as substance use disorders and psychopathy (7–11). The Gambling Task tests the ability to balance immediate rewards against long-term negative consequences.
Children acquire the ability to delay gratification as they learn to exercise such balance. By age 11–12 years, most children have reached the formal operational period (12), characterized by the ability to conceptualize and the acquisition of a sense of time that facilitates successful action planning. Similarly, by that age, they have developed the ability to deflect attention from irrelevant stimuli and to control perseverative responses (13).
Children with externalizing behavior disorders, such as attention deficit hyperactivity disorder (ADHD) and conduct disorder are likely to have impaired decision making as a result of impulsivity, delay aversion, heightened sensitivity to immediate reward, and propensity toward risk-taking behaviors (14–19). These conditions are thought to contribute to the greater incidence of substance use disorders in these adolescents than in the general population (20, 21). Both conduct disorder and ADHD have been associated with enhanced risk for substance use disorders (22–27). The association of conduct disorder with high rates of substance use disorders is well-established; rates of substance use disorders of 65% in boys and 94% in girls have been reported for subjects whose childhood conduct problems extend into adulthood (24). However, the association of ADHD with substance use disorders has been more controversial, with some data showing an increased risk of substance use disorders mediated by comorbid conduct disorder, and other data showing that ADHD is a risk factor in itself. A series of studies by Biederman’s group (22, 23, 26, 27) clarified this discrepancy by demonstrating that the excess in rates of substance use disorders related to ADHD could be reliably detected only after age 19 years, whereas conduct disorder was shown to increase the risk of substance use disorders throughout adolescence. Therefore, to detect an association between ADHD and substance abuse disorders, longitudinal studies of subjects beyond age 19 years are needed. In a retrospective study, adults with a diagnosis of childhood-onset ADHD had a lifetime rate of substance use disorders that was twice that of non-ADHD adults (52% versus 27%) (23). Onset of substance use disorders was reported to occur significantly earlier in ADHD adults (at age 19 years) than in non-ADHD adults (at age 22 years) (27). Prospectively, rates of substance use disorders were found to be similar in ADHD and non-ADHD adolescents at 15 years of age (15%) (22), whereas the prevalence of substance use disorders in ADHD adolescents at 17 years of age was 41%, compared to 16% in the age-matched non-ADHD group (26).
Previous studies have reported impaired performance on decision-making tasks in adults with substance use disorders (7, 9, 28, 29). Several studies that used the Gambling Task suggested performance deficits in this population, but none reported how performance changed with time (7, 9, 28). This question is particularly relevant to substance use disorders, which are characterized by a lack of adaptations to avoid negative consequences (DSM-IV). We expected that differences in performance on the Gambling Task between healthy subjects and subjects with substance use disorders would increase with time, as the healthy subjects improved their performance while the substance abusers did not.
The Wisconsin Card Sorting Task was used as a comparison test because it also requires intact executive functions, including choice and planning (30, 31), but it engages the dorsolateral (32–35) portion of the prefrontal cortex rather than the ventral portion that is more specific to performance on the Gambling Task (6, 36). Deficits on both tasks would suggest generalized prefrontal dysfunction, whereas a deficiency on only one task would suggest involvement of a specific prefrontal region.
We hypothesized that performance on the Gambling Task would 1) be similar or less adaptive in healthy adolescents than in healthy adults, 2) be worse in adolescents with behavior disorders than in healthy adolescents; and 3) improve over time in healthy adults but not in adults with substance use disorders. We predicted that performance on the Wisconsin Card Sorting Task in healthy adolescents would be similar to that in adults (37). We also expected that adolescents with behavior disorders would show deficits on this task, on the basis of evidence for impaired executive function in children with ADHD (38–40), although such impairments have not been found consistently (27, 41).
Method
Subjects and Experimental Design
The subjects included adults and adolescents. Part of the data collected for the adults (performance scores of the first administration of the Gambling Task) has been previously published (9). Adults and adolescents were assessed with identical cognitive paradigms, and the same experimenter (L.S.) administered the tests for both groups.
Male and female volunteers were recruited through newspaper advertisements and psychiatric clinics. The adults were 21–44 years old, and the adolescents were 12–14 years old. After receiving a detailed description of the research, adult volunteers and parents gave written informed consent, and adolescents gave written informed assent. The studies were approved by the National Institute on Drug Abuse Institutional Review Board.
Participants completed a medical screening, including a psychiatric evaluation (assessment with the Diagnostic Inventory Schedule [42] for the adults and with the Diagnostic Interview for Children and Adolescents [43] for the adolescents) and a test of intellectual functioning (Shipley Institute of Living Scale [44] for the adults and the abbreviated Wechsler Intelligence Scale for Children, 3rd ed. [WISC-III] [45] for the adolescents). Socioeconomic status was determined by using Hollingshead’s Four-Factor Index of Social Status (46). Exclusion criteria included a history of head trauma, medical illness, and IQ <80. Lifetime axis I psychiatric diagnoses were also exclusionary, except for substance use disorders in the adults, and ADHD with or without comorbid mood disorders and conduct disorder in the adolescents.
