Regional Patterns of Brain Activity in Adults With a History of Childhood-Onset Depression: Gender Differences and Clinical Variability
Abstract
OBJECTIVE: The study investigated the hypothesis that EEG asymmetry scores (indicating higher right and lower left frontal brain activity) are associated with vulnerability to negative mood states and depressive disorders. Gender and clinical history variables were examined as factors that may influence the relation between EEG and depression. METHOD: EEG measures of asymmetrical alpha frequency (7.5–12.5 Hz) suppression were analyzed in 55 young adults with a documented clinical history of childhood-onset depression and 55 comparison subjects with no history of major psychopathology. EEG patterns were examined in relation to operational diagnoses of mental disorders during childhood and adulthood. RESULTS: Differences in EEG asymmetry between childhood depression probands and comparison subjects varied with gender, diagnostic history, and current symptoms. Women with childhood depression had higher right midfrontal alpha suppression, and men with childhood depression had higher left midfrontal alpha suppression, relative to comparison subjects. At all scalp sites, women showed greater alpha power than men. Probands with a bipolar spectrum course had the most extreme midfrontal asymmetry. Frontal asymmetry was more extreme in probands with current depressive symptoms than in those without current symptoms. CONCLUSIONS: Regional brain activity is influenced by gender and variability in clinical course. The findings have implications for investigating brain correlates of mood disorder and may help to develop more refined phenotypes.
Several studies of quantitative electroencephalography have sought to relate emotion, temperament, and depression to individual differences in resting frontal alpha asymmetry (1). On the basis of the evidence, it has been proposed that asymmetry scores reflecting higher right and lower left frontal brain activity may signify vulnerability to negative mood and depressive disorders (2–4). Our ongoing program of research on risk for depression tested this hypothesis in a clinically well-defined group of young adults with a history of mood disorder beginning during childhood (5).
Characteristic patterns of lateralized brain activity have been associated with emotional tendencies across development, including separation distress during infancy (6), social competence in toddlers (7), self-reported emotional experience in adults (8), and responses to laboratory challenges in children (9) and adults (10). The primary framework for explaining such observations is the approach-withdrawal model of frontal brain asymmetry (1–3). According to this model, the left frontal cortex is part of a neural system that promotes approach-directed emotional responses, and the right frontal cortex is part of a system that facilitates withdrawal-directed emotional responses. Lateralized frontal brain activity may reflect the balance of activity in neurobehavioral systems for emotion regulation. When applied to depression, the model suggests that extreme patterns of relevant brain activity may maintain protracted negative mood in affective disorders (2).
The primary dependent measure in EEG asymmetry studies has been suppression of power in the alpha band. Alpha is the dominant frequency in the human EEG waveform, and it is suppressed at occipital and central sites during visual or motor stimulation, respectively (presumably from desynchronized electrical fields generated by pyramidal neurons) (11). Relative measures of alpha asymmetry minimize between-participant differences in skull morphology that influence absolute power (12) and are typically computed as logarithmic differences scores (e.g., ln F4 alpha – ln F3 alpha).
Studies of EEG alpha asymmetry and depression have examined juvenile and adult populations. For example, high-risk offspring of depressed mothers displayed left frontal hypoactivation and atypical power, relative to low-risk youngsters (13, 14). Decreased relative left midfrontal alpha suppression also has been observed in adults with current or remitted depression (15, 16). In posterior areas implicated in emotional perception, some patients displayed the reverse pattern of lower right and higher left parietal activity, relative to normal comparison subjects (15, 17). However, inconsistent findings for EEG asymmetry and depression have focused attention on possible mediating or moderating factors (18, 19). Major factors may include variability in clinical diagnoses (19), stability of asymmetry measures (12, 20), and differences between men and women (21).
The issue of diagnostic variability is particularly important in studies of depressive disorders, given the heterogeneity of clinical phenotypes and the high rates of comorbid psychiatric disorders. For example, Heller and Nitschke (22), focusing on comorbidity between depression and anxiety, argued that anxious arousal could offset effects of depression on alpha asymmetries, especially at posterior sites. Consistent with this perspective, Bruder and his colleagues (17) reported that depressed patients without anxiety disorders (N=25) showed posterior asymmetry consistent with lower activity over the right and higher activity over the left parietal sites, while patients with comorbid anxious depression (N=19) had higher activation over right frontal and parietal sites, relative to normal comparison subjects (N=26). Likewise, adolescent girls (N=8) with “pure” major depression reportedly displayed more extreme posterior alpha asymmetries than girls with comorbid depression and anxiety (N=11) and girls with anxiety disorders only (N=6) (23). In addition, relative right frontal asymmetry has been observed in girls but not in boys with oppositional defiant disorder (N=119) (24), but previous EEG studies have not examined behavior disorders comorbid with depression.
