Neuropsychological Correlates of Hippocampal Volumes in Patients Experiencing a First Episode of Schizophrenia
Abstract
OBJECTIVE: Despite evidence for hippocampal structural abnormalities in patients with schizophrenia, their functional correlates remain largely unknown. This study investigated the neuropsychological correlates of hippocampal volume in 43 men and 32 women experiencing a first episode of schizophrenia. METHOD: Posterior and anterior hippocampal volumes were computed from contiguous 3.1-mm magnetic resonance images and examined in relationship to six domains of neuropsychological functioning. Significant structure-function associations were investigated by examining the correlations between functioning and individual hippocampal slice volumes across the long axis of the hippocampus after interpolation to 10 equally spaced slice positions. RESULTS: Among men, worse executive and motor functioning correlated significantly with smaller anterior, but not posterior, hippocampal volume. The relationship between executive and motor functioning and hippocampal volume was not linear, however, when examined across the long axis of the hippocampus. Anterior hippocampal volume was more strongly correlated with both executive and motor functioning than with either memory or language functioning in men. None of the correlations between either posterior or anterior hippocampal volumes and the neuropsychological domains was significant among women. Anterior hippocampal volume was more strongly correlated with motor functioning in men than in women. CONCLUSIONS: Anterior hippocampal abnormalities associated with deficits on tests considered sensitive to frontal lobe functions implicate a defect in the integrated system linking frontal and mesiotemporal lobe regions. These findings further suggest that there are sex differences in structure-function relations in schizophrenia such that men may have more pronounced frontolimbic system abnormalities.
Neurodevelopmental models of schizophrenia posit an important role for aberrant hippocampal morphology in the pathophysiology of the disorder. There is considerable evidence from postmortem (1–6) and magnetic resonance imaging (MRI) (7–9) studies that patients with schizophrenia have hippocampal structural abnormalities. Moreover, a meta-analysis of 18 structural MRI studies (10) identified a 4% reduction in bilateral hippocampal volumes in patients with schizophrenia in relation to comparison subjects. Because hippocampal volume reductions have been identified in younger patients experiencing their first episode of schizophrenia (8), these abnormalities do not appear to be an artifact of long-term exposure to antipsychotic medications or to chronicity of the disorder.
Despite evidence for hippocampal pathology in patients with schizophrenia, few studies have examined their neuropsychological correlates using quantitative MRI. Several studies reported no significant associations of hippocampal volumes and neuropsychological functions (11–13). In contrast, Goldberg et al. (14) reported that in a group of monozygotic twins discordant for schizophrenia, intrapair differences in left anterior hippocampal volumes correlated significantly with intrapair differences in logical memory but not other functions. Bilder et al. (15) found that smaller anterior hippocampal volumes were associated with lower scores on measures of executive and motor functions considered sensitive to the integrity of the frontal lobes in patients experiencing a first episode of schizophrenia. This study was interpreted in the context of a neurodevelopmental defect within the medial frontolimbic system involving the anterior hippocampus, cingulate gyrus, and/or dorsal aspects of the premotor or prefrontal cortex. The medial frontolimbic system or dorsal archicortical trend (16) has been hypothesized to be important for redundancy-biased activation of cortical networks for executive functioning and forward planning; an abnormality in these brain structures and/or their connections may, at least in part, constitute the structural basis for frontal lobe dysfunction in schizophrenia (15, 17–20).
Several reviews (21, 22) have highlighted important neuroanatomical and functional differences between posterior and anterior hippocampal entorhinal-hippocampal projections (23). Specifically, the posterior hippocampus receives converging sensory input from posterior cortices, whereas the anterior hippocampus is connected primarily with the striatum and other parts of the limbic system. Empirical evidence for an anterior or posterior distinction of the hippocampus has been observed in functional MRI (24) and proton magnetic resonance spectroscopic imaging (25). In addition, in a meta-analysis of positron emission tomography (PET) studies of experimentally induced changes in blood flow (26), episodic memory encoding was found to be associated with anterior hippocampal activations, whereas episodic memory retrieval was associated with posterior hippocampal activations. A subsequent report (27), however, which included and excluded studies on the basis of criteria from the meta-analysis, reported that encoding activations in PET studies were observed frequently in both posterior and anterior mesiotemporal lobe regions. One possible reason for the apparent lack of a consistent relationship between hippocampal and neurocognitive measures in schizophrenia may be the lack of a distinction between the posterior and anterior hippocampus. Indeed, several studies that did distinguish between these regions implicated abnormalities in the anterior hippocampus compared to other parts of the hippocampus or other limbic structures (15, 28–31).
In this study we examined the neuropsychological correlates of posterior and anterior hippocampal volumes in large groups of men and women experiencing a first episode of schizophrenia. For significant correlations of hippocampal volume and neuropsychological functioning, we examined the pattern of correlations between that neuropsychological domain and individual slice volumes across the long axis of the hippocampus after cubic-spline interpolation to 10 evenly spaced slice positions. Correlations were examined separately by sex, given evidence that men with schizophrenia have been found to have more severe temporal (32, 33) and mesiotemporal (3, 8) lobe abnormalities than women with schizophrenia as well as reports noting sex differences between patient groups in patterns of structure-function relations (20, 34).
