Gender Differences in Temporal Lobe Structures of Patients With Schizophrenia: A Volumetric MRI Study
Abstract
OBJECTIVE: The temporal lobe and associated structures have been previously implicated in the neuroanatomy of schizophrenia. This study was designed to assess the potential influence of gender on the morphology of temporal lobe structures, including the superior temporal gyrus and the amygdala/hippocampal complex, in patients with schizophrenia and to examine whether schizophrenic patients differ morphologically in these structures from comparison subjects. METHOD: Magnetic resonance imaging was used to measure the volume of temporal lobe structures, including the superior temporal gyrus, the amygdala/hippocampal complex, and the temporal lobe (excluding the volumes of the superior temporal gyrus and amygdala/hippocampal complex), and two comparison areas—the prefrontal cortex and caudate—in 36 male and 23 female patients with schizophrenia and 19 male and 18 female comparison subjects. RESULTS: There was a significant main effect of diagnosis in the superior temporal gyrus and the amygdala/hippocampal complex, with smaller volumes in patients than in comparison subjects. There was a significant gender-by-diagnosis-by-hemisphere interaction for temporal lobe volume. Temporal lobe volume on the left was significantly smaller in male patients than in male comparison subjects. Female patients and female comparison subjects demonstrated no significant difference in temporal lobe volume. There were no statistically significant gender interactions for the superior temporal gyrus, the amygdala/hippocampal complex, or the comparison regions. CONCLUSIONS: These findings suggest that there may be a unique interaction between gender and the pathophysiologic processes that lead to altered temporal lobe volume in patients with schizophrenia.
There has recently been considerable interest in the role of gender in the expression of schizophrenic disease manifestations. This interest stems from observed differences in the clinical presentation of men and women with this illness. It has been reported that women with schizophrenia have a better response to pharmacological treatment, better premorbid social functioning, better long-term outcomes, and a later age at onset than men (1–4). Also, men with schizophrenia may be more likely to present with prominent negative symptoms, whereas women with schizophrenia are reported to present with proportionally more positive symptoms (5, 6). No compelling explanations have been provided for these variations in clinical presentation; however, they suggest that gender may be a significant factor in the pathophysiology of this illness.
Among normal individuals, gender differences have been observed in cognitive functioning, neuroanatomy, and brain metabolism. Men have been reported to perform better on tasks involving spatial abilities, mathematical reasoning, navigation, and target-directed motor skills. In contrast, women have been shown to have superior ability on tasks involving perceptual speed, verbal fluency, mathematical calculation, and recalling landmarks (7). In addition to differences in total brain weight, normal male brains are 10% larger than normal female brains; neuroanatomical studies have also identified several dimorphic areas of the brain (8). Witelson et al. (9) reported an 11% greater cell density in the posterior superior temporal gyrus of female brains in Brodmann area 22. The volume of the bed nucleus of the stria terminalis was reported to be 147% larger in male brains than in female brains (10), and the investigators could not attribute this finding to the greater brain size in men. Although the corpus callosum is smaller in female brains than in male brains, the posterior region has been reported to be larger in female brain specimens (11, 12). The anterior commissure has also been reported to be larger in female brains as compared with male brains (13). Functional imaging studies have shown significant metabolic differences between normal men and women. Gur et al. (14) demonstrated greater resting metabolism in men than in women in temporal-limbic regions and the cerebellum and lower resting metabolism in the cingulate cortex. In contrast, Andreason et al. (15) reported that women had greater resting glucose metabolic rates than men, and Baxter et al. (16) reported a trend of higher glucose metabolic rates in women than in men. Shaywitz et al. (17), using functional magnetic resonance imaging (MRI), reported that gender differences exist in the language areas of the brain, with men showing left inferior frontal gyrus activation during phonological tasks and women showing more diffuse and bilateral activation of the inferior frontal gyrus during such tasks. These examples of cognitive, structural, and functional dimorphisms suggest that there are fundamental differences in brain organization between men and women, which may have important implications for differences in clinical presentation and pathophysiology of psychiatric illnesses such as schizophrenia.