In addition to comorbid disorders, clinical phenotypes of depression include unipolar or bipolar course (25). Although one small EEG study examined women with bipolar seasonal affective disorder (26), no studies have compared subjects with unipolar and bipolar depression. Because major depression beginning during childhood is associated with conversion to bipolar disorder in up to a third of cases (27), such populations may be valuable for such comparisons.
In the present article, we report on the baseline patterning of EEG alpha asymmetry in young adults with a history of childhood-onset depression or no history of major psychopathology. The clinical probands had a documented history of childhood depression and well-characterized outcomes into adulthood. Most had recurrent depression and a positive family history for mood disorder (28). We tested the primary hypothesis that childhood depression probands have higher levels of right frontal brain activity and lower levels of left frontal brain activity, relative to comparison subjects without a history of major psychopathology. We also examined whether gender differences, diagnostic history, and current symptoms influence patterns of frontal brain asymmetry.
Method
Participants
Data were derived from 55 young adults with a history of childhood-onset depression and 55 adults with no psychiatric history who had been participants in a larger research program on risk for juvenile-onset mood disorders. Childhood depression probands were recruited through prior research studies or community media advertisements. Depression probands had to have clinical or research records supporting the presence of DSM-III and DSM-IV diagnostic criteria for major depressive disorder or dysthymic disorder by age 14.99 years. The comparison group was recruited through a Coles Directory search, a neighborhood program in a low-socioeconomic-status area, and previous research studies. Potential comparison subjects were excluded if they met criteria for and were impaired by psychopathology any time in their life or if they reported elevated depressive symptoms at the psychophysiology assessment. Written informed consent was obtained from all participants.
Groups were comparable on demographic characteristics. The childhood depression group included 27 men and 28 women, and their mean age was 26.0 years (SD=3.2). In this group, 65.5% of the subjects were Caucasian (N=36), 25.5% were African American (N=14), and 9.1% had other ethnic backgrounds (N=5). Approximately 85% (N=47) were right-handed, according to the Edinburgh Handedness Inventory (29). Nine (16.4%) were taking psychotropic medication at the time of the psychophysiology assessment, and 37 (67.3%) were treated with psychotropic medication during their lifetime. The comparison group consisted of 17 men and 38 women, and their mean age was 27.2 years (SD=5.8). In this group, 81.8% of the subjects were Caucasian (N=45), 16.4% were African American (N=9), and 1.8% had another ethnic background (N=1); 89.1% (N=49) were right-handed.
Procedures
Psychiatric diagnoses and symptom ratings
Information about lifetime psychiatric disorders was obtained through one of several means. For patients who had participated in a longitudinal naturalistic follow-up study since they were children (5), diagnoses had been derived through repeated assessments with the semistructured Interview Schedule for Children and Adolescents and Follow-Up Interview Schedule for Adults (30) in which the proband and the parent (or other adults) as the second informant had been interviewed. These diagnoses were supported by medical records, as needed. Other probands who had participated in research studies for a limited number of years while children, as well as the remaining subjects, were assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID) (31) adapted to include selected childhood and axis II diagnoses. For the SCID, a second informant also was required, as well as supporting clinical or related psychosocial records. Experienced research clinicians assessed each participant and each participant’s second informant. On the basis of all available SCID data and medical/research records, two independent senior psychiatrists, who were blind to group status, provided final diagnoses using DSM-IV and DSM-III criteria and Research Diagnostic Criteria. Disagreements were resolved by consensus.
All clinical probands had major depression or dysthymia during childhood. For the current analyses, other lifetime diagnoses of interest were designated as dichotomous variables (1=present, 0=absent), including 1) bipolar spectrum disorders (i.e., bipolar I or bipolar II disorder; 11 men and 10 women), 2) early-onset behavior disorders (i.e., conduct, oppositional, and attention deficit disorders by age 14.99 years; 13 men and nine women); and 3) early-onset anxiety disorders (i.e., separation anxiety, overanxious, atypical anxiety, phobia, obsessive-compulsive, panic, generalized anxiety, and posttraumatic stress disorders by age 14.99 years; 16 men and 15 women). Among the 21 probands who had developed some form of bipolar disorder, 14 (seven men and seven women) also had behavior or anxiety disorders, and most had both. Among the 34 probands with unipolar depression, 10 (four men and six women) had a behavior disorder, 19 (nine men and 10 women) had anxiety without behavior disorders, and five had neither a behavior nor an anxiety disorder. Notably, no probands with unipolar depression had both behavior and anxiety diagnoses during childhood.