The adult group included healthy comparison subjects and polydrug abusers with current histories of opioid or stimulant use evidenced by self-reports and by a positive urine drug test at study entry. The adolescent group included healthy comparison subjects and subjects with an externalizing behavior disorder (ADHD or conduct disorder) who were considered to be at risk for substance use disorders but did not have a current history of drug abuse. The adults with substance use disorders lived in a residential treatment unit for 36–48 hours before testing to eliminate the effects of acute intoxication. The other subjects were not living in a residential treatment setting when they were tested.
Two testing sessions, separated by 1 week, were conducted. Results on the Wisconsin Card Sorting Task only for week 1 were included in the analysis, because data from repeated administration of this task are not clinically meaningful. Adolescents treated with stimulants were asked to discontinue their medication for 48 hours before each testing session. There is no evidence that discontinuation of oral methylphenidate at therapeutic doses induces a state of withdrawal or an exacerbation of native symptoms (47–51). Symptoms usually return to baseline levels within 3–4 hours after administration of an oral dose of methylphenidate (see review in reference 52), suggesting that 48 hours is sufficiently long to expect a return to baseline states. Adolescents treated with other psychotropic medications with longer half-lives were not asked to discontinue treatment, because a longer period of medication withdrawal would have been necessary.
Cognitive Tasks
Decision-making task
The Gambling Task (5) is a card game in which participants are told to accumulate as much play money as possible by picking one card at a time from any of four decks (A, B, C, and D) until 100 cards have been selected. The decks (40 cards each) differ in representation of both the level of immediate gain and the level of risk of penalties. Every card from decks A and B yields a gain of $100, compared with $50 for every card from decks C and D. Some cards in each deck also carry penalties, such that the accumulated penalties exceed the accumulated gains in decks A and B, and the accumulated penalties are smaller than the accumulated gains in decks C and D. Thus, continued choice from decks C or D leads to a net gain ($250/10 cards), whereas continued choice from decks A or B leads to a net loss (–$250/10 cards). The optimal strategy is to avoid the short-term appeal of decks A and B in favor of the slower gain from decks C and D.
Performance on the Gambling Task is scored by a global outcome score (net score) and a score for each consecutive block of 20 cards. These scores correspond to the number of cards chosen from the advantageous decks (C and D) minus the number of cards chosen from the disadvantageous decks (A and B). The analysis of the Gambling Task performance by blocks of 20 cards provides information about the learning capacity and strategy used by participants. Bechara (53) identified four learning phases corresponding to changes in awareness or understanding of the task: guess, pre-hunch, hunch, and conceptual knowledge. Performance (net scores) improves across these phases. The analysis of performance at each phase provides a means to better qualify the influence of age and diagnosis on the learning process.
Wisconsin Card Sorting Task
The Wisconsin Card Sorting Task tests the ability to form abstract concepts and to shift between response sets (30). Four sample cards are presented, each bearing geometric designs that vary along the dimensions of color (red, green, yellow, blue), number (numbers 1–4), and shape (triangle, star, cross, circle). Participants sequentially select from a presorted deck of 64 cards that vary in the combinations of these dimensions, and they are asked to match each card to one of the sample cards. The criterion for matching is not stated, but the participant is told immediately whether each match was correct or incorrect. After each 10 consecutive correct matches, the criterion changes. Once all the cards in the first deck have been picked, a second deck of 64 cards presorted in the same order is used.
We analyzed the five scores most commonly used across studies: 1) number of completed sets of 10 consecutive correct matches; 2) number of perseverative errors; 3) number of nonperseverative errors, reflecting impulsivity and distractibility; 4) number of trials to complete the first category, a measure of conceptual ability; and 5) failure to maintain set, i.e., number of times an incorrect response was made after five successive correct responses.
Statistical Analysis
Subject characteristics are described as means and standard deviations for each group. Two-by-two (age group: adults and adolescents; diagnosis group: healthy and patients) analyses of variance (ANOVAs) were conducted to evaluate the main effects of age group and diagnosis group and the effects of the interaction of age group and diagnosis group on sex, ethnicity, socioeconomic status, and IQ. Age differences between diagnostic groups were tested for adults and adolescents separately.
To evaluate potentially confounding effects on group comparisons of the performance scores, the variables of age, sex, ethnicity, socioeconomic status, and IQ were entered into exploratory regression analyses for each performance score and main comparison group (three groups: healthy subjects [both adults and adolescents], adults [both healthy adults and adults with substance abuse disorders], and adolescents [both healthy adolescents and adolescents with a behavior disorder]). Only IQ remained in the regression analyses for both the Gambling Task (accounting for 11% to 19% of the variance in the net scores) and the Wisconsin Card Sorting Task (accounting for 8% to 33% of the variance in the scores). Therefore, IQ was used as a covariate in all subsequent analyses of covariance (ANCOVAs).