Method
Subjects
Patients with schizophrenia were participants in our larger Prospective Study of Psychobiology in First-Episode Psychosis and were recruited from patients consecutively admitted to the inpatient service at Hillside Hospital. Detailed information regarding the ascertainment, treatment, and clinical characteristics of the overall study group has been published elsewhere (35, 36). Inclusion criteria included admittance for a first episode of psychotic illness as defined as requiring hospital admission and having a current rating of 4 or more on at least one of the psychotic items of the Schedule for Affective Disorders and Schizophrenia (SADS), a diagnosis of definite or probable schizophrenia or of schizoaffective disorder, mainly schizophrenia, and fewer than 12 weeks cumulative (lifetime) antipsychotic treatment. Patients were excluded if there was any medical contraindication to the use of antipsychotic medications or if there was any history of serious neurologic or endocrine disorder. All procedures were approved by the institutional review board of Long Island Jewish Medical Center, and written informed consent was obtained from all patients.
The 75 patients (43 men, 32 women) described in this study were a subset of the 118 patients who participated in our larger study who had MRI scans available for measurement of the hippocampus and whole brain and who had completed at least one comprehensive neuropsychological examination after meeting operational criteria for clinical stabilization. Seventeen individuals in the present study were included in a prior study (15), but these patients were remeasured blindly as part of a larger series that included patients and healthy comparison subjects. The average number of weeks from study entry to the neuropsychological and MRI examinations is illustrated in Table 1. The men and women included in this study were not significantly different, respectively, from the male and female patients in the larger study who were not included in this study in terms of age, education, parental social class, baseline clinical global impression rating, age at onset of psychotic symptoms, and number of weeks of psychotic symptoms before study entry (all p>0.05). Diagnoses of patients in this study were based on the SADS interview and supplemental information from family members obtained by using the Research Diagnostic Criteria (37) and included schizophrenia (N=56; subtypes were paranoid [N=45], disorganized [N=4], and undifferentiated [N=7]) and schizoaffective disorder (N=19).
MRI Procedures
MRI scans were completed as soon as it was practical for patients after study entry; in most cases patients were receiving antipsychotic medication at the time of the MRI scan. The average numbers of weeks from the administration of antipsychotic medication to the MRI examination and from the MRI examination to the neuropsychological examination are reported in Table 1. MRI scans were acquired in the coronal plane by using a three-dimensional, gradient echo, fast low-angle-shots sequence, with a 50° flip angle, 40-msec TR, and 15-msec TE (38) on a 1.0-T whole-body superconducting imaging system (Siemens Magnetom, Erlangen, Germany). This sequence produced 63 contiguous coronal slices (slice thickness=3.1 mm) through the whole head in about 11 minutes, with a nominal in-plane resolution of 1.17 mm × 1.17 mm in a 256×256 matrix. Before the scan, head tilt was adjusted by using external landmarks. In addition, a two-dimensional fast low-angle-shots scan was used to position the head so that the floor of the fourth ventricle was parallel to the inferior-superior plane. The patients typically received 200–400 mg of sodium amytal orally before the procedure. Each of the scans was reviewed by a neuroradiologist and a member of the research team. Any scan with significant artifacts was repeated.
Measurement Criteria
All measurements were completed by using a semiautomated computerized mensuration system described previously (38). Before all measurements, scans of patients with schizophrenia were mixed together with those of healthy comparison subjects and were flipped randomly. No identifying information was available from the scan. Measurements were thus completed by an operator who was blind to group membership and hemisphere. Whole-brain measurements included both hemispheres, from the frontal to the occipital poles, the brainstem (i.e., mesencephalon, pons, and medulla), cerebellum, and ventricles but excluded the sulcal CSF. Interrater reliability (as assessed by intraclass correlations) between two operators regarding whole-brain measurements for 10 patients was 0.96. Measurement of the hippocampus complex was on the basis of operational criteria from postmortem histological work (2, 39). The anatomic regions used for measurement are illustrated elsewhere (8) and have been used in our previous MRI research (7, 8, 15). Two contiguous portions of the hippocampal complex in each hemisphere were analyzed: 1) the anterior hippocampus (including the mammillary body slice and the slice immediately posterior to this [postmammillary body slice]) and 2) the posterior hippocampus (from the slice posterior to the postmammillary slice to the slice at which the ascending fornix is interrupted by the surrounding pulvinar). Measurements of the hippocampal formation included all CA segments (CA1, CA2, CA3, and CA4), the dentate gyrus, the alveus, and the subicular region, which could not be separated in the scans. Intraclass correlations between two operators on scans for 15 patients ranged from 0.69 to 0.87 for the right and left posterior and anterior hippocampus and were 0.82 and 0.78 for the total posterior and total anterior hippocampus, respectively.
Hippocampus Slice Interpolation
The hippocampal slice volumes were resampled so that each individual’s measurements comprised 10 slices per hemisphere. This was implemented in MATLAB (40) by performing a cubic-spline interpolation of the measured hippocampal slice areas and resampling the spline curve at 10 equally spaced slice positions.
Neuropsychological Procedures
Because of the possibility that neuropsychological assessments could be confounded by factors typically associated with a first episode of schizophrenia, these tests were conducted after patient remission or when patients had a stable level of residual symptoms for the preceding 2 weeks, as measured by rating scale assessments (41). The patients were therefore receiving medication at the time of neuropsychological testing. The average number of weeks from the administration of antipsychotic medication to the neuropsychological examination is shown in Table 1. Administration of the neuropsychological tests was counterbalanced and took place only if the patients were clinically stable. The comprehensive test battery has been used extensively in our previous research (15, 20, 42) and includes 41 tests selected to characterize six domains of neuropsychological functioning: language, attention, memory, executive, motor, and visuospatial (Appendix 1).