At the time of the psychophysiology assessment, symptomatic status was assessed by self-rating and clinical evaluation. Participants self-rated their symptoms using the Beck Depression Inventory (32) and the Beck Anxiety Inventory (33), 21-item scales that quantify, respectively, depressive and anxious symptoms and attitudes. Trained raters used a structured interview format to complete the Follow-Up Depression and Anxiety Scales, composed of selected items from the Follow-Up Interview Schedule for Adults (30), which provided a broader clinical picture of a respondent’s symptomatic status.
Physiologic data acquisition
EEG acquisition followed standard guidelines (34). An electrode cap (ElectroCap, Eaton, Ohio) was positioned according to the expanded 10–20 International System (35). Electrodes were placed at sites F3, F4, AF3, AF4, F7, F8, FC1, FC2, FC5, FC6, C3, C4, T7, T8, P3, P4, P7, P8, O1, and O2. Recordings were made with a vertex (Cz) reference. The isolated-common ground was AFz. The electro-oculogram (EOG) was recorded with a bipolar reference. Six-mm tin electrodes were placed above and below the right eye to record blinks and vertical eye movements and on the outer canthi to record horizontal eye movements. Scalp electrode impedances were below 10 kΩ throughout the session (with left-right pairs generally within 0.5 kΩ), resulting in a very low level of electrical noise in the EEG signals.
EEG data were collected during six 1-minute resting baseline periods with the eyes either open (O) or closed (C). Two counterbalanced orders were used (OCCOCO/COOCOC). Data were collected with equipment and software from the James Long Company (Caroga Lake, N.Y.). The bioamplifier was set for band-pass filtering with half power cutoff frequencies of 0.01 and 100 Hz (12 dB/octave roll-off). The gain was 5000 for the EEG channels and 2500 for the EOG channels. Data were digitized continuously at 512 Hz.
Physiology data reduction
Automated regression-based algorithms minimized blink artifacts in the EEG (10), and manual scoring excluded periods confounded by movements, muscle tension, and large saccades. The data were transformed from a Cz- to average-reference montage, and then Fourier analyses were applied to each 1-minute baseline epoch. Hanning windows identified 1-second periods of artifact-free data in each epoch (50% overlap). Spectral power from 1-Hz bins was clustered into broad bands. The band of primary interest was alpha (7.5–12.5 Hz), given that previous asymmetry and emotion results pertained specifically to this band (1). Alpha power (in pico-watt ohms or microvolts squared) was averaged across baselines and weighted by the number of artifact-free windows in each epoch. Power values were then transformed to natural logarithms to normalize the distribution for statistical analyses. Asymmetry metrics were computed as the natural logarithm of alpha power at the right recording site minus the left recording site (e.g., ln F4 – ln F3). The logarithmic difference scores are equivalent to ratiometric scores of the raw data and control for individual differences in skull morphology (12). Given that alpha power varies inversely with cortical activation, higher asymmetry values represent greater left relative to right brain activity.
Statistical Analyses
Primary analyses used repeated measures multivariate analysis of variance and the Pillai’s trace statistic. For a priori hypotheses, the alpha level was 0.05, with Bonferroni corrections for post hoc comparisons. We report values of p (i.e., probability of making a type I error) and η2 (i.e., proportion of accounted variance). On the basis of previous research, a priori hypotheses focused on alpha suppression at midfrontal sites (F3, F4), and follow-up analyses were conducted with all sites. For example, the initial analysis included group (childhood depression, comparison) and gender (male, female) as between-subjects factors and hemisphere (left, right) as a within-subjects repeated measure. The dependent variable was midfrontal alpha power (microvolts squared), transformed to a natural logarithmic scale (ln). Interactions for hemisphere as a repeated measure in analyses of site-specific power are equivalent to main effects for analyses of asymmetry metrics (e.g., ln F4 – ln F3).
Results
Gender Differences
In initial analyses for midfrontal sites F3 and F4, we found main effects for gender (F=12.70, df=1, 106, p<0.001, η2=0.11) and hemisphere (F=4.90, df=1, 106, p<0.03, η2=0.04). In addition, we observed a group-by-gender-by-hemisphere interaction (F=5.47, df=1, 106, p<0.02, η2=0.05). Figure 1 illustrates the mean values for midfrontal asymmetry for men and women in each group. Higher scores reflect greater relative left midfrontal alpha suppression. The figure shows that women with childhood depression had greater relative right midfrontal alpha suppression, whereas men with childhood depression had greater relative left midfrontal alpha suppression. The magnitude of asymmetry was greater for men than for women. Contrast analyses of data for subjects with childhood depression revealed that midfrontal asymmetry differed between men and women (t=2.00, df=53, p<0.05, η2=0.07). Gender differences depended on both left midfrontal (F3) alpha power (t=–2.50, df=108, p<0.01, η2=0.06) and right midfrontal (F4) alpha power (t=–2.64, df=108, p<0.009, η2=0.06). Men had lower power than women at all sites.
