Slower Treatment Response in Bipolar Depression Predicted by Lower Pretreatment Thyroid Function
Abstract
OBJECTIVE: Because treatment of the depressed phase of bipolar disorder is a clinical challenge and hypothyroidism is known to be associated with depression, the authors examined the relationship between pretreatment thyroid values and response to antidepressant treatment. It was hypothesized that subjects with lower thyroid function, even within the normal range, would have a poorer response to initial treatment. METHOD: The subjects were 65 patients in the depressed phase of bipolar I disorder who were enrolled in a larger ongoing study. A panel of thyroid measures, including thyroid-stimulating hormone (TSH), thyroxine, triiodothyronine resin uptake, and free thyroxine index (FTI), were determined before initiation of algorithm-guided treatment. The effect of each thyroid measurement on time to remission was estimated by using the Cox proportional hazards model. RESULTS: Both lower values of FTI and higher values of TSH were significantly associated with longer times to remission, i.e., slower response to treatment. Outcomes were relatively poor unless patients had FTI values above the median and TSH values below the median. Patients with this optimal profile experienced remission 4 months faster than the remainder of the study group. CONCLUSIONS: This study provides further evidence that patients with bipolar disorder are particularly sensitive to variations in thyroid function within the normal range. Our results suggest that nearly three-quarters of patients with bipolar disorder have a thyroid profile that may be suboptimal for antidepressant response. It remains to be seen whether pharmacological enhancement of thyroid function will facilitate recovery from bipolar depression.
Treating the depressed phase of bipolar affective disorder is a significant clinical challenge. Use of antidepressant medications carries the risk of inducing mania and increasing the frequency of mood cycling (1, 2). Further, the sparse published reports of research in this area suggest that response to treatment may be poorer for bipolar depression than for unipolar depression (3–5). Proposals to improve outcomes have included multiple strategies, such as monotherapy and combination therapy using both established and putative mood stabilizers, addition of a variety of antidepressants, and other augmentation strategies, including thyroid augmentation.
An important question is whether modifiable risk factors are involved in the treatment resistance of bipolar depression. One topic that has generated considerable interest is the role of thyroid function. It has long been recognized that frank hypothyroidism can cause depressive symptoms (6), and it almost invariably does so in severe cases (7). In the early 1970s the development of assays for thyroid-stimulating hormone (TSH) provided the means to assess more subtle forms of hypothyroidism and thus to define subclinical hypothyroidism. In 1974, Wenzel et al. (8) introduced a system of grading hypothyroidism. In this classification, grade 1 defines overt hypothyroidism, and grades 2 and 3 define subclinical hypothyroidism. With the TSH assay and new definitions for subclinical hypothyroidism, it was then possible to gather epidemiological data to document that the prevalence of subclinical hypothyroidism is greater than that of overt hypothyroidism (9, 10). Furthermore, patients with subclinical hypothyroidism were noted to have a higher than normal lifetime prevalence of depression (11–13). The converse, that depressed populations demonstrate a high prevalence of subclinical hypothyroidism, also has been demonstrated (14–16). For instance, the rates of subclinical hypothyroidism reported for depressed populations are higher (8%–17%) than the rate for the general population (5%). In refractory depression, the presence of thyroid abnormalities is even greater. Studies reviewed by Howland (17) consistently demonstrate a higher than normal risk of subclinical hypothyroidism, with prevalence rates of 30% and above in patients with refractory depression.
Over the past decade, research findings have extended the relationship between thyroid function and mood disorders and have called into question the appropriateness of the accepted normal ranges for values on the standard thyroid function tests. For example, Prange et al. (18) found that the pretreatment free thyroxine index (FTI) was lower in patients who had poorer responses to treatment than in antidepressant responders, even though all FTI values were within the normal range. Frye et al. (19) assessed thyroid function prospectively in 52 outpatients with bipolar disorder. The patients were given monthly assessments, including thyroid function tests, during 1 year of lithium treatment. Even though their FTI values were within the normal range, patients with lower mean FTI values had more affective episodes and more severe depressive symptoms.
In this study we examined the relationship between pretreatment thyroid values and antidepressant response in bipolar depression. We predicted that patients with lower values within the normal range would have poorer outcomes of initial antidepressant treatment.
Method
Patients
The patients included in this report were participants in a larger, ongoing study, Maintenance Therapies in Bipolar Disorder. Referral sources included the inpatient units at Western Psychiatric Institute and Clinic, affiliated outpatient programs, community clinicians, and patient response to radio and newspaper advertisements. The assessment procedures included structured diagnostic interviews (to be specified), physical examination with medical history, laboratory assays, and an electrocardiogram. If a patient was eligible to participate in the study, explicit written informed consent was obtained.