The possibility that differences in the clinical presentations of men and women with schizophrenia are related to morphological differences in particular structures of the brain is an important, ongoing area of investigation. We chose to focus on the temporal lobe on the basis of previous MRI and postmortem studies, which have consistently implicated altered structure of the temporal lobe and its components (superior temporal gyrus and amygdala/hippocampal complex) in the pathophysiology of schizophrenia (18–36).
Several previous MRI studies have examined the issue of gender differences in temporal lobe structures of patients with schizophrenia. Bogerts et al. (18) reported significant differences in the left posterior hippocampus, the right and left amygdala/hippocampus complex, the left anterior temporal horn of the ventricular system, and the right temporal lobe in male patients with schizophrenia as compared with male control subjects. There were no such findings in female patients and control subjects. Cowell et al. (19) reported decrements in the left temporal lobe total volume in men, but not in women, with schizophrenia. Schlaepfer et al. (20) examined 32 male and 14 female patients with schizophrenia and found reductions in gray matter in the superior temporal and middle temporal regions; these findings were most marked in the female patients. However, these authors did not state whether male/female differences were significant. In contrast, Flaum et al. (21) examined 70 male and 32 female patients with schizophrenia and 45 male and 42 female comparison subjects, assessing several structures including the temporal lobe. They found that the patients with schizophrenia had smaller hippocampal and superior temporal gyrus volumes than the comparison subjects; however, no gender differences were found in this study. One major reason for differences in the results of the studies mentioned above may be differences in MRI acquisition parameters and slice thickness.
In the present study, volumes of the superior temporal gyrus, the amygdala/hippocampal complex, and the temporal lobe (excluding volumes of the superior temporal gyrus and amygdala/hippocampal complex) were assessed by MRI for gender differences in morphology in patients with schizophrenia and comparison subjects. Prefrontal cortex total volume, gray and white matter volumes, and caudate volume were also measured to test the specificity of temporal lobe results.
METHOD
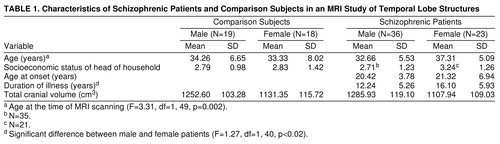
Fifty-nine right-handed patients (23 women and 36 men) were selected from the Maryland Psychiatric Research Center outpatient research program (table 1). The patients met the DSM-III-R criteria for chronic schizophrenia. A best-estimate diagnostic approach was used that incorporated all available sources of information, including direct assessment, family informants, and past medical and psychiatric records. Patients who had a DSM-III-R diagnosis of alcohol or substance abuse or dependence, organic brain disorder, or mental retardation or had a medical condition that may affect brain function and structure were excluded from the study. All patients were clinically stable on a regimen of antipsychotic medication. Thirty-seven right-handed comparison subjects (18 women and 19 men) were selected from the general population (table 1). All comparison subjects were interviewed with the Structured Clinical Interview for DSM-III-R—Patient Version (37). Potential comparison subjects with a history of a DSM-III-R axis I or axis II disorder, a family history of psychotic illness, or any medical condition known to affect brain function or structure were excluded from the study.
Handedness for all subjects was determined with the use of the Annett Handedness Scale (38). All subjects in the study were between the ages of 21 and 45 years. Seventeen male comparison subjects were white and two were black; 13 female comparison subjects were white, three were black, and two were Asian. Twenty-seven male patients were white and nine were black; 14 female patients were white, eight were black, and one was Asian. After a complete description of the proposed study was discussed with each potential participant, written informed consent was obtained. Some subjects were part of an earlier MRI study that included 44 patients with schizophrenia (29 male and 15 female) and 29 comparison subjects (20 male and nine female) (25).
MRI scans were obtained on a 2-T Siemens scanner operating at 1.5 T at the University of Maryland School of Medicine, Baltimore. A sagittal scout series was acquired first to correct for obvious head tilt and to localize imaging coordinates. Next, the whole brain was scanned with spin echo T1-weighted images. These T1-weighted images were obtained in the coronal plane with 3-mm contiguous sections. TR was 600 msec and TE was 17 msec, with two excitations and a 256×256-pixel matrix. MRI data were directly transferred from magnetic tape to optical disks for archiving. All identifying information was removed from the images for blinded assessment of morphology.