Clinical History
Participants were hierarchically sorted on the basis of whether they had a history of 1) lifetime bipolar spectrum disorder (N=21), 2) unipolar depression with early-onset behavior disorders (N=10), or 3) unipolar depression with early-onset anxiety disorders (N=19). The five participants who did not meet criteria for these categories were not included in these analyses.
Specifically at midfrontal sites, we observed a diagnostic group-by-gender-by-hemisphere interaction (F=3.38, df=3, 97, p<0.02, η2=0.10) (Figure 2). Among probands with bipolar spectrum disorder, men and women had particularly extreme relative left and relative right midfrontal asymmetry, respectively. Contrast effects showed that midfrontal asymmetry for the bipolar disorder group differed from that for other groups (t=–2.76, df=103, p<0.007, η2=0.07). In addition, there was an interaction with gender (t=3.17, df=103, p<0.002, η2=0.09). When men and women were considered separately, differences between the bipolar disorder and comparison groups remained for both men (t=2.13, df=26, p<0.04, η2=0.15) and women (t=–2.95, df=46, p<0.005, η2=0.16).
Current Depressive Symptoms
The childhood depression proband group, relative to the comparison group, had a higher mean current Beck Depression Inventory score (childhood depression group: mean=9.1, SD=7.6; comparison group: mean=2.5, SD=2.3) (F=35.05, df=1, 106, p<0.0001, η2=0.25). When the data were viewed categorically, 19 of the 55 childhood depression probands had a high level of depressive symptoms (i.e., Beck Depression Inventory score ≥10). This group included both probands with a history of unipolar depression and those with a history of bipolar disorder and probands who were and were not taking psychotropic medication (nonsignificant chi-square for group membership). Probands who were taking psychotropic medication (N=9) and those who were not (N=46) did not differ in self-reported level of depressive symptoms (F=0.47, df=1, 53, p=0.49, η2=0.009) or in midfrontal EEG asymmetry (F=0.33, df=1, 53, p=0.56, η2=0.006).
Men and women with childhood depression did not differ significantly in self-reported severity of depression, as measured with the Beck Depression Inventory (men: mean=8.6, SD=6.6; women: mean=9.5, SD=8.5) (F=0.18, df=1, 53, p=0.67, η2=0.003) or in the proportion of symptomatic subjects (eight men and 11 women) (F=0.55, df=1, 53, p=0.46, η2=0.01). Among the comparison subjects, there also were no gender differences in severity of depressive symptoms (F=0.58, df=1, 53, p=0.45, η2=0.01). Yet, despite comparable self-reported depressive symptoms in men and women, gender differences in midfrontal asymmetry were more extreme in the symptomatic than in the nonsymptomatic probands.
In particular, we found a depressive symptom group-by-gender-by-hemisphere interaction at midfrontal sites (F=5.46, df=2, 104, p<0.006, η2=0.10). As illustrated in Figure 3, symptomatic men with a history of childhood depression had greater relative left midfrontal asymmetry (t=1.99, df=42, p<0.05, η2=0.09), and symptomatic women with childhood depression had greater relative right midfrontal asymmetry (t=–2.81, df=64, p<0.007, η2=0.11). Within the childhood depression group, midfrontal asymmetry in symptomatic women tended to differ from that in nonsymptomatic women (t=2.17, df=26, p<0.04, η2=0.15), but symptomatic and nonsymptomatic men did not differ from each other in this feature (t=–0.91, df=25, p=0.4, η2=0.03).
We also examined the effects of current levels of anxiety as measured by the Beck Anxiety Inventory. The childhood depression proband group, relative to the comparison group, reported higher levels of anxiety (childhood depression group: mean=7.8, SD=7.3; comparison group: mean=3.3, SD=3.0) (F=16.85, df=1, 106, p<0.0001, η2=0.14). When viewed categorically, 21 of the clinical probands had elevated anxiety symptoms (i.e., Beck Anxiety Inventory score ≥8). No gender differences were present in the childhood depression group (t=–1.20, df=53, p=0.23, η2=0.03) or the comparison group (t=0.005, df=53, p=0.99, η2=0.0001).
Midfrontal and parietal asymmetries were comparable for participants with and without anxiety symptoms. However, currently anxious childhood depression probands, but not the probands without current anxiety symptoms, differed from comparison subjects in their midfrontal-central (FC1, FC2) asymmetry (F=4.96, df=2, 104, p<0.009, η2=0.09). Among the childhood depression probands with elevated anxiety, both men (N=8) and women (N=13) had relative right midfrontal-central asymmetry (ln FC2 – ln FC1=–0.0554 for men and –0.0814 for women). In addition, for occipital (O1, O2) asymmetry, we observed an interaction of current anxiety symptom group and gender (F=4.68, df=2, 104, p<0.01, η2=0.08). In contrast to the findings for frontal asymmetry, particularly extreme relative left occipital asymmetry was found in anxious women (ln O2 – ln O1=0.3057) and relative right occipital asymmetry was found in anxious men (ln O2 – ln O1=–0.0333).