Each participant was required to be in a current episode of major depression, mania, or mixed state of bipolar I disorder. From 1991 to 1995, the diagnoses were determined according to the Research Diagnostic Criteria (20) on the basis of the Schedule for Affective Disorders and Schizophrenia—Lifetime Version (21). Thereafter, diagnoses were based on the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (22). The index episode must have been at least the third lifetime episode of mood disorder or the second episode of mania. At least 12 weeks of remission must have occurred between the prior mood episode and the onset of the current episode. The most recent mood episode before the index episode must have occurred within 5 years of the onset of the index episode.
An index episode of major depression was required to have a rating of 7 or higher on the Raskin Severity of Depression Scale (23) and of 15 or higher on the 17-item Hamilton Depression Rating Scale (24). An index episode of mania was required to have a rating of 7 or higher on the Raskin Severity of Mania Scale (23) and 15 or higher on the Bech-Rafaelsen Mania Scale (25).
Exclusions for the study protocol included the diagnosis of rapid cycling (four or more mood episodes per year) and any active axis I disorder other than an anxiety disorder. Patients with clear-cut borderline or antisocial personality disorder also were excluded. Potential subjects were also excluded for the presence of any serious medical condition (as determined by the assessment procedures already noted) or for concomitant treatment considered to be incompatible with the standard pharmacological management of bipolar disorder. Specific medical exclusions included pregnancy, congestive heart failure, epilepsy, and unstable endocrine illnesses, such as frank untreated hypothyroidism. Patients currently being treated and stabilized with thyroid replacement therapy were eligible for the larger Maintenance Therapies in Bipolar Disorder protocol.
The first goal of the protocol was remission of the index mood episode. During the initial treatment phase, study psychiatrists used individualized guideline-based algorithms to select medications of first, second, and third choice for sequential therapy. For depression, lithium monotherapy was the treatment of first choice; however, divalproex and carbamazepine were permitted if clinically indicated. Initially, the sequence in the algorithm for antidepressants was imipramine first followed by tranylcypromine. Given poor results with imipramine and increasing experience with selective serotonin reuptake inhibitors, imipramine was replaced by paroxetine, although psychiatrists were free to select any other antidepressant if there was sufficient clinical justification. The patients were seen weekly until they satisfied the criteria for remission, defined as 4 consecutive weeks during which the average score on both the Hamilton depression scale and the Bech-Rafaelsen scale was 7 or less. After remission, the participants proceeded to the 2-year maintenance phase, during which time their medications were not changed.
At the outset of the initial treatment phase, the participants were randomly assigned to one of two psychotherapeutic options, interpersonal and social rhythm therapy (26) or a supportive medication management condition, referred to as clinical status and symptom review therapy.
Current Study Group
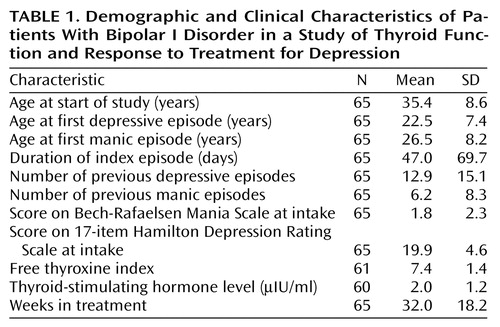
A total of 70 bipolar I patients entered the study in a major depressive episode. Among this group, five patients had a history of treated hypothyroidism. While such patients were included in the larger Maintenance Therapies in Bipolar Disorder protocol, they were excluded from this smaller study since the use of thyroid replacement therapy might complicate the interpretation of the results on pretreatment thyroid function tests. Demographic and pretreatment clinical characteristics of the study group are reported in Table 1.
All 65 patients received mood stabilizers; 57 received lithium carbonate as the sole mood stabilizer. Divalproex sodium was the sole mood stabilizer for three patients. Four patients were treated with both lithium and divalproex. One lithium nonresponder was treated with carbamazepine. About two-thirds of the patients (N=44) did not respond to monotherapy with a mood stabilizer and required the addition of an antidepressant. A majority of the patients (N=26) had more than one antidepressant trial. The antidepressants prescribed included tranylcypromine (17 trials), paroxetine (14 trials), imipramine (13 trials), bupropion (seven trials), fluoxetine (five trials), sertraline (four trials), venlafaxine (four trials), phenelzine (one trial), fluvoxamine (one trial), desipramine (one trial), and nefazodone (one trial).