The Gambling Task data were analyzed by using three separate groups of two-way ANCOVAs, with IQ as a covariate, to evaluate the interactions and main effects of 1) age group (adults, adolescents) and time (week 1, week 2) for the comparison of healthy adolescents and healthy adults, 2) diagnosis group (healthy, patients) and time (week 1, week 2) for the comparisons of adults with substance use disorders and healthy adults, and 3) diagnosis group (healthy, patients) and time (week 1, week 2) for the comparisons of healthy adolescents with adolescents with behavior disorders. Pooling the data for the adolescents with behavior disorders and the adults with substance use disorders (i.e., creating an overall patient group) was considered inappropriate because of the substantial clinical differences between the two groups. Therefore, a single three-way ANCOVA with diagnosis, age group, and time as factors was not used.
In addition, an exploratory analysis was performed on data for the five 20-card blocks of the Gambling Task to evaluate which phase of awareness (guess, pre-hunch, hunch, and conceptual knowledge) (53) might be most sensitive to age group and diagnosis group. Individual ANCOVAs were performed for each of the 10 blocks by using the group comparisons described earlier. Given the exploratory nature of this analysis, the criterion for statistical significance was set at p<0.10, after Bonferroni correction (p<0.01×10 comparisons).
Results
Demographic Characteristics
The study group comprised 52 adults (22 healthy comparison subjects and 30 adults with substance use disorders) and 64 adolescents (31 healthy comparison subjects and 33 adolescents with an externalizing behavior disorder) (Table 1). Data from two adult comparison participants were removed from an analysis published previously (9) because their Gambling Task scores indicated that they did not engage in the task.
Behavior disorders in adolescents consisted of ADHD without comorbidity (N=21), conduct disorder without comorbidity (N=5), and ADHD comorbid with a history of mood disorder without acute symptoms (N=7, four with unipolar depression, two with dysthymia, and one with adjustment disorder). An ANCOVA, with IQ as covariate, showed no significant differences in Gambling Task scores among the three groups of adolescents with behavior disorders. Thus, data for the three groups were analyzed together, to represent a single group of at-risk adolescents. About half of the subjects with behavior disorders (18 of 33) were treated with stimulants and were tested 48 hours after stimulant discontinuation. Performance on the decision-making task (net scores) and on the Wisconsin Card Sorting Task did not differ between the 18 adolescents who discontinued treatment and the 15 adolescents who were not treated with stimulants (net score, week 1: F=0.70, df=1, 30, p=0.41; net score, week 2: F=0.03, df=1, 30, p=0.86; Wisconsin Card Sorting Task: 0.01<F<0.74, df=1, 30, 0.40<p<0.93). Nine adolescents with behavior disorders were also receiving tricyclic antidepressants (N=2), bupropion (N=4), or sertraline (N=3). These medications were not discontinued because their longer half-lives would have required a longer discontinuation period. The performance scores of these nine adolescents did not differ significantly from that of the other 24 adolescents with behavior disorders (net score, week 1: F=0.02, df=1, 30, p=0.88; net score, week 2: F=1.72, df=1, 30, p=0.20; Wisconsin Card Sorting Task: 0.34<F<2.34, df=1, 30, 0.14<p<0.56).
Most adults with substance use disorders (28 of 30) used an average of 2.5 g/week (SD=0.7) of cocaine, more than half (19 of 30) used an average of 253 mg/week (SD=76) of heroin, and 29 used marijuana, alcohol, and/or nicotine weekly. The average duration of use was about 7 years for the cocaine and heroin users, and between 14 and 17 years for the users of marijuana, alcohol, and cigarettes. None of the comparison subjects had ever used cocaine or heroin or were currently smoking marijuana or nicotine cigarettes.
Data on the subjects’ age, sex, ethnicity, socioeconomic status, and IQ are listed in Table 1. The healthy adolescents and the adolescents with behavior disorders were similar in age, whereas the healthy adults were younger than the adults with substance use disorders (t=2.56, df=50, p=0.01). Socioeconomic status was lower in the adults than in the adolescents (F=35.16, df=1, 113, p<0.0001) and lower in the adults with substance use disorders than in the healthy adults (t=3.81, df=50, p=0.0004). There was no significant difference in socioeconomic status between the healthy adolescents and the adolescents with behavior disorders. Finally, IQ was significantly higher in the healthy adults than in the adults with substance use disorder (t=–2.97, df=50, p=0.005) and significantly lower in the adults with substance use disorders than in the adolescents with behavior disorders (t=–2.36, df=48.4, p=0.02), but there was no significant difference in IQ between the healthy adolescents and the adolescents with behavior disorder or between the healthy adolescents and the healthy adults.