A system of rules based on brain atlases and published MRI studies was used to establish landmarks for delineating the boundaries of the regions of interest (39–43). These landmarks served as general guidelines to supplement the information derived from visual inspection of the MRI images. Landmark descriptions, measurement rules, and interrater reliability statistics for the prefrontal cortex total volume, the volumes of the prefrontal cortex white matter and gray matter, caudate, and amygdala/hippocampal complex, and total cranial volume have been previously established and reported (25, 43). Landmark descriptions and measurement rules for the temporal lobe and the superior temporal gyrus were established in the same manner for the current study (figure 1). The anterior boundary of the temporal lobe began at the temporal pole, and the posterior boundary was defined by the slice anterior to the trigone of the lateral ventricles. The superior boundary of the temporal lobe was demarcated by the Sylvan fissure, and the lateral and posterior boundaries by the outline of the superior, middle, and inferior gyri. The medial temporal lobe boundary was defined by drawing a line perpendicular to the axis of the temporal stem from the inferior aspect of the insula around medial temporal lobe structures. The anterior slice of the superior temporal gyrus began at the temporal stem, and the most posterior slice was defined by the slice anterior to the trigone of the lateral ventricles. The superior temporal gyrus was bounded superiorly by the Sylvan fissure, inferiorly by the superior temporal sulcus, laterally by the gyral outline, and medially by a line connecting the superior temporal sulcus to the circular sulcus. Interrater reliability for the temporal lobe and superior temporal gyrus was based on 20 area measurements of each region conducted by two raters (N.L.B. and R.W.B.). The intraclass correlation coefficients were greater than 0.95 for both structures.
Morphometric analyses were performed with the use of the Loats image analysis system (44). The sample function of the system was used to determine volumes of the temporal lobe, superior temporal gyrus, caudate, and amygdala/hippocampus. This function enables the investigator to outline the region of interest directly on the computerized MRI image and calculates the area of the demarcated region. The threshold function of the system was used to determine volumes of prefrontal gray and white matter. This function enables the investigator to partition gray matter from both CSF and white matter by assigning nonoverlapping signal intensity ranges to each tissue compartment. The threshold function was not used to partition the temporal lobe and superior temporal gyrus into gray and white matter components because of problems with radio frequency inhomogeneity.
A three-way repeated measures analysis of covariance was performed on all measures, with gender and diagnostic group (comparison subjects versus schizophrenic patients) as between-group factors and hemisphere (right versus left) as a within-group factor. Age at the time of MRI scanning and total cranial volume were used as covariates to correct for differences in age, duration of illness, and head size (table 1). Post hoc analysis was performed using the Bonferroni method. Chi-square tests and t tests were used to compare total cranial volume, demographic variables, and clinical variables of the patients and comparison subjects. Volumes of the superior temporal gyrus and amygdala/hippocampal complex were excluded from temporal lobe volume to prevent redundancy within statistical analyses. Prefrontal cortex total volume and volumes of the prefrontal cortex gray and white matter and caudate were used to assess the specificity of temporal lobe results.
RESULTS
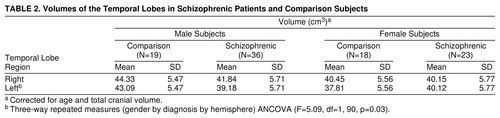
For temporal lobe volume (table 2, figure 2), there was not a significant main effect of diagnosis (F=1.79, df=1, 90, p=0.18), but there was a significant main effect of gender (F=6.50, df=1, 90, p=0.01). There was a significant three-way interaction (gender by diagnostic group by hemisphere). The male patients had significantly smaller volumes on the left than the male comparison subjects (t=3.2, df=182, p<0.01, Bonferroni post hoc test). There were no significant differences between the female patients and female comparison subjects; however, the female patients demonstrated nonsignificantly greater left temporal lobe volume.
