Alcohol and ADH2 in Israel: Ashkenazis, Sephardics, and Recent Russian Immigrants
Abstract
OBJECTIVE: Jews drink less than other Caucasians and have a higher prevalence of ADH2*2, an allele of an alcohol dehydrogenase gene that protects against heavy drinking. The relationship of ADH2 polymorphisms to lifetime maximum number of drinks per occasion was investigated in recent Russian immigrants to Israel (exposed to heavier drinking in their country of origin), other Israeli Ashkenazis, and Sephardics. METHOD: Seventy-five randomly sampled Israelis participated in a structured interview. ADH2 was genotyped for 68 subjects. RESULTS: ADH2*2 predicted less drinking; however, associations between ADH2 and drinking appeared to differ across the groups, consistent with differences in environmental exposure to heavy drinking. CONCLUSIONS: The findings support a protective effect for ADH2*2 against heavy drinking in Jewish samples but also suggest the importance of environment. Future work should investigate interactions between genes and the environment in larger samples.
Jewish individuals have low rates of alcoholism (1), but little is known about influences on their drinking behavior, which may ultimately prove informative about alcoholism in general. Alcohol dehydrogenase is the principle enzyme for ethanol oxidation (2). A functional polymorphism of the alcohol dehydrogenase genes, ADH2*2, has been shown to protect against alcoholism (2). ADH2*2 occurs in approximately 30% of Jewish individuals (3, 4), suggesting an explanatory role for ADH2 in drinking by Jews. However, the relationship of ADH2*2 to drinking in Jews varies (3, 4), possibly because of environmental influences or methodological variation. Studying contrasting Jewish groups with consistent methods should provide clarification.
Russia’s per capita alcohol consumption level is very high, while Israel’s is very low. Until recently, the two main subgroups of Israeli Jews were Ashkenazis (European/Russian background) and Sephardics (Middle Eastern/North African background), who differ in drinking patterns (5). Since 1989, approximately 720,000 new immigrants from the former Soviet Union have arrived in Israel, now forming a third group. Recent Russian immigrants drink more than other Israelis (6). Therefore, ADH2*2 and drinking were studied in these three contrasting Israeli groups.
Method
Three neighborhoods with predominantly Ashkenazi, Sephardic, and recent Russian immigrant residents, respectively, were identified within a city. Buildings were randomly selected within each neighborhood, and households were randomly selected within those buildings. Introduction letters were mailed to 103 households, followed by a call in which one household member aged 22–65 was randomly selected (men were oversampled) and invited to participate in an interview. Nurses or physicians conducted private in-person interviews with 75 subjects (response rate, 73%) and obtained DNA for genotyping from 68 (91% of interviewees). Enzymatic amplification of genomic DNA followed by hybridization with allele-specific oligonucleotides determined ADH2 genotype (7). After complete description of the study to the subjects, written informed consent was obtained.
Sections from a structured interview (8) were translated into Hebrew and Russian (available from D.H.). In this report, we present an analysis of the lifetime maximum number of drinks per occasion, a measure suitable for alcoholics and nonalcoholics (9). In an interrater reliability study of 23 Israeli subjects, the interrater correlation coefficient for maximum drinks per occasion was excellent (0.96). While biochemical drinking validators were not used, these might prove useful in future studies.
Because of some mixing within the three neighborhoods, final determination of Ashkenazi, Sephardic, or recent Russian immigrant status was based on self-report (5). Ashkenazi origins (N=23) included Europe, the Americas, Australia, South Africa, and the former Soviet Union for subjects who had arrived before 1989; Sephardic origins (N=25) included North Africa and the Middle East. Recent Russians immigrants (N=27) were those who had emigrated from the former Soviet Union since 1989. Approximately 70% of the subjects were male, and most were married and working. The Ashkenazis were older (mean=48.7 years, SD=9.8) than the new Russian immigrants (mean=42.3 years, SD=11.0) or Sephardics (mean=36.3 years, SD=10.8).