Scaled scores for each domain for the patients in this study are from Bilder et al. (42) and were computed by averaging the z scores for each contributing test variable. A combined group of 36 healthy comparison subjects (24 men and 12 women) was used to generate z scores for the patients on the contributing neuropsychological variables. There were significant performance differences between the healthy men and women on the Finger Tapping test; thus z scores for the patients on these tests were computed by using a same-sex comparison group rather than the combined group. Functional scales were computed so that higher values indicated better performance. Additional information regarding the use of transformations to stabilize variances and the validity of a priori assignments of variables to scales by confirmatory factor analysis is provided elsewhere (42).
Handedness was assessed by using a modified version of the Edinburgh Inventory (43) consisting of 20 items. Total number of right- and left-hand items were scored, and the laterality quotient was computed according to the following formula: (total right – total left)/(total right + total left). This yielded a total laterality quotient for each participant that ranged from 1.00 (totally dextral) to –1.00 (totally nondextral). Subjects with a laterality quotient greater than 0.70 were classified as dextral and the rest as nondextral (44). Information regarding handedness classifications is provided in Table 1.
Statistical Analyses
To minimize type I errors, we made several predictions regarding the direction of the correlations between the neuropsychological domains and hippocampal volumes on the basis of prior empirical and theoretical research. Specifically, we hypothesized that smaller anterior hippocampal volume would be significantly correlated with lower scores on the executive and motor functional domains. Therefore, although the main hypotheses focused on the associations of anterior hippocampal volume with executive and motor performance, we examined all the neuropsychological correlates of both posterior and anterior hippocampal volumes to determine specificity of the findings. Given that distributions of variables were judged to have a normal shape, Pearson’s product-moment correlations (two-tailed; alpha=0.05) were used for investigation of structure-function relations. Partial correlation analysis was used to investigate the possible effects of age at onset and parental social class on significant findings. For significant correlations of hippocampal volume and neuropsychological functioning, we tested the hypothesis that there is a linear relationship between performance on that neuropsychological domain and individual slice volumes across the long axis of the hippocampus after cubic-spline interpolation to 10 evenly spaced slice positions. Curve estimation was used to model the pattern of correlations between structure and function across the long axis of the hippocampus; hippocampal slice number was the independent variable, and the structure-function correlation was the dependent variable.
We compared correlated correlation coefficients using the method described by Meng et al. (45). In this method two correlations obtained in a single group of subjects can be compared where each correlation is between one predictor variable (X1 or X2) and a single common dependent variable (Y). Its purpose, therefore, is to test the null hypothesis that two correlation coefficients are not significantly different and not to test whether any single correlation is statistically significant. Tests of the difference between correlation coefficients employed r-to-z transformations to test the null hypothesis that a structure-function correlation obtained for the male patients did not differ significantly from the same structure-function correlation obtained for the female patients.
Differences in group characteristics, brain volumes, and neuropsychological performance were examined by using either independent-groups t tests or chi-square analyses (Table 1). Differences in the average number of weeks between the various time points in the study were examined by using nonparametric Mann-Whitney U tests because of violations in the homogeneity-of-variance assumption (Table 1). To control for nonspecific size differences among study participants, anterior and posterior hippocampal volumes and hippocampal slices were adjusted for total brain volume through linear regression; these analyses were conducted separately for male and female patients.
Results
Group characteristics and the numbers of weeks between various time points in the study are reported in Table 1. Male and female patients did not differ significantly at the time of the MRI or neuropsychological examinations in age, years of education, parental social class, handedness, or performance in any of the neuropsychological domains. In addition, there were no significant differences between male and female patients in the number of weeks between the various time points in the study (i.e., study entry, administration of antipsychotic medications, MRI examination, and neuropsychological examination). The groups did differ significantly, however, in age at onset of psychotic symptoms and in racial or ethnic composition (Table 1). We also compared demographic variables for the 75 patients in this study and the 36 healthy comparison subjects who were used to standardize patient scores on the neuropsychological domains. Similar to our previous study (42), the patient and healthy comparison groups were well matched on distributions of sex, age, and handedness (all p>0.05) but differed in parental social class (χ2=6.2, df=1, p=0.01). Possible effects of this difference were investigated in subsequent analyses.
The correlations between total posterior and total anterior hippocampal volumes and the six neuropsychological domains are illustrated in Figure 1 and Figure 2. Figure 1 shows that posterior hippocampal volume was not significantly correlated with functioning on any of the neuropsychological domains among men or women with a first episode of schizophrenia (all p>0.05). Anterior hippocampal volume was significantly correlated only with executive (r=0.33, df=43, p=0.03) and motor (r=0.38, df=42, p=0.01) functioning among men; none of the correlations between anterior hippocampal volume and neuropsychological functioning was statistically significant among women with a first episode of schizophrenia (Figure 2). Figure 3 and Figure 4 provide the scatter plots for the relation of anterior hypocampal volume to executive or to motor functioning among men with a first episode of schizophrenia. The use of Spearman’s rank-order correlations did not alter any of the significant (or nonsignificant) findings.
Additional analyses examined the possible effects of racial or ethnic group composition by comparing the coefficients of determination (r2) for the Caucasian subjects, the only racial or ethnic group that was large enough for analysis, and the entire group of male patients. The coefficients of determination for the correlations of anterior hippocampal volume and executive functioning and anterior hippocampal volume and motor functioning were comparable between groups. We also examined the possible effects of parental social class and age at onset (by using partial correlations) on the observed findings; these analyses did not differ substantively from the original analyses.