For superior temporal gyrus volume (table 3), there was a significant main effect of diagnosis. The patients with schizophrenia had significantly smaller volumes bilaterally than the comparison subjects. There were no statistically significant interactions. There was a significant hemisphere effect, with right volume greater than left volume in both diagnostic groups (F=10.97, df=1, 92, p=0.001).
For amygdala/hippocampal complex volume (table 3), there was a significant main effect of diagnosis. The patients with schizophrenia had significantly smaller volumes bilaterally than the comparison subjects. There were no statistically significant interactions, and there was no significant hemisphere effect for either diagnostic group (F=0.24, df=1, 92, p=0.63).
For prefrontal cortex total volume (table 3), there was a significant main effect of diagnosis. The patients with schizophrenia had significantly smaller volumes bilaterally than the comparison subjects. There were no statistically significant interactions. There was a significant hemisphere effect, with right volume greater than left volume in both diagnostic groups (F=12.49, df=1, 91, p=0.005).
For prefrontal cortex white matter volume (table 3), there was a significant main effect of diagnosis. The patients with schizophrenia had significantly smaller volumes bilaterally than the comparison subjects. There were no statistically significant interactions. There was a significant hemisphere effect, with left volume greater than right volume in both diagnostic groups (F=39.70, df=1, 89, p=0.005).
For prefrontal cortex gray matter volume (table 3), there was no significant main effect of diagnosis (F=0.48, df=1, 89, p=0.49). There was a significant hemisphere effect, with right volume greater than left in both diagnostic groups (F=132.23, df=1, 89, p=0.005). There were no statistically significant interactions.
Caudate volume (table 3) was significantly larger on the left in the patients than in the comparison subjects (F=5.60, df=1, 88, p<0.02), and there was a nonsignificantly greater volume on the right in the patients with schizophrenia versus the comparison subjects. There were no statistically significant interactions. There was a significant effect of hemisphere.
DISCUSSION
These results demonstrate that in our outpatient clinic population, abnormal volumes of the temporal lobe were evident in the patients with schizophrenia in comparison with the healthy subjects. There was a significantly smaller left temporal lobe volume in the male patients than in the male comparison subjects, but there was no significant difference in temporal lobe volume between the female patients and the female comparison subjects. The latter finding is consistent with the report of Cowell et al. (19). The male and female patients had smaller volumes of the amygdala/hippocampal complex and superior temporal gyrus as compared with the male and female comparison subjects. There were no gender differences in these structures. These findings have been reported by some, but not all, previous MRI studies (18, 21, 22, 24–27, 29).
Prefrontal cortex total volume, volumes of the prefrontal cortex gray matter and white matter, and caudate volume were important for determining the specificity of the influence of gender on temporal lobe volume in the patients versus the comparison subjects. There were no significant gender interactions for these structures. Prefrontal cortex white matter and total volumes were significantly smaller bilaterally in the male and female patients than in the comparison subjects, which was reported in an earlier study that used a smaller study group (25). The caudate was significantly larger on the left in the male and female patients than in the male and female comparison subjects, and there was a nonsignificant increase on the right in the patients versus the comparison subjects. This finding was also reported in the earlier study with a smaller study group (25). Taken together, these results suggest that the influence of gender on abnormal structure in patients with schizophrenia is limited to the temporal lobe, an effect that is not localized to either the superior temporal lobe or the amygdala/hippocampal complex.
The female patients were significantly older than the female comparison subjects, the male patients, and the male comparison subjects. There was also a significantly greater duration of illness among the female patients as compared with the male patients (table 1). Age at the time of MRI scanning was used as a covariate to correct for these differences. There were no other significant differences in demographic variables.
We were unable to differentiate volumes of the temporal lobe and superior temporal gyrus gray and white matter. This would have been beneficial in assessing whether group differences were related to alterations in gray matter volume, white matter volume, or both. Several studies have reported significant gray matter abnormalities in temporal lobe structures in schizophrenic subjects (20, 24, 27, 28, 42). The majority of these gray matter abnormalities, however, have been reported in male patients. The extent of the presence of these abnormalities in female patients remains to be determined.