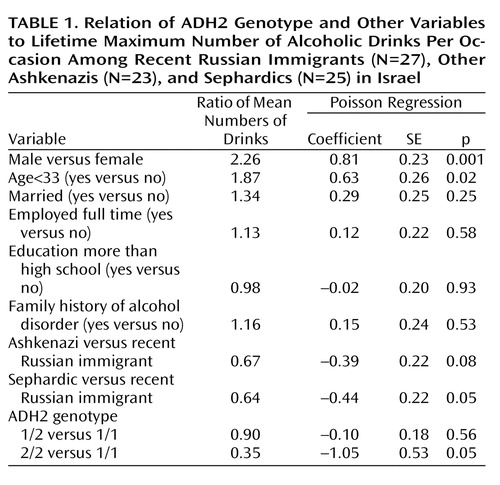
The association between population group and ADH2*2 allele frequency was determined by chi-square test. The maximum number of drinks per occasion, a count variable, had a skewed distribution and heterogeneous variances across groups, so group differences were determined with the Kruskal-Wallis test. Poisson regression (10), appropriate for count outcomes with skewed distributions that violate variance assumptions for linear regression, indicated the relationship of ADH2 to maximum drinks per occasion while controlling for group, gender, and other variables. For each predictor, a mean ratio estimate was derived, the interpretation of which is similar to that for an odds ratio. Within-group associations between genotype and maximum drinks per occasion were explored with Spearman’s rank correlation coefficient. The genotypes ADH2*1/1, ADH2*1/2, and ADH2*2/2 indicated the ascending ranked order of the ADH2 effect. The presented p levels reflect two-tailed tests.
Results
The mean maximum number of drinks per occasion differed significantly among the recent Russian immigrants (mean=5.3, SD=3.3), other Ashkenazis (mean=2.7, SD=2.5), and Sephardics (mean=3.0, SD=0.4) (Kruskal-Wallis χ2=13.92, df=2, p<0.001). The prevalences of ADH2*2 were 17%, 20%, and 41% in the Russians, other Ashkenazis, and Sephardics, respectively (χ2=22.14, df=2, p<0.001). The distribution of genotypes was consistent with Hardy-Weinberg equilibrium in all three groups. The mean maximum number of drinks per occasion differed significantly among subjects with the ADH2*1/1 genotype (mean=3.89, SD=3.24), the ADH2*2/1 genotype (mean=3.29, SD=2.49), and the ADH2*2/2 genotype (mean=1.00, SD=0.63) (Kruskal-Wallis χ2=7.69, df=2, p=0.02). Controlling for gender, group, and other characteristics (Table 1), we found that ADH2*2/2 was negatively related to maximum drinks per occasion, indicating a protective effect.
While the sample size was limited for within-group tests of ADH2 and maximum number of drinks, we did a preliminary exploration. Within the Sephardics, the mean maximum number of drinks per occasion differed in the predicted direction among those with the ADH2*1/1 genotype (mean=4.3, SD=4.5), the ADH2*1/2 genotype (mean=2.1, SD=2.2), and the ADH2*2/2 genotype (mean=1.0, SD=0.7) (rs=0.52, N=23, p=0.01). Within the Ashkenazis, the maximum number of drinks also differed in the predicted direction for the ADH2*1/1 genotype (mean=3.0, SD=2.7), the ADH2*1/2 genotype (mean=2.9, SD=2.3), and the ADH2*2/2 genotype (mean=1.0, SD=0), although the difference was not significant (rs=0.07, N=22, p=0.77). In contrast, for the recent Russian immigrants, the mean maximum numbers of drinks were 4.5 (SD=2.9) for ADH2*1/1 and 5.0 (SD=2.3) for ADH2*1/2 (rs=–0.13, N=23, p=0.57). None of the Russians had the ADH2*2/2 genotype.
Discussion
We examined genotype and drinking in contrasting Jewish Israeli groups. Greater drinking among the Russian immigrants supported the validity of the drinking measure. The prevalence of the ADH2*2 allele was high, especially among Sephardics, and was found to protect against heavy drinking when we controlled for confounders.
The prevalences of a phenotypic trait or disease and an allele can both be elevated in a population subgroup for unrelated reasons. When this is undetected in a sample, population stratification can confound research results (11). In our study, confounding due to population stratification was unlikely because we controlled for three main groups. Further, since a higher number of strata lowers the likelihood of confounding (12), the fact that our subjects reported 18 different countries of origin minimized the likelihood of confounding due to population stratification.