Cognitive Performance
The four groups’ mean scores on the Gambling Task and Wisconsin Card Sorting Task, after correction for differences in IQ, are presented in Table 2.
Healthy adolescents versus healthy adults
As Figure 1 and Figure 2 show, the mean overall net scores and the block net scores on the Gambling Task did not differ between the adults and the adolescents at week 1 or at week 2.
Of the five Wisconsin Card Sorting Task scores, only the number of trials to complete the first category (a measure of conceptual ability) showed a significant main effect of age group (F=11.66, df=1, 45, p=0.001), indicating that the adolescents scored better than the adults (Table 2).
Healthy adolescents versus adolescents with behavior disorders
For the overall scores on the Gambling Task, the time-by-diagnosis group interaction was statistically significant (F=4.97, df=1, 61, p=0.03): the healthy adolescents improved from week 1 to week 2, and the adolescents with behavior disorders did not (Figure 1). In addition, there was a main effect of diagnosis group on net scores (F=4.79, df=1, 61, p=0.03), which was mainly due to the worse performance of the adolescents with behavior disorders compared to the healthy adolescents at week 2 (F=7.42, df=1, 61, p=0.008). No significant differences were found between the healthy adolescents and those with behavior disorders on the Gambling Task block net scores.
The adolescents with behavior disorders had significantly worse scores than the healthy adolescents on the Wisconsin Card Sorting Test measure of failure to maintain set (F=7.12, df=1, 61, p=0.01) (Table 2). Performance on the measure of failure to maintain set at week 1 was not correlated with Gambling Task performance at week 1 or week 2 in the adolescents with behavior disorders (week 1: r=–0.15, df=31, p=0.40; week 2: r=–0.21, df=31, p=0.23) or in the healthy adolescents (week 1: r=–0.07, df=29, p=0.72; week 2: r=0.12, df=29, p=0.53).
Healthy adults versus adults with substance use disorders
No significant time-by-diagnosis group interaction and no significant main effects of time and diagnosis group were found in the comparison of overall Gambling Task scores between the healthy adults and the adults with substance use disorder (Figure 1). The Gambling Task block net scores showed that only performance in block 3 (cards 41–60) at week 1 differed significantly between the healthy adults and the adults with substance use disorders (F=6.55, df=1, 47, p=0.01, p=0.10, with Bonferroni correction), with the adults with substance use disorders scoring worse than the healthy adults.
There were no significant differences on any of the Wisconsin Card Sorting Test scores between the healthy adults and the adults with substance use disorders.
Discussion
The key findings regarding adolescents in this study are that 1) Gambling Task performance is similar in healthy adolescents and in healthy adults and 2) Gambling Task performance after the task is learned (i.e., at the second administration of the task) is worse in adolescents with behavior disorders (who are at risk for substance use disorders) than in healthy adolescents. In addition, healthy adults and adults with substance use disorders showed no difference in their change in performance on the Gambling Task between week 1 and week 2. It is noteworthy that the adults with substance use disorders performed worse than healthy adults during the “hunch” period (block 3 of week 1), when participants presumably begin to understand the reward/penalty differences between the Gambling Task decks but are not sure of the actual risk of incurring penalties (53).
Healthy Adolescents Versus Healthy Adults
The finding that healthy adolescents and healthy adults performed similarly on the Gambling Task suggests that this measure can be used in adolescents to probe deficits in decision making and that performance in adolescents can be compared to that in adults. Overall, our findings support the idea that prefrontal neural organization at ages 12–14 years has reached a developmental stage that permits adult levels of cognitive ability on the Gambling Task.
As expected (37), performance on the Wisconsin Card Sorting Task did not differ between the adolescents and the adults on most scores. However, the worse performance of the adults compared to the adolescents on the number of trials to complete the first category was not anticipated. The adolescents’ performance score for trials to complete the first category (mean score=12.2, SD=5.0) was better than that of the normative sample for the same age group (normative score for 13-year-olds: mean=18.7, SD=17.1, N=29), whereas the adults’ performance (mean score=20.0, SD=11.5) was worse than that for the normative sample of adults (normative score for 30–39-year-olds: mean=12.2, SD=4.8, N=63) (31). However, the normative scores for this subtest varied substantially with age (e.g., 11-year-olds: mean score=13.3, SD=5.7, N=50; 14-year-olds: mean score=19.1, SD=23.4, N=27; 15-year-olds: mean score=12.9, SD=5.1, N=32; 40–49-year-olds: mean score=14.0, SD=15.3, N=63) (31). On the basis of this observation and on the fact that scores on all other subscales were within the expected range, the difference between the adults and the adolescents in performance on a single subscale is not likely to reflect overall abnormal performance in the adults.