Tests of the difference between correlated correlation coefficients indicated that among the male patients (N=43), anterior hippocampal volume was significantly more strongly correlated with executive functioning than with either memory (difference: z=1.98, p=0.02) or language (difference: z=2.47, p=0.007) functioning. Similarly, among the male patients (N=42), anterior hippocampal volume was significantly more strongly correlated with motor functioning than with either memory (difference: z=2.03, p=0.02) or language (difference: z=2.11, p=0.02) functioning. Neither executive nor motor functioning was more strongly correlated with anterior hippocampal volume than with posterior hippocampal volume (p>0.05). Anterior hippocampal volume was more strongly correlated with motor functioning among male (N=42) than female (N=31) patients (difference: z=2.34, p=0.01).
We tested the hypothesis that there would be a linear relationship between executive and motor performance and hippocampal volume across its long axis (after cubic-spline interpolation to 10 evenly spaced slice positions). The correlations between executive and motor functioning and volumes of individual hippocampal slices are illustrated in Figure 5 and Figure 6, respectively. Results of these analyses, however, indicated that when examined across its long axis, the relationships between hippocampal volume and executive performance (F=1.37, df=1, 8, p=0.28) and hippocampal volume and motor performance (F=1.05, df=1, 8, p=0.34) were not linear.
Discussion
The main finding of this study is that anterior hippocampal volume correlates significantly with neuropsychological functions in male patients experiencing a first episode of schizophrenia who were studied soon after illness onset and before extensive pharmacological intervention. Among male patients, anterior hippocampal volume was more strongly correlated with both executive and motor functioning than with either memory or language functioning. These findings also suggest that the pattern of correlations between structure and function differ between male and female patients.
Despite evidence for hippocampal abnormalities in patients with schizophrenia, few MRI studies have investigated their neuropsychological correlates. In one such study, Hoff et al. (11) reported no significant associations of hippocampal volume with neuropsychological measures in a sample of patients experiencing a first episode of schizophreniform illness. Similarly, DeLisi et al. (12) and Nestor et al. (13) reported no significant neuropsychological correlates of hippocampal volumes in their patient samples, although significant associations were identified with the parahippocampal gyrus. In contrast to these studies, Goldberg et al. (14) implicated the left anterior hippocampus in the inability of patients to repeat stories read to them. Differences between our study findings and those of previous reports may be related to selection and diagnostic issues and methodological differences in measuring the hippocampus, including our distinction between the posterior and anterior hippocampus. In addition, if neuropsychological deficits are more strongly tied to anterior hippocampal abnormalities, studies investigating the entire hippocampus might not detect these associations.
The finding that smaller anterior hippocampal volume was associated with deficits in performance of executive and motor tasks considered sensitive to the integrity of frontal lobe functioning may seem paradoxical in light of research that has implicated the hippocampus in memory formation and consolidation (46, 47). Moreover, we did not observe any significant correlation between hippocampal volume and memory functioning in patients. It should be noted, however, that although studies have identified memory deficits as the most conspicuous result of hippocampal lesions, such findings were often obtained following normal development in healthy humans after they sustained some focal lesion or in temporal lobe epilepsy. In schizophrenia, hippocampal abnormalities are more likely the result of some neurodevelopmental abnormality rather than the result of an acquired lesion, and therefore, the adult-lesion model may not be applicable (48, 49). Animal studies have indicated that early developmental lesions to the hippocampal formation may not yield evidence of memory deficits; rather, these animals demonstrated both pharmacological and behavioral abnormalities that are more consistent with frontal lobe lesions in adult animals (50–53). Particularly noteworthy is that these animals with developmental lesions demonstrated evidence of “frontal dysfunction” only when they matured into adolescence or adulthood. Our results are therefore consistent with the hypothesis that some neurodevelopmental abnormality affecting the anterior hippocampus may later disrupt frontolimbic functioning in adulthood, thereby yielding a pattern of frontal lobe dysfunction. Thus, the adult-lesion model (with its implication that memory deficits are strongly tied to hippocampal integrity) may therefore be less relevant to our understanding of structure-function relations in schizophrenia, given that neurodevelopmental mechanisms are hypothesized to play a significant role in the pathophysiology of the disorder.
Given the extensive anatomic connections between frontal and mesiotemporal regions, a defect in connectivity between these regions may therefore be implicated in the pathophysiology of schizophrenia (17, 26). Csernansky et al. (54) found that the superior and lateral aspects of the hippocampal head, which have strong connections with the medial prefrontal regions, had the greatest shape abnormalities in patients with schizophrenia. Similarly, Weinberger et al. (55) found that differences in anterior hippocampal volume computed between monozygotic twins who were discordant for schizophrenia were significantly correlated with the difference between the twins in regional CSF to the prefrontal cortex during performance of the Wisconsin Card Sorting Test. It is therefore plausible that neurodevelopmental abnormalities in the medial frontolimbic system—which includes the dorsal aspects of the premotor and prefrontal cortex, cingulate gyrus, and anterior hippocampus—may comprise the structural basis for the observation of executive and motor dysfunction in schizophrenia. The medial frontolimbic system, in which the frontal lobes and the limbic system are linked by the cingulate bundle (17), is isomorphic with the medial/dorsal archicortical system and can be distinguished from the ventral or lateral paleocortical system that comprises the olfactory cortex and the peri-insular and ventral neocortices, including the orbital frontal cortex (16). The dorsal archicortical system has been hypothesized to be important for projectional control of behavior (56), including the types of executive and motor functions that correlated significantly with anterior hippocampal volume in this study (57).