This study indicates a protective role for ADH2*2 against heavy drinking in Jewish individuals, which may partially explain their low levels of alcoholism. However, in the recent Russian immigrants, exposed to an environment of heavy drinking before immigration, the effect of ADH2*2 appeared different. Future studies with larger samples should specifically investigate the interaction of ADH2*2 and environmental influences on alcoholism. Such research may enhance the understanding of both genetic and environmental causes of disease.
 |
Received June 4, 2001; revision received Jan. 22, 2002; accepted Feb. 28, 2002. From the Division of Epidemiology and Division of Biostatistics, Mailman School of Public Health, Columbia University, New York; the Department of Psychiatry, College of Physicians and Surgeons, Columbia University, New York; the New York State Psychiatric Institute; the Ness-Ziona Psychiatric Hospital, Ness-Ziona, Israel; and the Department of Medicine, Indiana University School of Medicine, Indianapolis. Address reprint requests to Dr. Hasin, Columbia University/New York State Psychiatric Institute, Box 123, 1051 Riverside Dr., New York, NY 10032; [email protected] (e-mail). Supported by grants AA-00161 (D.H.) and AA-07611 (L.C. and T.-K.L.) from the National Institute on Alcohol Abuse and Alcoholism.
1. Levav I, Kohn R, Golding JM, Weissman MM: Vulnerability of Jews to affective disorders. Am J Psychiatry 1997; 154:941-947Link, Google Scholar
2. Li T-K: Pharmacogenetics of responses to alcohol and genes that influence alcohol drinking. J Stud Alcohol 2000; 61:5-12Crossref, Medline, Google Scholar
3. Neumark YD, Friedlander Y, Thomasson HR, Li T-K: Association of the ADH2*2 allele with reduced ethanol consumption in Jewish men in Israel: a pilot study. J Stud Alcohol 1998; 59:133-139Crossref, Medline, Google Scholar
4. Shea SH, Wall TL, Carr LG, Li T-K: ADH2 and alcohol-related phenotypes in Ashkenazic Jewish American college students. Behav Genet 2001; 31:231-239Crossref, Medline, Google Scholar
5. Aharonovich E, Hasin D, Rahav G, Meydan J, Neumark Y: Differences in drinking patterns among Ashkenazi and Sephardic Israeli adults. J Stud Alcohol 2001; 62:301-305Crossref, Medline, Google Scholar
6. Rahav G, Hasin D, Paykin A: Drinking patterns of recent Russian immigrants to Israel and other Israelis: national survey results. Am J Public Health 1999; 89:1212-1215Crossref, Medline, Google Scholar
7. Xu Y, Carr LG, Bosron WF, Li T-K, Edenberg HJ: Genotyping of human alcohol dehydrogenase at the ADH2 and ADH3 loci following DNA sequence amplification. Genomics 1988; 2:209-214Crossref, Medline, Google Scholar
8. Grant BF, Harford TC, Dawson D, Chou P, Pickering R: The Alcohol Use Disorder and Associated Disabilities Schedule (AUDADIS): reliability of alcohol and drug modules in a general population sample. Drug Alcohol Depend 1995; 39:37-44Crossref, Medline, Google Scholar
9. Saccone NL, Kwon JM, Corbett J, Goate A, Rochberg N, Edenberg HJ, Foroud T, Li T-K, Begleiter H, Reich T, Rice JP: A genome screen of maximum number of drinks as an alcoholism phenotype. Am J Med Genet 2000; 96:632-637Crossref, Medline, Google Scholar
10. McCullagh P, Nelder JA: Generalized Linear Models, 2nd ed. London, Chapman & Hall, 1989Google Scholar
11. Lander E, Schork N: Genetic dissection of complex traits. Science 1996; 265:2037-2048Crossref, Google Scholar
12. Wacholder S, Rothman N, Caporaso N: Population stratification in epidemiologic studies of common genetic variants and cancer: quantification of bias. J Natl Cancer Inst 2000; 92:1151-1158Crossref, Medline, Google Scholar