Healthy Adolescents Versus Adolescents With Behavior Disorders
The adolescents with behavior disorders and the healthy adolescents performed similarly on the Gambling Task at week 1. However, the performance of the adolescents with behavior disorders did not improve by week 2, unlike the performance of the healthy adolescents. This finding suggests that adolescents with behavior disorders, who are considered to be at risk for substance use disorders, show deficits in decision making similar to those found in substance abusers (7, 9, 28, 29). However, the specificity and sensitivity of this deficit remain to be determined. If this impairment in decision making represents a vulnerability for the development of substance abuse, the qualitative difference of the deviance in Gambling Task performance—significantly worse performance on block 3 at week 1 for the adults with substance use disorders than for the healthy adults and significantly worse performance at week 2 for the adolescents with behavior disorders than for the healthy adolescents—suggests an evolution of the deficits with age or with exposure to drugs of abuse. Only longitudinal studies can clarify the link between maladaptive decision making in adolescents with behavior disorders and the development of substance abuse disorders. In addition, the adolescents with behavior disorders had poorer performance than the healthy adolescents on the Wisconsin Card Sorting Task score for failure to maintain set. This difference probably reflects the relatively high degree of impulsivity and distractibility of this group or a reduced responsivity to reinforcement in guiding problem-solving behavior. This deficit on the Wisconsin Card Sorting Task was not correlated with performance on the Gambling Task, suggesting that Gambling Task performance was independent of the cognitive processes engaged in the Wisconsin Card Sorting Task.
Healthy Adults Versus Adults With Substance Use Disorders
In contrast to the results of our previous work (9), overall performance on the Gambling Task across week 1 and week 2 did not differ significantly between adults with substance use disorders and healthy adults. Adults with substance use disorders performed worse than the healthy adults, but this difference did not reach significance (F=2.99, df=1, 47, p=0.09). In addition, contrary to our hypothesis, changes in Gambling Task performance between week 1 and week 2 did not differ between adults with substance use disorders and healthy adults. Although Figure 2 shows a pattern of improvement in mean scores during week 2 in the healthy adults but not in the adults with substance use disorders, the net score on block 3 at week 1 was the only score that was significantly worse in the adults with substance use disorders. This block corresponds to the time when participants begin to understand the pattern of rewards and penalties associated with the decks of cards and begin to shift preference toward the advantageous decks (53). The distribution of scores across time suggests that adults with substance use disorders either reached the hunch period later than healthy adults or did not adapt their strategies based on knowledge of costs for rewards. As already reported (9), maladaptive performance on the Gambling Task is contrasted to normal performance on the Wisconsin Card Sorting Task, suggesting a specific deficit in decision making involving conflicts between immediate rewards and long-term penalties.
One caveat to the interpretation of this work concerns the use of IQ as a covariate in cognitive studies of healthy versus substance use disorders groups. This approach is conservative because the removal of the variance related to IQ is likely to also remove a portion of the variance due to substance use disorders. Indeed, neuropsychological studies of subjects with substance use disorders have tended to show cognitive deficits that are likely to affect IQ (54, 55). In fact, group differences in Gambling Task mean scores across week 1 and week 2 were 10 times more robust before IQ correction (F=6.90, df=1, 48, p=0.01) than after IQ correction (F=2.99, df=1, 47, p=0.09). Our results corrected for IQ, however, indicate that Gambling Task deficits (in block 3 at week 1) are present above and beyond differences in global cognitive function and thus are specific to the cognitive processes involving the weighting of rewards and penalties for deciding a course of action. Another caveat concerns the inability to ascertain a specific relationship between the cognitive deficits found in the adolescents with behavior disorders and a vulnerability for substance use disorders. Follow-up evaluation of these adolescents will provide the means to assess directly the predictive value of cognitive performance for the development of substance use disorders. Because Gambling Task performance did not differ among the adolescents with ADHD, those with conduct disorder, and those with comorbid ADHD and mood disorder, we did not explore further the relative contributions of individual diagnostic groups to the findings. Finally, inclusion of adolescents treated with psychotropic medications for their behavior symptoms may have introduced an artifact in this study. The effect of this artifact, however, is expected to be minimal because the performance of the adolescents who received medication did not differ significantly from that of those who did not receive medication.
Further studies are needed to elucidate which elemental cognitive processes contribute to decision-making deficits in adults with substance use disorders and adolescents with behavior disorders. Functional magnetic resonance imaging is at present the best tool to examine the neural substrates of these deficits. The present work suggests that the optimal windows to capture compromised performance on the Gambling Task correspond to the hunch period of block 3 (cards 41–60) during a first administration of the Gambling Task for adults with substance use disorders and during readministration of the task for adolescents with behavior disorders.
 |
 |
Received Feb. 11, 2002; revision received June 26, 2002; accepted Sept. 26, 2002. From the Mood and Anxiety Disorders Program, NIMH; the National Institute on Drug Abuse, Rockville, Md., and Baltimore, Md.; and the Departments of Psychiatry and Biobehavioral Sciences and Molecular and Medical Pharmacology, University of California, Los Angeles. Address reprint requests to Dr. Ernst, NIH NIMH/MAP, 15K North Dr., Room 118, MSC 2670, Bethesda, MD 20892-2670; [email protected] (e-mail). The authors thank Jennifer Schroeder for statistical support and Lyal Tressler, Neir Eshel, and Sarah Davariah for help with manuscript preparation.