The present study, conducted with large groups of men and women with a first episode of schizophrenia, suggested sex differences in the functional correlates of anterior hippocampal volumes. Specifically, anterior hippocampal volume was more strongly correlated with motor dysfunction in male than in female patients. It is important to acknowledge a possible selection bias, however, in that female patients who were functioning better overall may have dropped out of the study earlier than women who were not functioning as well and, therefore, did not complete the neuropsychological assessment (41). It is difficult to compare our results with prior research because few studies have investigated sex differences in structure-function relations in patients with schizophrenia. In one such study, Flaum et al. (34) found that full-scale IQ was uncorrelated with brain regions of interest for their entire group of patients with schizophrenia. When patients were divided by sex, however, female patients had a pattern of correlations similar to that observed in normal comparison subjects, while no such relationship was evident among the male patients. In another study, Szeszko et al. (20) found that smaller anterior cingulate gyrus volume correlated significantly with worse executive functioning in male, but not female, patients experiencing a first episode of schizophrenia. Future research would benefit from the investigation of possible sex differences in analyses of structure-function relations in schizophrenia, as this may have implications for better understanding the pathophysiology of the disorder. For example, it may be that male patients have more pronounced abnormalities in the medial frontolimbic system, and this may be reflected in their neuropsychological test performance.
Although executive and motor functioning correlated significantly with anterior hippocampal volume and not with posterior hippocampal volume in the current study, these correlations were not statistically different from one another. This lack of anatomic specificity was most evident in the slice-by-slice analysis of hippocampal volumes in the male patients, which revealed that the relationship between executive and motor performance and hippocampal volume along its long axis did not fit a linear model. It was not possible to determine in this study, however, whether correlations involving the posterior hippocampal slices reflected morphologic abnormalities in the hippocampus or possibly in adjacent subcortical structures. Moreover, hippocampal tissue posterior to where the crus of the fornix is interrupted by the surrounding pulvinar was not included in the posterior hippocampal volume measurement, and consequently, we were unable to determine whether the most posterior part of the hippocampal formation would also show a similar pattern of correlations.
It is important to note several limitations of our hippocampal delineation methods that may have influenced the observed pattern of correlations. First, although we used the MRI scan that contained the mammillary bodies to assist in making the distinction between the posterior and anterior hippocampus before analysis, the use of this landmark to differentiate these regions is arguably somewhat arbitrary and may, therefore, have only approximated true functional differences between these regions. Second, because precise separation of the anterior hippocampus from the amygdala was not possible in these MRI scans, the anterior hippocampal volume measurement may have included the most caudal parts of the amygdala, thereby lowering the correlations of anterior hippocampal volume with the executive and motor domains.
In summary, this study indicated that there are neuropsychological correlates of hippocampal volumes in male patients experiencing a first episode of schizophrenia and that this relationship may not be linear along the long axis of the hippocampus. The results of the present study also suggest that our prior findings (15) were confirmed in a larger group of patients, even after independent remeasurement of the hippocampal formation. Strengths of this study include the large groups of male and female patients who were studied soon after illness onset, our assessment with a comprehensive neuropsychological test battery, and the distinction between the posterior and anterior hippocampus. Future studies should employ more precise methods for separation of the anterior hippocampus from the amygdala to better elucidate the functional correlates of these brain structures.
 |
Received April 2, 2001; revision received Aug. 14, 2001; accepted Aug. 24, 2001. From the Department of Psychiatry Research, Hillside Hospital, a division of North Shore–Long Island Jewish Health System; and the Department of Radiology, Long Island Jewish Medical Center, New Hyde Park, N.Y. Address reprints to Dr. Szeszko, Department of Psychiatry Research, Hillside Hospital, 75-59 263rd St., Glen Oaks, NY 11004; [email protected] (e-mail). Supported by NIMH grants from the Clinical Research Center for the Study of Schizophrenia (MH-41960) and MH-41646 (to Dr. Lieberman).
 |
Appendix 1.
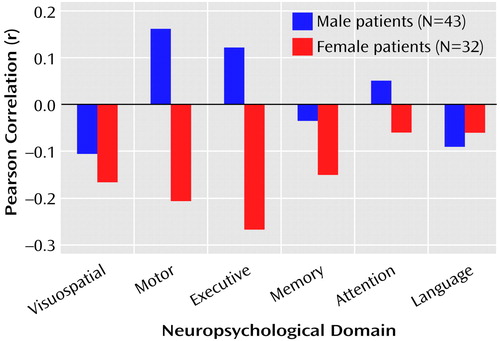
Figure 1. Relation of Posterior Hippocampal Volume to Neuropsychological Functional Domains in Patients Experiencing a First Episode of Schizophrenia
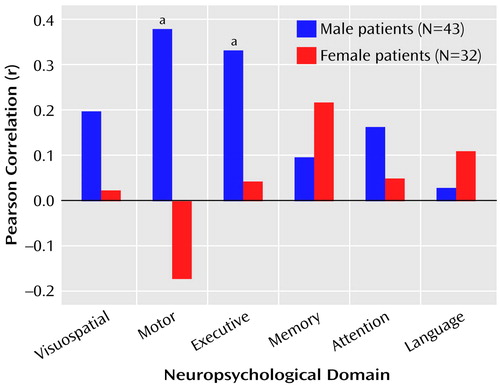
Figure 2. Relation of Anterior Hippocampal Volume to Neuropsychological Functional Domains in Patients Experiencing a First Episode of Schizophrenia
aSignificant correlation for men (p<0.05).