The results of the current study suggest that gender does not exert a major influence on volumes of the superior temporal gyrus or the amygdala/hippocampal complex. The finding of volumetric differences in the temporal lobe (excluding volumes of the superior temporal gyrus and amygdala/hippocampal complex) suggests that there may be gender-specific abnormalities in the middle temporal gyrus, the inferior temporal gyrus, and/or the fusiform gyrus in male and female patients with schizophrenia. Positron emission tomography (PET), electrical stimulation, and electrophysiologic studies provide evidence that the middle temporal, the inferior temporal, and the fusiform gyri are important in performing several complex cognitive and sensory integrative functions. PET studies have shown that the left middle temporal gyrus and inferior temporal gyrus are important components in language processing, and the right middle temporal gyrus is an important component in tonal memory in normal subjects(45, 46). Studies of aphasic patients have shown that the posterior middle temporal gyrus and inferior temporal gyrus, along with the inferior parietal lobule, may play an important role in auditory comprehension, naming, oral reading, and repetition (46–48). Electrical stimulation studies of the inferior temporal and the fusiform gyri have been shown to result in significant impairments in language production (49, 50). Suzuki et al. (51), using intracranial EEG, demonstrated a direct connection between the left fusiform gyrus and Wernicke’s area. Visual evoked potential and clinicopathologic studies suggest that the fusiform and the inferior temporal gyri are also involved in color perception, color integration, visuospatial processing, and facial recognition (52–54). Patients with schizophrenia demonstrate substantial difficulties with many of these cognitive processes, and it seems likely that volumetric increments or decrements in the middle temporal, the inferior temporal, or the fusiform gyri may potentially reflect some of these difficulties and, in turn, identify specific neuroanatomical substrates of cognitive pathology based on gender.
Future studies will focus on examining these structures by using more advanced MRI volumetric techniques with three-dimensional images to correlate morphology with neuropsychological testing and clinical symptoms.
Received March 11, 1997; revision received June 19, 1998; accepted Oct. 5, 1998. From the Maryland Psychiatric Research Center, Department of Psychiatry, University of Maryland School of Medicine; and the University of Maryland Medical System, Department of Radiology, Section of Neuroradiology, University of Maryland School of Medicine . Address reprint requests to Dr. Bryant, Maryland Psychiatric Research Center, Maple and Locust Streets, P.O. Box 21247, Baltimore, MD 21228. Supported by NIMH grants MH-19126, MH-48225, MH-40279, MH-45074, and MH-55588.
 |
 |
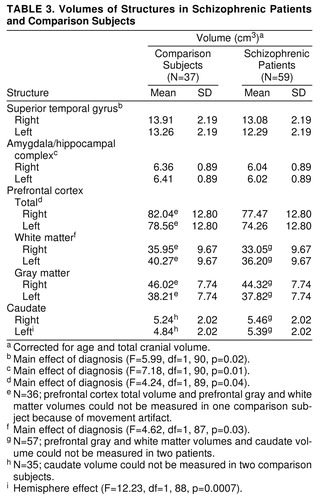 |
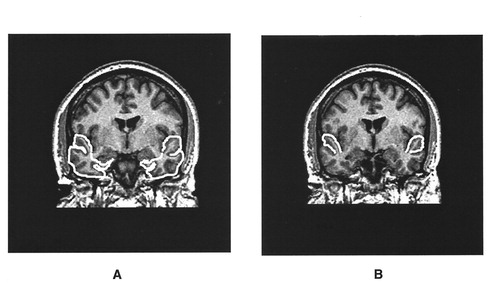
FIGURE 1. Examples of Temporal Lobe Total Volume (A) and Superior Temporal Gyrus Volume (B)
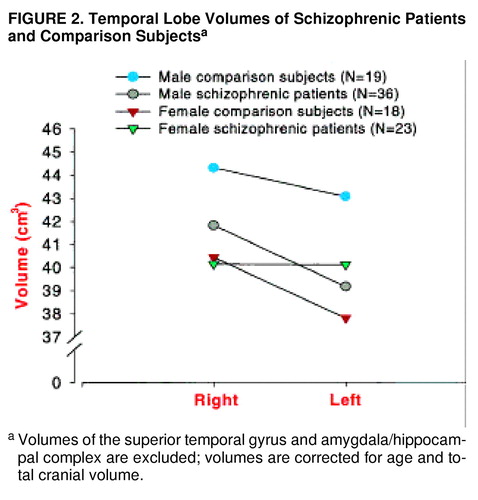
FIGURE 2. Temporal Lobe Volumes of Schizophrenic Patients and Comparison Subjectsa
aVolumes of the superior temporal gyrus and amygdala/hippocampal complex are excluded; volumes are corrected for age and total cranial volume.