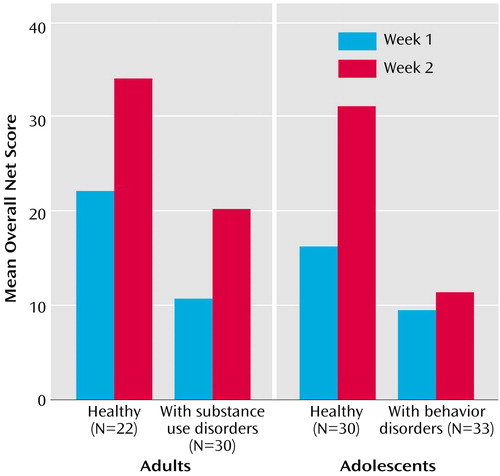
Figure 1. Overall Net Scores on the Gambling Task in Healthy Adults, Adults With Substance Use Disorders, Healthy Adolescents, and Adolescents With Behavior Disordersa
aNet score is the number of advantageous cards minus number of disadvantageous cards, corrected for between-group differences in IQ. The task was administered on two occasions separated by 1 week. Net scores were similar in healthy adults and healthy adolescents. They were significantly lower in adolescents with behavior disorders than in healthy adolescents (F=4.79, df=1, 61, p=0.03), mostly because of differences in week 2 performance (F=7.42, df=1, 61, p=0.008).
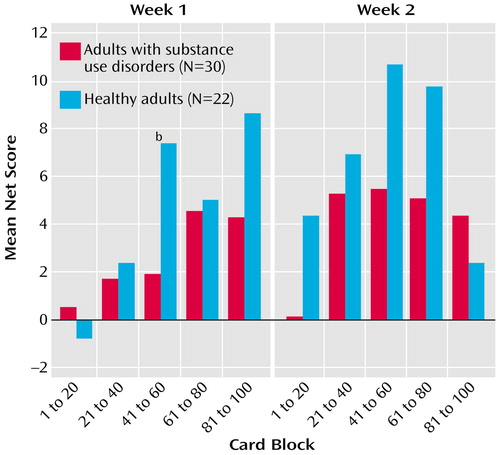
Figure 2. Net Scores on the Gambling Task Over Five Blocks of 20 Card Choices in Healthy Adults and Adults With Substance Use Disordersa
aNet score is the number of advantageous cards minus number of disadvantageous cards, corrected for between-group differences in IQ. The task was administered on two occasions separated by 1 week. The blocks of cards correspond to phases of awareness or understanding of the task, as follows: guess (cards 1 to 20), pre-hunch (cards 21 to 40), hunch (cards 41 to 60), and conceptual knowledge (cards 61 to 100).
bSignificant difference between groups (F=6.55, df=1, 47, p<0.10 Bonferroni corrected).
1. Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P: Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 2001; 30:619-639Crossref, Medline, Google Scholar
2. Critchley HD, Mathias CJ, Dolan RJ: Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron 2001; 29:537-545Crossref, Medline, Google Scholar
3. Elliott R, Frith CD, Dolan RJ: Differential neural response to positive and negative feedback in planning and guessing tasks. Neuropsychologia 1997; 35:1395-1404Crossref, Medline, Google Scholar
4. Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED: Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology 2002; 26:682-691Crossref, Medline, Google Scholar
5. Bechara A, Damasio AR, Damasio H, Anderson SW: Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994; 50:7-15Crossref, Medline, Google Scholar
6. Bechara A, Damasio H, Damasio AR, Lee GP: Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci 1999; 19:5473-5481Crossref, Medline, Google Scholar
7. Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE: Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia 2001; 39:376-389Crossref, Medline, Google Scholar
8. Blair RJ, Colledge E, Mitchell DG: Somatic markers and response reversal: is there orbitofrontal cortex dysfunction in boys with psychopathic tendencies? J Abnorm Child Psychol 2001; 29:499-511Crossref, Medline, Google Scholar
9. Grant S, Contoreggi C, London ED: Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia 2000; 38:1180-1187Crossref, Medline, Google Scholar
10. Mazas CA, Finn PR, Steinmetz JE: Decision-making biases, antisocial personality, and early-onset alcoholism. Alcohol Clin Exp Res 2000; 24:1036-1040Crossref, Medline, Google Scholar
11. Petry NM, Bickel WK, Arnett M: Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction 1998; 93:729-738Crossref, Medline, Google Scholar
12. Piaget J: Science of Education and the Psychology of the Child. New York, Orion Press, 1970Google Scholar
13. Pineda D, Ardila A, Rosselli M, Cadavid C, Mancheno S, Mejia S: Executive dysfunctions in children with attention deficit hyperactivity disorder. Int J Neurosci 1998; 196:177-196Crossref, Google Scholar
14. Carlson CL, Tamm L: Responsiveness of children with attention deficit-hyperactivity disorder to reward and response cost: differential impact on performance and motivation. J Consult Clin Psychol 2000; 68:73-83Crossref, Medline, Google Scholar
15. Douglas VI, Parry PA: Effects of reward on delayed reaction time task performance of hyperactive children. J Abnorm Child Psychol 1983; 11:313-326Crossref, Medline, Google Scholar