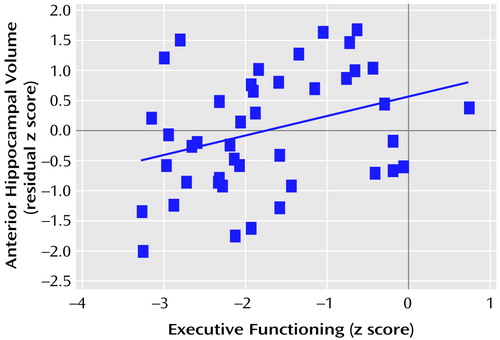
Figure 3. Relation of Executive Functioning to Anterior Hippocampal Volume in 43 Men Experiencing a First Episode of Schizophreniaa
aThe blue line denotes the best fit.
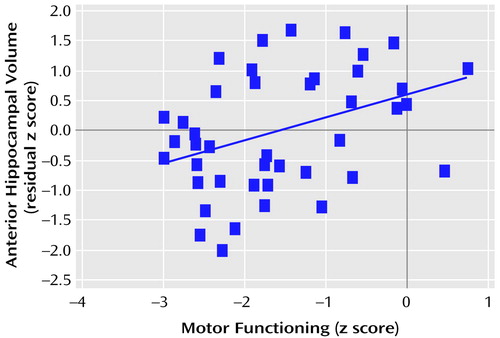
Figure 4. Relation of Motor Functioning to Anterior Hippocampal Volume in 42 Men Experiencing a First Episode of Schizophreniaa
aThe blue line denotes the best fit.
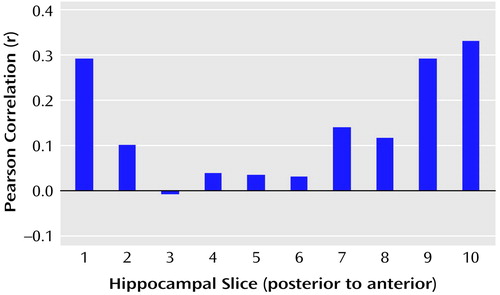
Figure 5. Relation of Executive Functioning to Interpolated Hippocampal Slice Volumes for 43 Men Experiencing a First Episode of Schizophreniaa
aCubic-spline interpolation of the measured volumes was used to provide data representing 10 equally spaced slice positions.
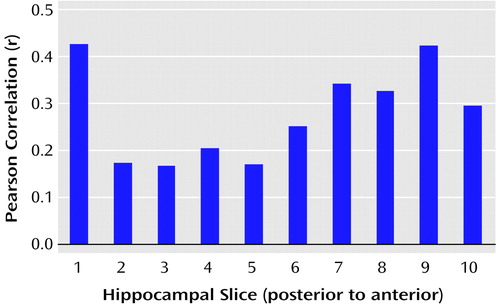
Figure 6. Relation of Motor Functioning to Interpolated Hippocampal Slice Volumes for 42 Men Experiencing a First Episode of Schizophreniaa
aCubic-spline interpolation of the measured volumes was used to provide data representing 10 equally spaced slice positions.
1. Kovelman JA, Schiebel AB: A neurohistological correlate of schizophrenia. Biol Psychiatry 1984; 19:1601-1621Medline, Google Scholar
2. Bogerts B, Meertz E, Schonfeld-Bausch R: Basal ganglia and limbic system pathology in schizophrenia. Arch Gen Psychiatry 1985; 42:784-791Crossref, Medline, Google Scholar
3. Bogerts B, Falkai P, Haupts M, Greve B, Ernst S, Tapernon-Franz L, Heinzmann U: Post-mortem volume measurements of limbic system and basal ganglia structures in chronic schizophrenics: initial results from a new brain collection. Schizophr Res 1990; 3:295-301Crossref, Medline, Google Scholar
4. Jeste DV, Lohr JB: Hippocampal pathologic findings in schizophrenia. Arch Gen Psychiatry 1989; 46:1019-1024Crossref, Medline, Google Scholar
5. Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL: Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry 1991; 48:996-1001Crossref, Medline, Google Scholar
6. Arnold SE, Franz BR, Gur RC, Gur RE, Shapiro RM, Moberg PJ, Trojanowski JQ: Smaller neuron size in schizophrenia in hippocampal subfields that mediate cortical-hippocampal interactions. Am J Psychiatry 1995; 152:738-748Link, Google Scholar
7. Bogerts B, Lieberman JA, Ashtari M, Bilder RM, Degreef G, Lerner G, Johns C, Masiar S: Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biol Psychiatry 1993; 33:236-246Crossref, Medline, Google Scholar
8. Bogerts B, Ashtari M, Degreef G, Alvir JMJ, Bilder RM, Lieberman JA: Reduced temporal limbic structure volumes on magnetic resonance images in first-episode schizophrenia. Psychiatry Res Neuroimaging 1990; 35:1-13Crossref, Medline, Google Scholar
9. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604-612Crossref, Medline, Google Scholar
10. Nelson MD, Saykin AJ, Flashman LA, Riordan HJ: Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry 1998; 55:433-440Crossref, Medline, Google Scholar
11. Hoff AL, Riordan H, O’Donnell D, Stritzke P, Neale C, Boccio A, Anand AK, DeLisi LE: Anomalous lateral sulcus asymmetry and cognitive function in first-episode schizophrenia. Schizophr Bull 1992; 18:257-272; correction, 1994; 20:248Google Scholar
12. DeLisi LE, Hoff AL, Schwartz JE, Shields GW, Halthore SN, Gupta SM, Henn FA, Anand AK: Brain morphology in first-episode schizophrenic-like psychotic patients: a quantitative magnetic resonance imaging study. Biol Psychiatry 1991; 29:159-175Crossref, Medline, Google Scholar
13. Nestor PG, Shenton ME, McCarley RW, Haimson J, Smith RS, O’Donnell B, Kimble M, Kikinis R, Jolesz FA: Neuropsychological correlates of MRI temporal lobe abnormalities in schizophrenia. Am J Psychiatry 1993; 150:1849-1855Link, Google Scholar
14. Goldberg TE, Torrey EF, Berman KF, Weinberger DR: Relations between neuropsychological performance and brain morphological and physiological measures in monozygotic twins discordant for schizophrenia. Psychiatry Res Neuroimaging 1994; 55:51-61Crossref, Medline, Google Scholar
15. Bilder RM, Bogerts B, Ashtari M, Wu H, Alvir J, Ma Jody D, Reiter G, Bell L, Lieberman JA: Anterior hippocampal volume reductions predict frontal lobe dysfunction in first episode schizophrenia. Schizophr Res 1995; 17:47-58Crossref, Medline, Google Scholar
16. Sanides F: Comparative architectonics of the neocortex of mammals and their evolutionary interpretation. Ann NY Acad Sci 1969; 167:404-423Crossref, Google Scholar
17. Bilder RM, Degreef G: Morphologic markers of neurodevelopmental paths to schizophrenia, in Developmental Neuropathology of Schizophrenia. Edited by Mednick SA, Canon TD, Barr CE, LaFosse JM. New York, Plenum, 1991, pp 167-190Google Scholar