Discussion
This study examined frontal brain asymmetry among young adults with a history of childhood-onset depression who were well characterized clinically and a comparison group with no history of major psychopathology. Our findings supported the hypothesized relation between depression and midfrontal (F3, F4) asymmetry scores reflecting higher right and lower left frontal brain activity, but only for women. Men with a history of childhood depression showed the opposite pattern of extreme relative left midfrontal asymmetry. Gender differences in midfrontal brain asymmetry were most extreme in childhood depression probands with an eventual history of a bipolar spectrum disorder and probands with elevated depressive symptoms at the time of the psychophysiology assessment. Among clinical probands with currently elevated anxiety symptoms, gender differences were present specifically at occipital sites (O1, O2). Anxious women showed relative left occipital asymmetry, and anxious men displayed relative right occipital asymmetry. Within the currently anxious group, both men and women displayed greater relative right activity at frontal-central sites (FC1, FC2). We did not find group differences in parietal EEG asymmetry.
The importance of gender differences in EEG asymmetry is one of the crucial findings of our study. It is particularly notable in relation to findings by Bruder and his colleagues (36) that extreme relative right alpha asymmetry predicts nonresponse to fluoxetine only in depressed women. The importance of gender differences in EEG studies of depression has not been fully appreciated because previous studies of this disorder typically have examined only female subjects (18, 23) or included small study groups with insufficient power to detect gender differences (15, 16). However, our findings as well as those of Bruder et al. (36) are consistent with prior reports on nonclinical subjects that demonstrated large differences in EEG asymmetry between women and men (21). Additional data are needed to clarify the psychological and physiological mechanisms whereby differences between women and men influence regional brain activity implicated in emotion and depression (see reference 10).
In the current study, we examined gender differences in relation to current symptoms and diagnostic history. Previous research has established that population base rates of depression are higher in women than in men (37) and that women and men differ in longitudinal patterns of comorbidity (38). Although women have higher rates of depression, men with early-onset depression have higher rates of comorbid behavior disorders and substance abuse; over time these disorders tend to become uncoupled in men but not in women (38). Such data raise the possibility that differences in clinical symptoms and history between men and women may account for gender differences in EEG asymmetry. However, in the current study group, men and women had relatively similar levels of current depressive symptoms and did not differ in early diagnostic history, and yet gender differences in midfrontal asymmetry were particularly extreme in participants with a history of bipolar spectrum disorders or current depressive symptoms.
The findings suggest that men and women with childhood depression may differ in their biological propensities for emotion regulation. Right frontal asymmetry in women with childhood depression may reflect regulatory tendencies characterized by withdrawal and anhedonia. Left frontal asymmetry in men with childhood depression may reflect regulatory dispositions primed more toward approach-related responses for distracting oneself or fighting off challenges. Such patterns may maintain protracted negative mood and dysfunctional coping. Possibly, extreme right or left frontal asymmetry may be maladaptive (rather than left frontal asymmetry being protective in all cases), such that individual differences in the relative imbalance of lateralized regulatory systems may predict depressive history or risk for subsequent episodes.
Our asymmetry finding for bipolar disorder appears consistent with the emotional lability and dysregulation that characterize this variant of mood disorder. Further work is needed to examine the polarity of depression in a larger group of subjects. For example, modeling variability between unipolar and bipolar depression would be facilitated by additional categorical data on diagnostic trajectories as well as continuous measures of affective temperament (39).
The observed relation between midfrontal asymmetry and current depressive symptoms warrants further assessment of the stability of asymmetry measures across time and in relation to changing emotional state. Asymmetry differences between symptomatic and nonsymptomatic women, but not men, could signify that asymmetry may be more state-dependent in women than men. Longitudinal data across sessions and during emotion challenge conditions may help to assess within-subject changes in affective responses and symptoms.
In summary, our results show that patterns of resting frontal EEG asymmetry in adults with a history of childhood-onset depression are influenced by substantial gender differences and natural clinical variability. Attending closely to such factors may increase the replicability of findings regarding CNS markers of depression and may provide clues about shared mechanisms of commonly comorbid psychiatric disorders.