Thyroid Function Tests
The thyroid panel usually included TSH, thyroxine (T4), triiodothyronine (T3) resin uptake, and FTI. However, four patients received only a screening test of TSH level, whereas five patients had all pretreatment measures except TSH. The assays were performed by the clinical laboratory at the University of Pittsburgh Medical Center with commercially available radioimmunoassay methods. The thyroid measures for which correlations were determined were FTI and T4 (r=0.81, df=59, p<0.0001), FTI and TSH (r=–0.19, df=54, p=0.16), T4 and TSH (r=0.02, df=54, p=0.89), T3 resin uptake and T4 (r=–0.38, df=59, p<0.003), and T3 resin uptake and TSH (r=–0.36, df=54, p=0.007).
Data Analysis
The effect of each thyroid measure on time to remission was estimated by using the Cox proportional hazards model (27). Time to remission was defined as the number of weeks needed to achieve a 4-week period of euthymia (i.e., score of 7 or less on the 17-item Hamilton depression scale and no signs or symptoms of hypomania or mania). For patients who withdrew from the study prematurely or who did not achieve remission within 52 weeks, data at the time of withdrawal or study termination were used. Because lithium treatment affects synthesis and release of thyroid hormone (28–32) and has been shown to affect thyroid laboratory assays in prospective studies (19, 33–37), we controlled for pretreatment lithium use by constructing a categorical variable indicating whether or not a subject had been taking lithium for at least 1 month before entering the study.
Results
The thyroid values for all patients were within the normal ranges, with the single exception of a patient with an elevated TSH level, 7.7 μIU/mL (normal range=0.4–5.0 μIU/mL). A total of 28 patients were receiving lithium salts at the time of study entry. Of these, 23 had taken lithium for at least 1 month, and the mean duration of treatment was 29 months (SD=33 months, range=1 month to 9 years, median=18 months).
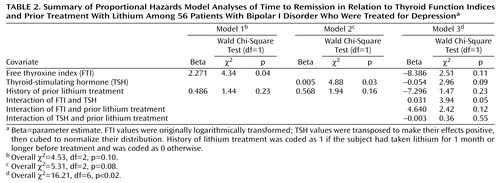
The remission rate based on intent to treat was 49% (32 of 65). The mean time to remission was 30 weeks (SD=11, range=15–52, median=30). Nine patients experienced remission with mood stabilizers alone, 18 responded to the first antidepressant trial, and five responded to the second antidepressant trial. The intent-to-treat remission rate among the patients receiving both a mood stabilizer and an antidepressant was only 52% (23 of 44). Results of the Cox proportional hazards model analyses are summarized in Table 2. There were no associations between time to remission and type of psychosocial treatment (i.e., interpersonal and social rhythm therapy versus clinical status and symptom review therapy); this term was dropped from subsequent analyses. Model 1 addresses the relationship between FTI and remission. Model 2 addresses the relationship between TSH and remission. Lower values of FTI and higher values of TSH were both significantly associated with longer time to stabilization, i.e., a slower response to treatment. In neither case was a history of recent lithium treatment significantly associated with the results.
Given the correlated nature of FTI and TSH, we conducted a third analysis including FTI, TSH, prior treatment with lithium, and their interactions. The three-way interaction was not significant and, therefore, was dropped from the model. The two-way interactions of each thyroid measure with prior lithium treatment were nonsignificant. However, the two-way interaction between FTI and TSH was significant (Table 2).
To explicate this interaction, we divided the study group according to a median split of each thyroid measure. The median value for FTI was 7.1. The median value for TSH was 1.7 μIU/mL. Survival analyses were performed for patients with FTI values above (top panel of Figure 1) and below (bottom panel) the median. This pair of survival curves illustrates that response was relatively poor unless the FTI was above the median and the TSH level was below the median. Patients with this optimal thyroid profile experienced remission an average of at least 4 months earlier than the remainder of the study group.
Discussion
We found that lower FTI values and higher TSH values within the normal range were significantly associated with a poorer response in bipolar depression during the initial phase of treatment. Conversely, the combination of lower pretreatment TSH and higher pretreatment FTI was associated with a markedly more rapid remission of depression. These results add to the growing literature in support of the idea that thyroid measures in the low normal range may result in a less than optimal outcome in mood disorders. Two previous studies similarly showed an association of marginal thyroid values to poorer response to antidepressant treatment in nonbipolar depression (18) and to a greater frequency and severity of mood episodes in bipolar disorder (19).