16. Farmer JE, Peterson L: Injury risk factors in children with attention deficit hyperactivity disorder. Health Psychol 1995; 14:325-332Crossref, Medline, Google Scholar
17. Oosterlaan J, Sergeant JA: Effects of reward and response cost on response inhibition in AD/HD, disruptive, anxious, and normal children. J Abnorm Child Psychol 1998; 26:161-174Crossref, Medline, Google Scholar
18. Solanto MV, Wender EH, Bartell SS: Effects of methylphenidate and behavioral contingencies on sustained attention in attention-deficit hyperactivity disorder: a test of the reward dysfunction hypothesis. J Child Adolesc Psychopharmacol 1997; 7:123-136Crossref, Medline, Google Scholar
19. Tripp G, Alsop B: Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. J Clin Child Psychol 1999; 28:366-375Crossref, Medline, Google Scholar
20. Biederman J, Wilens TE, Mick E, Faraone SV, Spencer T: Does attention-deficit hyperactivity disorder impact the developmental course of drug and alcohol abuse and dependence? Biol Psychiatry 1998; 44:269-273Crossref, Medline, Google Scholar
21. Bukstein OG: Disruptive behavior disorders and substance use disorders in adolescents. J Psychoactive Drugs 2000; 32:67-79Crossref, Medline, Google Scholar
22. Biederman J, Wilens T, Mick E, Faraone SV, Weber W, Curtis S, Thornell A, Pfister K, Jetton JG, Soriano J: Is ADHD a risk factor for psychoactive substance use disorders? findings from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry 1997; 36:21-29Crossref, Medline, Google Scholar
23. Biederman J, Wilens T, Mick E, Milberger S, Spencer TJ, Faraone SV: Psychoactive substance use disorders in adults with attention deficit hyperactivity disorder (ADHD): effects of ADHD and psychiatric comorbidity. Am J Psychiatry 1995; 152:1652-1658Link, Google Scholar
24. Disney ER, Elkins IJ, McGue M, Iacono WG: Effects of ADHD, conduct disorder, and gender on substance use and abuse in adolescence. Am J Psychiatry 1999; 156:1515-1521Link, Google Scholar
25. Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M: Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry 1998; 155:493-498Link, Google Scholar
26. Milberger S, Biederman J, Faraone SV, Wilens T, Chu MP: Associations between ADHD and psychoactive substance use disorders: findings from a longitudinal study of high-risk siblings of ADHD children. Am J Addict 1997; 6:318-329Medline, Google Scholar
27. Wilens TE, Biederman J, Mick E, Faraone SV, Spencer T: Attention deficit hyperactivity disorder (ADHD) is associated with early onset substance use disorders. J Nerv Ment Dis 1997; 185:475-482Crossref, Medline, Google Scholar
28. Petry NM: Substance abuse, pathological gambling, and impulsiveness. Drug Alcohol Depend 2001; 63:29-38Crossref, Medline, Google Scholar
29. Rogers RD, Everitt BJ, Baldacchino A, Blackshawc AJ, Swainsona R, Bakera NB, Huntera J, Carthya T, Bookera E, London M, Deakin JFW, Sahakian BJ, Robbins TW: Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology 1999; 20:322-339Crossref, Medline, Google Scholar
30. Berg E: A simple objective technique for measuring flexibility in thinking. J Gen Psychol 1948; 39:15-22Crossref, Medline, Google Scholar
31. Heaton R, Chelune G, Talley J, Kay G, Curtiss G: Wisconsin Card Sorting Test Manual USA. Lutz, Fla, Psychological Assessment Resources (PAR), 1993Google Scholar
32. Anderson SW, Damasio H, Jones RD, Tranel D: Wisconsin Card Sorting Test performance as a measure of frontal lobe damage. J Clin Exp Neuropsychol 1991; 13:909-922Crossref, Medline, Google Scholar
33. Axelrod BN, Goldman RS, Heaton RK, Curtiss G, Thompson LL, Chelune GJ, Kay GG: Discriminability of the Wisconsin Card Sorting Test using the standardization sample. J Clin Exp Neuropsychol 1996; 18:338-342Crossref, Medline, Google Scholar
34. Berman KF, Ostrem JL, Randolph C, Gold J, Goldberg TE, Coppola R, Carson RE, Herscovitch P, Weinberger DR: Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: a positron emission tomography study. Neuropsychologia 1995; 33:1027-1046Crossref, Medline, Google Scholar