Presented in part at the 40th annual meeting of the Society for Psychophysiological Research, San Diego, Oct. 18–22, 2000. Received July 19, 2001; revision received Dec. 4, 2001; accepted Jan. 2, 2002. From the Departments of Psychiatry and Psychology, University of Pittsburgh; and the Department of Human Development, University of Maryland, College Park. Address reprint requests to Dr. Miller, Department of Psychiatry, University of Pittsburgh, Western Psychiatric Institute and Clinic, TDH Suite E469, 3811 O’Hara St., Pittsburgh, PA 15213; [email protected] (e-mail). Supported by NIMH grants MH-56193, MH-18951, and MH-30915 and a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression/Educational Foundation of America. The authors thank the staff of program project MH-56193 for assistance with participant scheduling, data collection, and statistical consultation.
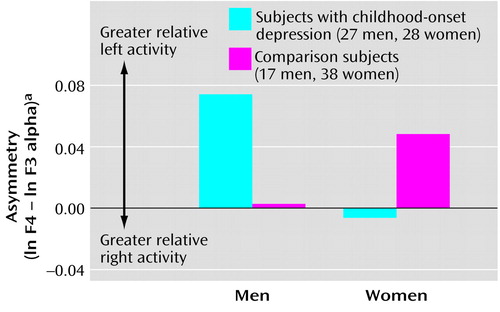
Figure 1. Asymmetry Between EEG Measures for Right and Left Midfrontal Regions in Subjects With Childhood-Onset Depression and in Healthy Comparison Subjects, by Gendera
aAlpha band (7.5–12.4 Hz) power at right and left midfrontal EEG recording sites (F4 and F3) was measured in microvolts squared and transformed to natural logarithms. Asymmetry was computed as the difference between the natural logarithms of alpha power at the right and left recording sites (i.e., ln F4 – ln F3). Values for ln F4 and ln F3 used to compute asymmetry were as follows: men with childhood-onset depression: 1.516 and 1.442, male comparison subjects: 1.301 and 1.298, women with childhood-onset depression: 2.090 and 2.096, female comparison subjects: 2.021 and 1.972. Given that alpha power varies inversely with cortical activation, higher asymmetry values represent greater relative left frontal brain activity.
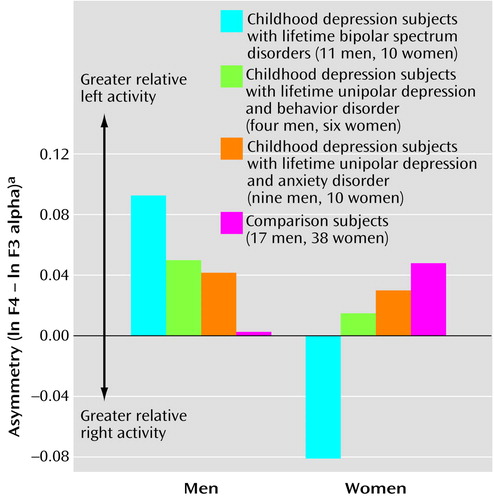
Figure 2. Asymmetry Between EEG Measures for Right and Left Midfrontal Regions in Subjects With Childhood-Onset Depression and Other Lifetime Diagnoses and in Healthy Comparison Subjects, by Gendera
aAlpha band (7.5–12.4 Hz) power at right and left midfrontal EEG recording sites (F4 and F3) was measured in microvolts squared and transformed to natural logarithms. Asymmetry was computed as the difference between the natural logarithms of alpha power at the right and left recording sites (i.e., ln F4 – ln F3). Values for ln F4 and ln F3 used to compute asymmetry were as follows: men with childhood-onset depression: 1.576 and 1.483 for those with lifetime bipolar spectrum disorders, 1.397 and 1.347 for those with lifetime unipolar depression and behavior disorder, and 1.287 and 1.245 for those with lifetime unipolar depression and anxiety disorder; male comparison subjects: 1.301 and 1.298; women with childhood-onset depression: 1.790 and 1.871 for those with lifetime bipolar spectrum disorders, 2.180 and 2.165 for those with lifetime unipolar depression and behavior disorder, and 2.213 and 2.182 for those with lifetime unipolar depression and anxiety disorder; female comparison subjects: 2.021 and 1.972. Given that alpha power varies inversely with cortical activation, higher asymmetry values represent greater relative left frontal brain activity.