1. Delisi LE, Dauphinais DI, Hauser P: Gender differences in the brain: are they relevant to the pathogenesis of schizophrenia? Compr Psychiatry 1989; 30:197–208Google Scholar
2. Loranger AW: Sex difference in age at onset of schizophrenia. Arch Gen Psychiatry 1984; 41:157–161Crossref, Medline, Google Scholar
3. Childers SE, Harding CM: Gender, premorbid social functioning, and long-term outcome in DSM-III schizophrenia. Schizophr Bull 1990; 16:309–318Crossref, Medline, Google Scholar
4. Goldstein JM: Gender differences in the course of schizophrenia. Am J Psychiatry 1988; 145:684–689Link, Google Scholar
5. Shtasel DL, Gur RE, Gallacher F, Heimberg C, Gur RC: Gender differences in the clinical expression of schizophrenia. Schizophr Res 1992; 7:225–231Crossref, Medline, Google Scholar
6. Rector NA, Seeman MV: Auditory hallucinations in women and men. Schizophr Res 1992; 7:233–236Crossref, Medline, Google Scholar
7. Kimura D: Sex differences in the brain. Sci Am 1992; 267:118–125Crossref, Medline, Google Scholar
8. Dekaban AS, Sadowsky D: Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol 1978; 4:345–356Crossref, Medline, Google Scholar
9. Witelson SF, Glezer II, Kigar DL: Women have greater density of neurons in the posterior temporal cortex. J Neurosci 1995; 15:3418–3428Crossref, Medline, Google Scholar
10. Allen LS, Gorski RA: Sex difference in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol 1990; 302:697–706Crossref, Medline, Google Scholar
11. Witelson SF: Hand and sex differences in the isthmus and genu of the human corpus callosum: a postmortem morphological study. Brain 1989; 112:799–835Crossref, Medline, Google Scholar
12. Habib M, Gayraud D, Oliva A, Regis J, Salamon G, Khalil R: Effects of handedness and sex on the morphology of the corpus callosum: a study with brain magnetic resonance imaging. Brain Cogn 1991; 16:41–61Crossref, Medline, Google Scholar
13. Allen LS, Gorski RA: Sexual dimorphism of the anterior commissure and massa intermedia of the human brain. J Comp Neurol 1991; 312:97–104Crossref, Medline, Google Scholar
14 Gur RC, Mozley LH, Mozley PD, Resnick SM, Karp JS, Alavi A, Arnold SE, Gur RE: Sex differences in regional cerebral glucose metabolism during a resting state. Science 1995; 267:528–531Crossref, Medline, Google Scholar
15. Andreason PJ, Zametkin AJ, Guo AC, Baldwin P, Cohen RM: Gender-related differences in regional cerebral glucose metabolism in normal volunteers. Psychiatry Res 1994; 51:175–183Crossref, Medline, Google Scholar
16. Baxter LR, Schwartz JM, Mazziotta JC, Phelps ME, Pahl JJ, Guze BH, Fairbanks L: Cerebral glucose metabolic rates in normal human females versus normal males. Psychiatry Res 1987; 21:237–245Crossref, Medline, Google Scholar
17. Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher JM, Shankweller DP, Katz L, Gore JC: Sex differences in the functional organization of the brain for language. Nature 1995; 373:606–609Crossref, Google Scholar
18. Bogerts B, Ashtari M, Degreef G, Alvir JM, Bilder RM, Lieberman JA: Reduced temporal limbic structure volumes on magnetic resonance images in first-episode schizophrenia. Psychiatry Res Neuroimaging 1990; 35:1–13Crossref, Medline, Google Scholar
19. Cowell PE, Kostianovsky DJ, Gur RC, Turetsky BI, Gur RE: Sex differences in neuroanatomical and clinical correlations in schizophrenia. Am J Psychiatry 1996; 153:799–805Link, Google Scholar