35. Robinson AL, Heaton RK, Lehman RA, Stilson DW: The utility of the Wisconsin Card Sorting Test in detecting and localizing frontal lobe lesions. J Consult Clin Psychol 1980; 48:605-614Crossref, Medline, Google Scholar
36. Bechara A, Damasio H, Tranel D, Anderson SW: Dissociation of working memory from decision making within the human prefrontal cortex. J Neurosci 1998; 18:428-437Crossref, Medline, Google Scholar
37. Chelune GJ, Baer RA: Developmental norms for the Wisconsin Card Sorting test. J Clin Exp Neuropsychol 1986; 8:219-228Crossref, Medline, Google Scholar
38. Chelune GJ, Ferguson W, Koon R, Dickey TO: Frontal lobe disinhibition in attention deficit disorder. Child Psychiatry Hum Dev 1986; 16:221-234Crossref, Medline, Google Scholar
39. Seidman LJ, Biederman J, Faraone SV, Weber W, Ouellette C: Toward defining a neuropsychology of attention deficit-hyperactivity disorder: performance of children and adolescents from a large clinically referred sample. J Consult Clin Psychol 1997; 65:150-160Crossref, Medline, Google Scholar
40. Shue KL, Douglas VI: Attention deficit hyperactivity disorder and the frontal lobe syndrome. Brain Cogn 1992; 20:104-124Crossref, Medline, Google Scholar
41. Loge DV, Staton RD, Beatty WW: Performance of children with ADHD on tests sensitive to frontal lobe dysfunction. J Am Acad Child Adolesc Psychiatry 1990; 29:540-545Crossref, Medline, Google Scholar
42. Robins LN, Helzer JE, Croughan J, Ratcliff KS: The National Institute of Mental Health Diagnostic Interview Schedule: its history, characteristics, and validity. Arch Gen Psychiatry 1981; 38:381-389Crossref, Medline, Google Scholar
43. Reich W: Diagnostic Interview for Children and Adolescents (DICA). J Am Acad Child Adolesc Psychiatry 2000; 39:59-66Crossref, Medline, Google Scholar
44. Zachary RA, Paulson MJ, Gorsuch RL: Estimating WAIS IQ from the Shipley Institute of Living Scale using continuously adjusted age norms. J Clin Psychol 1985; 41:820-831Crossref, Medline, Google Scholar
45. Wechsler D: Wechsler Intelligence Scale for Children, 3rd ed. San Antonio, Tex, Psychological Corp (Harcourt), 1991Google Scholar
46. Hollingshead AB: Four-Factor Index of Social Status. New Haven, Conn, Yale University, Department of Sociology, 1975Google Scholar
47. Greenhill LL, Findling RL, Swanson JM: A double-blind, placebo-controlled study of modified-release methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 2002; 109:E39Google Scholar
48. Solanto MV, Conners CK: A dose-response and time-action analysis of autonomic and behavioral effects of methylphenidate in attention deficit disorder with hyperactivity. Psychophysiology 1982; 19:658-667Crossref, Medline, Google Scholar
49. Greenhill LL, Halperin JM, Abikoff H: Stimulant medications. J Am Acad Child Adolesc Psychiatry 1999; 38:503-512Crossref, Medline, Google Scholar
50. Ernst M, Earle A, Zametkin AJ: Brain imaging studies comparing the action of methylphenidate and cocaine in human brain, in Ritalin: Theory and Practice. Edited by Greenhill LL, Osman B. New York, Mary Ann Liebert, 1999Google Scholar
51. Pelham WE, Schnedler RW, Bologna NC, Contreras JA: Behavioral and stimulant treatment of hyperactive children: a therapy study with methylphenidate probes in a within-subject design. J Appl Behav Anal 1980; 13:221-236Crossref, Medline, Google Scholar
52. Swanson JM, Volkow ND: Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behav Brain Res 2002; 130:73-78Crossref, Medline, Google Scholar
53. Bechara A: Neurobiology of decision-making: risk and reward. Semin Clin Neuropsychiatry 2001; 6:205-216Crossref, Medline, Google Scholar
54. Bolla KI, Cadet JL, London ED: The neuropsychiatry of chronic cocaine abuse. J Neuropsychiatry Clin Neurosci 1998; 10:280-289Crossref, Medline, Google Scholar
55. Weinrieb RM, O’Brien CP: Persistent cognitive deficits attributed to substance abuse. Neurol Clin 1993; 11:663-691Crossref, Medline, Google Scholar