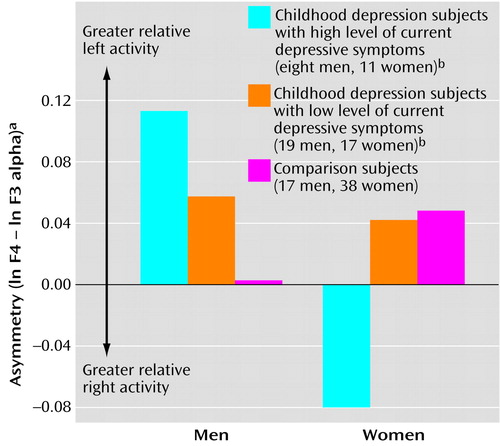
Figure 3. Asymmetry Between EEG Measures for Right and Left Midfrontal Regions in Subjects With Childhood-Onset Depression and High or Low Level of Current Depressive Symptoms and in Healthy Comparison Subjects, by Gendera
aAlpha band (7.5–12.4 Hz) power at right and left midfrontal EEG recording sites (F4 and F3) was measured in microvolts squared and transformed to natural logarithms. Asymmetry was computed as the difference between the natural logarithms of alpha power at the right and left recording sites (i.e., ln F4 – ln F3). Values for ln F4 and ln F3 used to compute asymmetry were as follows: men with childhood-onset depression: 1.694 and 1.580 for those with a high level of current depressive symptoms and 1.442 and 1.384 for those with a low level of current depressive symptoms; male comparison subjects: 1.301 and 1.298; women with childhood depression: 2.087 and 2.167 for those with a high level of current depressive symptoms and 2.093 and 2.051 for those with a low level of current depressive symptoms; female comparison subjects: 2.021 and 1.972. Given that alpha power varies inversely with cortical activation, higher asymmetry values represent greater relative left frontal brain activity.
bCurrent depressive symptoms were assessed with the Beck Depression Inventory (32), on which scores ≥10 indicated a high level and <10 a low level of symptoms.
1. Davidson RJ: Cerebral asymmetry, emotion, and affective style, in Brain Asymmetry. Edited by Davidson RJ, Hugdahl K. Cambridge, Mass, MIT Press, 1995, pp 361-387Google Scholar
2. Tomarken AJ, Keener-Miller AD: Frontal brain asymmetry and depression: a self-regulatory perspective. Cognition Emotion 1998; 12:387-420Crossref, Google Scholar
3. Marshall PJ, Fox NA: Emotion regulation, depression, and hemispheric asymmetry, in Stress, Coping, and Depression. Edited by Johnson SL, Hayes AM. Mahwah, NJ, Lawrence Erlbaum Associates, 2000, pp 35-50Google Scholar
4. Debener S, Beauducel A, Nessler D, Brocke B, Heilemann H, Kayser J: Is resting anterior EEG alpha asymmetry a trait marker for depression? findings for healthy adults and clinically depressed patients. Neuropsychobiology 2000; 41:31-37Crossref, Medline, Google Scholar
5. Kovacs M, Feinberg TL, Crouse-Novak MA, Paulauskas SL, Finkelstein R: Depressive disorders in childhood, I: a longitudinal prospective study of characteristics and recovery. Arch Gen Psychiatry 1984; 41:229-237Crossref, Medline, Google Scholar
6. Davidson RJ, Fox NA: Frontal brain asymmetry predicts infants’ response to maternal separation. J Abnorm Psychol 1989; 98:127-131Crossref, Medline, Google Scholar
7. Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, Porges SW, Long JM, Stewart S: Frontal activation asymmetry and social competence at four years of age. Child Dev 1995; 66:1770-1784Crossref, Medline, Google Scholar
8. Tomarken AJ, Davidson RJ, Wheeler RE, Doss RC: Individual differences in anterior asymmetry and fundamental dimensions of emotion. J Person Soc Psychol 1992; 62:676-687Crossref, Medline, Google Scholar
9. Davidson RJ, Fox NA: Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science 1982; 218:1235-1237Crossref, Medline, Google Scholar
10. Miller A, Tomarken AJ: Task-dependent changes in frontal brain asymmetry: effects of incentive cues, outcome expectancies, and motor responses. Psychophysiology 2001; 38:500-511Crossref, Medline, Google Scholar
11. Pfurtscheller G, Stancak A, Neuper C: Event-related synchronization (ERS) in the alpha band—an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol 1996; 24:39-46Crossref, Medline, Google Scholar
12. Tomarken AJ: Methodological issues in psychophysiological research, in Handbook of Research Methods in Clinical Psychology, 2nd ed. Edited by Kendall PC, Butcher JN, Holmbeck GN. New York, John Wiley & Sons, 1999, pp 251-275Google Scholar
13. Dawson G, Frey K, Panagiotides H, Osterling J: Infants of depressed mothers exhibit atypical frontal brain activity: a replication and extension of previous findings. J Child Psychol Psychiatry 1997; 38:179-186Crossref, Medline, Google Scholar
14. Field T, Fox NA, Pickens J, Nawrocki T: Relative right frontal EEG activation in 3-month-old to 6-month-old infants of depressed mothers. Dev Psychol 1995; 31:358-363Crossref, Google Scholar
15. Henriques JB, Davidson R: Regional brain electrical asymmetry discriminates between previously depressed and healthy control subjects. J Abnorm Psychol 1990; 99:22-31Crossref, Medline, Google Scholar