20. Schlaepfer TE, Harris GJ, Tien AY, Peng LW, Lee S, Federman EB, Chase GA, Barta PE, Pearlson GD: Decreased regional cortical gray matter volume in schizophrenia. Am J Psychiatry 1994; 151:842–848Link, Google Scholar
21. Flaum M, Swayze VW II, O’Leary DS, Yuh WTC, Ehrhardt JC, Arndt SV, Andreasen NC: Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am J Psychiatry 1995; 152:704–714Link, Google Scholar
22. Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR: Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med 1990; 322:789–794Crossref, Medline, Google Scholar
23. Dauphinais D, DeLisi LE, Crow TJ, Alexandropoulos K, Colter N, Tuma I, Gershon ES: Reduction in temporal lobe size in siblings with schizophrenia: a magnetic resonance imaging study. Psychiatry Res 1990; 35:137–147Crossref, Medline, Google Scholar
24. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604–612Crossref, Medline, Google Scholar
25. Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F: Brain morphology and schizophrenia: an MRI study of limbic prefrontal cortex and caudate structures. Arch Gen Psychiatry 1992; 49:921–926Crossref, Medline, Google Scholar
26. Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE: Auditory hallucinations and smaller temporal gyrus volume in schizophrenia. Am J Psychiatry 1990; 147:1457–1462Link, Google Scholar
27. Menon RR, Barta PE, Aylward EH, Richards SS, Vaughn DD, Tien AY, Harris GJ, Pearlson GD: Posterior superior temporal gyrus in schizophrenia: grey matter changes and clinical correlates. Schizophr Res 1995; 16:127–135Crossref, Medline, Google Scholar
28. Zipursky RB, Marsh L, Lim KO, DeMent S, Shear PK, Sullivan EV, Murphy GM, Csernansky JG, Pfefferbaum A: Volumetric MRI assessment of temporal lobe structures in schizophrenia. Biol Psychiatry 1994; 35:501–516Crossref, Medline, Google Scholar
29. Marsh L, Suddath RL, Higgins N, Weinberger DR: Medial temporal lobe structures in schizophrenia: relationship of size to duration of illness. Schizophr Res 1994; 11:225–238Crossref, Medline, Google Scholar
30. Rossi A, Stratta P, D’Albenzio L, Tartaro A, Schiazza G, di Michele V, Bolino F, Casacchia M: Reduced temporal lobe areas in schizophrenia: preliminary evidence from a controlled multiplanar magnetic resonance imaging study. Biol Psychiatry 1990; 27:61–68Crossref, Medline, Google Scholar
31. Johnstone EC, Owens DGC, Crow TJ, Frith CD, Alexandropolis K, Bydder G, Colter N: Temporal lobe structure as determined by nuclear magnetic resonance in schizophrenia and bipolar affective disorder. J Neurol Neurosurg Psychiatry 1989; 52:736–741Crossref, Medline, Google Scholar
32. Arnold SE: Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry 1991; 48:625–632Crossref, Medline, Google Scholar
33. Jakob H, Beckman H: Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transm 1986; 65:303–326Crossref, Medline, Google Scholar
34. Falkai P, Bogerts B: Limbic pathology in schizophrenia: the entorhinal region: a morphometric study. Biol Psychiatry 1988; 24:515–521Crossref, Medline, Google Scholar
35. Jeste DV, Lohr JB: Hippocampal pathologic findings in schizophrenia: a morphometric study. Arch Gen Psychiatry 1989; 46:1019–1024Crossref, Medline, Google Scholar
36. Kovelman JA, Scheibel AB: A neurohistological correlate of schizophrenia. Biol Psychiatry 1984; 19:1601–1620Medline, Google Scholar
37. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P). New York, New York State Psychiatric Institute, Biometrics Research, 1989Google Scholar
38. Annett M: A classification of hand preference by association analysis. Br J Psychiatry 1970; 61:303–321Google Scholar
39. Matsui T, Hirano A: An Atlas of the Human Brain for Computerized Tomography. New York, Igaku-Shoin, 1978Google Scholar
40. Schnitzlein HN, Murtagh FR: An Atlas of the Human Brain for Computerized Tomography. Baltimore, Urban & Schwarzenberg, 1985Google Scholar
41. Kelsoe JR, Cadet JL, Pickar D, Weinberger DR: Quantitative neuroanatomy in schizophrenia. Arch Gen Psychiatry 1988; 45:533–541Crossref, Medline, Google Scholar
42. Suddath RL, Casanova MF, Goldberg TE, Daniel DG, Kelsoe JR Jr, Weinberger DR: Temporal lobe pathology in schizophrenia: a quantitative magnetic resonance imaging study. Am J Psychiatry 1989; 146:464–472Link, Google Scholar
43. Buchanan RW, Breier A, Kirkpatrick B, Elkashef A, Munson RC, Gellad F, Carpenter WT Jr: Structural abnormalities in deficit and nondeficit schizophrenia. Am J Psychiatry 1993; 150:59–65Link, Google Scholar
44. Loats HL, Lloyd DG, Pittenger M, Tucker RW, Unnerstall JR: Biomedical image analysis applications, in Imaging Techniques in Biology and Medicine. Edited by Conklin JJ, Swenberg CE. New York, Academic Press, 1988, pp 1–75Google Scholar
45. Demonet F, Chollet F, Ramsay S, Cardebat D, Nespoulous J-L, Wise R, Rascol A, Frackowiak R: The anatomy of phonological and semantic processing in normal subjects. Brain 1992; 115:1753–1768Crossref, Medline, Google Scholar
46. Mazziotta JC, Phelps ME, Carson RE, Kuhl DE: Tomographic mapping of human cerebral metabolism: auditory stimulation. Neurology 1982; 32:921–937Crossref, Medline, Google Scholar
47. Metter EJ, Riege WH, Hansen W, Phelps ME, Kuhl DE: Correlation of metabolic and language abnormalities in aphasia (abstract). Ann Neurol 1981; 10:102Crossref, Google Scholar
48. Metter EJ, Wasterlain CG, Kuhl DE, Hanson WR, Phelps ME: FDG positron emission computed tomography in a study of aphasia. Ann Neurol 1981; 10:173–183Crossref, Medline, Google Scholar
49. Burnstine TH, Lasser RP, Hart J, Uematsu S, Zinreich SJ, Krauss GL, Fisher RS, Vining EPG, Gordon B: Characterization of the basal temporal language area in patients with left temporal lobe epilepsy. Neurology 1990; 40:966–970Crossref, Medline, Google Scholar
50. L�ders H, Lesser RP, Hahn J, Dinner DS, Morris H, Resor S, Harrison M: Basal temporal language area demonstrated by electrical stimulation. Neurology 1986; 36:505–510Crossref, Medline, Google Scholar
51. Suzuki I, Shimizu H, Ishijima B, Tani K: Aphasic seizure caused by focal epilepsy in the left fusiform gyrus. Neurology 1992; 42:2207–2210Crossref, Medline, Google Scholar
52. Bogousslavsky J, Miklossy J, Deruaz JP, Assal G, Regli F: Lingual and fusiform gyri in visual processing: a clinico-pathologic study of superior altitudinal hemianopia. J Neurol Neurosurg Psychiatry 1987; 50:607–614Crossref, Medline, Google Scholar
53. Allison T, Begleiter A, McCarthy G, Roessler E, Nobre AC, Spencer DD: Electrophysiological studies of color processing in human visual cortex. Electroencephalogr Clin Neurophysiol 1993; 88:343–355Crossref, Medline, Google Scholar
54. Gross CG: Representation of visual stimuli in inferior temporal cortex. Philos Trans R Soc Lond 1992; 335:3–10Crossref, Medline, Google Scholar