18. Bilder RM, Szeszko PS: Structural neuroimaging and neuropsychological impairments, in The Neuropsychology of Schizophrenia. Edited by Pantellis C, Nelson HE, Barnes TRE. Sussex, UK, John Wiley & Sons, 1996, pp 279-298Google Scholar
19. Bilder RM: Neurocognitive impairment in schizophrenia and how it affects treatment options. Can J Psychiatry 1997; 42:255-264Crossref, Medline, Google Scholar
20. Szeszko PR, Bilder RM, Lencz T, Ashtari M, Goldman RS, Reiter S, Wu H, Lieberman JA: Reduced anterior cingulate gyrus volume correlates with executive dysfunction in men with first-episode schizophrenia. Schizophr Res 2000; 43:97-108Crossref, Medline, Google Scholar
21. Moser EI, Moser MB: Is learning blocked by saturation of synaptic weights in the hippocampus? Neurosci Biobehav Rev 1999; 5:661-672Crossref, Google Scholar
22. Strange B, Dolan R: Functional segregation within the human hippocampus. Mol Psychiatry 1999; 4:508-511Crossref, Medline, Google Scholar
23. Nieuwenhuys R, Voogd J, van Huijzen C: The Human Central Nervous System: A Synopsis and Altas, 3rd ed. Berlin, Springer-Verlag, 1988Google Scholar
24. Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ: Segregating the functions of the human hippocampus. Proc Natl Acad Sci USA 1999; 96:4034-4039Crossref, Medline, Google Scholar
25. Vermathen P, Laxer KD, Matson GB, Weiner MW: Hippocampal structures: anteroposterior-acetylaspartate differences in patients with epilepsy and control subjects as shown with proton MR spectroscopic imaging. Radiology 2000; 124:403-410Crossref, Google Scholar
26. Lepage M, Habib R, Tulving E: Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus 1998; 8:313-322Crossref, Medline, Google Scholar
27. Schacter DL, Wagner AD: Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 1999; 9:7-24Crossref, Medline, Google Scholar
28. Weinberger DR, Berman KF, Suddath R, Torrey EF: Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry 1992; 149:890-897Link, Google Scholar
29. Suddath RL, Casanova MF, Goldberg TE, Daniel DG, Kelsoe JR Jr, Weinberger DR: Temporal lobe pathology in schizophrenia: a quantitative magnetic resonance imaging study. Am J Psychiatry 1989; 146:464-472Link, Google Scholar
30. Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR: Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med 1990; 322:789-794Crossref, Medline, Google Scholar
31. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604-612Crossref, Medline, Google Scholar
32. Bryant NL, Buchanan RW, Vladar K, Breier A, Rothman M: Gender differences in temporal lobe structures of patients with schizophrenia: a volumetric MRI study. Am J Psychiatry 1999; 156:603-609Abstract, Google Scholar
33. Cowell PE, Kostianovsky DJ, Gur RC, Turetsky BI, Gur RE: Sex differences in neuroanatomical and clinical correlations in schizophrenia. Am J Psychiatry 1996; 153:799-805Link, Google Scholar
34. Flaum M, Andreasen NC, Swayze VW II, O’Leary DS, Alliger RJ: IQ and brain size in schizophrenia. Psychiatry Res 1994; 53:243-257Crossref, Medline, Google Scholar
35. Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Lieberman JA: Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999; 56:241-247Crossref, Medline, Google Scholar
36. Robinson DG, Woerner MG, Alvir JMJ, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Bilder R, Goldman R, Lieberman JA: Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry 1999; 156:544-549Link, Google Scholar
37. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria (RDC) for a Selected Group of Functional Disorders, 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1977Google Scholar
38. Ashtari M, Zito JL, Gold BI, Lieberman JA, Borenstein M, Herman PG: Computerized volume measurement of brain structure. Invest Radiol 1990; 25:798-805Crossref, Medline, Google Scholar
39. Falkai P, Bogerts B: Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci 1986, 236:154-161Google Scholar
40. MATLAB, version 5.3. Natick, Mass, MathWorks, 1999Google Scholar
41. Lieberman JA, Alvir JMJ, Woerner MG, Degreef G, Bilder RM, Ashtari M, Bogerts B, Mayerhoff DI, Geisler SH, Loebel A, Levy DL, Hinrichsen GA, Szymanski S, Chakos MH, Koreen A, Borenstein MT, Kane JM: Prospective study of psychobiology in first-episode schizophrenia at Hillside Hospital. Schizophr Bull 1992; 18:351-371Crossref, Medline, Google Scholar
42. Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JMJ, Woerner MG, Geisler S, Kane JM, Lieberman JA: Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry 2000; 157:549-559Link, Google Scholar
43. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97-113Crossref, Medline, Google Scholar