16. Henriques JB, Davidson RJ: Left frontal hypoactivation in depression. J Abnorm Psychol 1991; 100:535-545Crossref, Medline, Google Scholar
17. Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, McGrath PJ, Quitkin FM: Regional brain asymmetries in major depression with and without an anxiety disorder: a quantitative electroencephalographic study. Biol Psychiatry 1997; 41:939-948Crossref, Medline, Google Scholar
18. Reid SA, Duke LM, Allen JJ: Resting frontal electroencephalographic asymmetry in depression: inconsistencies suggest the need to identify mediating factors. Psychophysiology 1998; 35:389-404Crossref, Medline, Google Scholar
19. Davidson RJ: Anterior electrophysiological asymmetries, emotion, and depression: conceptual and methodological conundrums. Psychophysiology 1998; 35:607-614Crossref, Medline, Google Scholar
20. Tomarken AJ, Davidson RJ, Wheeler RE, Kinney L: Psychometric properties of resting anterior EEG asymmetry: temporal stability and internal consistency. Psychophysiology 1992; 29:576-592Crossref, Medline, Google Scholar
21. Davidson RJ, Schwartz GE, Pugash E, Bromfield E: Sex differences in patterns of EEG asymmetry. Biol Psychol 1976; 4:119-138Crossref, Medline, Google Scholar
22. Heller W, Nitschke J: The puzzle of regional brain activity in depression and anxiety: the importance of subtypes and comorbidity. Cognition Emotion 1998; 12:421-447Crossref, Google Scholar
23. Kentgen LM, Tenke CE, Pine DS, Fong R, Klein RG, Bruder GE: Electroencephalographic asymmetries in adolescents with major depression: influence of comorbidity with anxiety disorders. J Abnorm Psychol 2000; 109:797-802Crossref, Medline, Google Scholar
24. Baving L, Laucht M, Schmidt MH: Oppositional children differ from healthy children in frontal brain activation. J Abnorm Child Psychol 2000; 28:267-275Crossref, Medline, Google Scholar
25. Angst J, Sellaro R: Historical perspective and natural history of bipolar disorder. Biol Psychiatry 2000; 48:445-457Crossref, Medline, Google Scholar
26. Allen JJ, Iacono WG, Depue RA, Arbisi P: Regional electroencephalographic asymmetries in bipolar seasonal affective disorders before and after exposure to bright light. Biol Psychiatry 1993; 33:642-646Crossref, Medline, Google Scholar
27. Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL: Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry 2001; 158:125-127Link, Google Scholar
28. Kovacs M, Devlin B, Pollock M, Richards C, Mukerji M: A controlled family history study of childhood-onset depressive disorder. Arch Gen Psychiatry 1997; 54:613-623Crossref, Medline, Google Scholar
29. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97-113Crossref, Medline, Google Scholar
30. Sherrill JT, Kovacs M: Interview Schedule for Children and Adolescents (ISCA). J Am Acad Child Adolesc Psychiatry 2000; 39:67-75Crossref, Medline, Google Scholar
31. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
32. Beck AT, Steer RA, Garbin MG: Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988; 8:77-100Crossref, Google Scholar
33. Beck AT, Epstein N, Brown G, Steer RA: An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988; 56:893-897Crossref, Medline, Google Scholar
34. Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox N, Nuwer MR: Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology 1993; 30:547-558Crossref, Medline, Google Scholar
35. American Electroencephalographic Society: Guideline thirteen: guidelines for standard electrode position nomenclature. J Clin Neurophysiol 1994; 11:111-113Crossref, Medline, Google Scholar
36. Bruder GE, Stewart JW, Tenke CE, McGrath PJ, Leite P, Bhattacharya N, Quitkin FM: Electroencephalographic and perceptual asymmetry differences between responders and nonresponders to an SSRI antidepressant. Biol Psychiatry 2001; 49:416-425Crossref, Medline, Google Scholar
37. Kessler RC, McGonagle KA, Swartz M, Blazer NG, Nelson CB: Sex and depression in the National Comorbidity Survey, I: lifetime prevalence, chronicity and recurrence. J Affect Disord 1993; 29:85-96Crossref, Medline, Google Scholar
38. Kovacs M, Obrosky DS, Sherrill J: Developmental changes in the phenomenology of depression in girls and young women from childhood onward. J Affect Disord (in press)Google Scholar
39. Cassano GB, Akiskal HS, Musetti L, Perugi G, Soriani A, Mignani V: Psychopathology, temperament, and past course in primary major depressions. Psychopathology 1989; 22:278-288Crossref, Medline, Google Scholar