44. Schachter SC, Ransil BJ, Geschwind N: Associations of handedness with hair color and learning disabilities. Neuropsychologia 1987; 25:269-276Crossref, Medline, Google Scholar
45. Meng X, Rosenthal R, Rubin DB: Comparing correlated correlation coefficients. Psychol Bull 1992; 111:172-175Crossref, Google Scholar
46. Sutherland GR, McNaughton B: Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol 2000; 10:180-186Crossref, Medline, Google Scholar
47. McGaugh JL: Memory—a century of consolidation. Science 2000; 286:248-251Google Scholar
48. Bilder RM, Goldberg E: Motor perseverations in schizophrenia. Arch Clin Neuropsychol 1987; 2:195-214Crossref, Medline, Google Scholar
49. Goldberg E, Bilder RM: The frontal lobes and the hierarchic organization of cognitive control, in The Frontal Lobes Revisited. Edited by Perecman E. New York, IRBN Press, 1987, pp 159-187Google Scholar
50. Sams-Dodd F, Lipska BK, Weinberger DR: Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behavior in adulthood. Psychopharmacology (Berl) 1997; 132:303-310Crossref, Medline, Google Scholar
51. Bachevalier J, Beauregard M: Maturation of medial temporal lobe memory functions in rodents, monkeys, and humans. Hippocampus 1993; 3:191-201Medline, Google Scholar
52. Bachevalier J: Medial temporal lobe structures and autism: a review of clinical and experimental findings. Neuropsychologia 1994; 32:627-648Crossref, Medline, Google Scholar
53. Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR: Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology (Berl) 1995; 122:35-43Crossref, Medline, Google Scholar
54. Csernansky JG, Joshi S, Wang L, Haller JW, Gado M, Miller JP, Grenander U, Miller MI: Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci USA 1998; 95:11406-11411Crossref, Medline, Google Scholar
55. Weinberger DR, Berman KF, Suddath R, Torrey EF: Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry 1992; 149:890-897Link, Google Scholar
56. Goldberg G: Supplementary motor area structure and function: review and hypothesis. Behav Brain Sci 1985; 8:567-616Crossref, Google Scholar
57. Christensen BK, Bilder RM: Dual cytoarchitectonic trends: an evolutionary model of frontal lobe functioning and its application to psychopathology. Can J Psychiatry 2000; 45:247-256Crossref, Medline, Google Scholar
58. Wechsler D: Wechsler Memory Scale—Revised. New York, Psychological Corp, 1987Google Scholar
59. Delis DC, Kramer JH, Kaplan E, Ober BA: The California Verbal Learning Test Manual. New York, Psychological Corp, 1987Google Scholar
60. Osterrieth PA: Le test de copie d’une figure complex: contribution à l’étude de la perception et de la memoire. Arch Psychol 1944; 30:286-350Google Scholar
61. Wechsler D: Wechsler Adult Intelligence Scale—Revised Manual. New York, Psychological Corp, 1981Google Scholar
62. Goldberg E, Bilder RM, Jaeger J: The Goldberg Frontal Lobe Battery. Philadelphia, Medical College of Pennsylvania, 1987Google Scholar
63. Goldberg E, Jaeger J, Bilder RM: The Executive Control Battery and its applications (abstract). J Clin Exp Neuropsychol 1987; 9:38Google Scholar
64. Heaton RK: The Wisconsin Card Sorting Test Manual. Odessa, Fla, Psychological Assessment Resources, 1981Google Scholar
65. Reitan RM: Manual for the Administration of Neuropsychological Test Batteries for Adults and Children. Tucson, Ariz, Reitan Neuropsychology Laboratories, 1979Google Scholar
66. Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery. Tucson, Ariz, Neuropsychology Press, 1985Google Scholar
67. Matthews CG, Klove H: Instruction Manual for the Adult Neuropsychology Test Battery. Madison, University of Wisconsin Medical School, 1964Google Scholar
68. Benton AL, Hamsher K: Multilingual Aphasia Examination Manual. Iowa City, University of Iowa, 1978Google Scholar
69. Goodglass H, Kaplan E: The Assessment of Aphasia and Related Disorders. Philadelphia, Lee & Febiger, 1983Google Scholar
70. Borod JC, Goodglass H, Kaplan E: Normative data on the Boston Diagnostic Aphasia Examination, Parietal Lobe Battery, and the Boston Naming Test. J Clin Exp Neuropsychol 1980; 2:209-216Crossref, Google Scholar
71. Spreen O, Benton AL: Neurosensory Center Comprehensive Examination for Aphasia (NCCEA) Manual of Instructions. Victoria, BC, Canada, University of Victoria, Department of Psychology, Neuropsychology Laboratory, 1977Google Scholar
72. Terman LM, Merrill MA: Stanford-Binet Intelligence Scale: Manual for the Third Revision Form L-M. Boston, Houghton Mifflin, 1973Google Scholar
73. Weintraub S, Mesulam MM: Mental state assessment of young and elderly adults in behavioral neurology, in Principles of Behavioral Neurology. Edited by Mesulam MM. Philadelphia, FA Davis, 1985Google Scholar