Although most patients in this series had a prior history of lithium treatment, the relationship between speed of remission of depression and current thyroid function was not mediated by a history of lithium treatment. Moreover, the subgroup of patients with optimal thyroid profiles was not distinguishable by sociodemographic, clinical, or illness course variables. Thus, the combination of above-median FTI and below-median TSH levels did not identify patients with a better inherent prognosis or a less severe illness.
These findings are consistent with the hypothesis that lower levels of thyroid hormones may represent an inadequate compensatory homeostatic response of the central nervous system to the stress of a depressive episode (38–41). Briefly, this hypothesis posits that depression may be associated with diminished availability of norepinephrine, that the normal compensatory response is an increase in receptor sensitivity, and that elevation of circulating thyroid hormones is one mechanism by which this compensatory response may be achieved. It is also possible that such inadequate thyroid response to depression may be a harbinger of early thyroid failure, even though the FTI and TSH values are in the normal range (42). While there are divergent views (43), it appears likely that patients with mood disorders are particularly vulnerable to even minor variations in thyroid hormone levels. For example, Kraus et al. (42) observed that 38% of depressed patients with baseline TSH values between 3.0 and 5.5 μIU/ml (i.e., above the mean normal value) had abnormal results on the thyrotropin-releasing hormone stimulation test.
Studies of adjunctive treatment with thyroid hormones date back more than 30 years (44) and include augmentation of both antidepressants (45) and electroconvulsive therapy (46). Adjunctive thyroid hormones also have been used with some success in rapid-cycling bipolar disorder (47, 48). Although findings from studies of the efficacy of thyroid augmentation have not been uniformly positive (49), on balance, these strategies continue to be accepted treatment options for treatment-resistant nonbipolar and bipolar depressions (50–52).
In our study, patients with bipolar depression who did not have the optimal combination of high normal FTI and low normal TSH had a median time to remission of nearly 1 year. This normal but apparently suboptimal thyroid state, which occurred in two-thirds of our patients, may represent a modifiable risk factor in bipolar depression. A prospective study is now needed to determine whether pharmacological augmentation can optimize thyroid function to facilitate clinical recovery.
 |
 |
Received Aug. 9, 2000; revisions received May 2 and June 21, 2001; accepted Aug. 11, 2001. From the Department of Psychiatry, University of Pittsburgh School of Medicine. Address reprint requests to Dr. Thase, Western Psychiatric Institute and Clinic, 3811 O’Hara St., Pittsburgh, PA 15213; [email protected] (e-mail). Supported in part by Mental Health Intervention Research Center grant MH-30915 from NIMH, by grant MH-29618 from NIMH to Drs. Frank, Kupfer, and Thase; by contract MH-80001 from the NIMH Systematic Treatment Enhancement Program for Bipolar Disorder to Drs. Thase, Kupfer, and Frank; and by a grant from the Theodore and Vada Stanley Foundation.
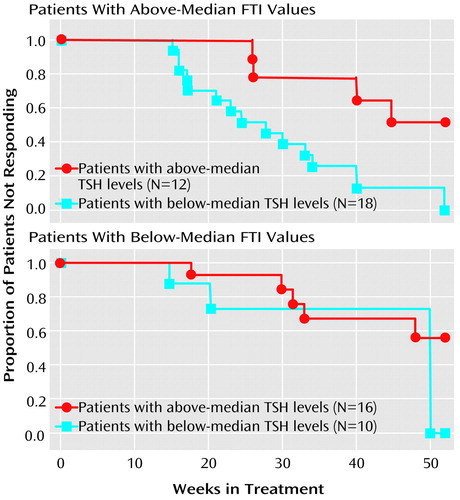
Figure 1. Time to Remission in Relation to Free Thyroxine Index (FTI) and Thyroid-Stimulating Hormone (TSH) Values in Patients With Bipolar I Disorder Who Were Treated for Depressiona
aThe median value for FTI was 7.1. The median value for TSH was 1.7 μIU/mL. The top panel illustrates that, among subjects with FTI values above the median, lower TSH values were associated with a shorter time to remission (Wilcoxon χ2=6.24, df=1, p=0.01). The bottom panel illustrates that there was no significant association between TSH values and time to remission in patients with FTI values below the median (Wilcoxon χ2=0.45, df=1, p>0.50).
1. Altshuler LL, Post RM, Leverich GS, Mikalauskas K, Rosoff A, Ackerman L: Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry 1995; 152:1130-1138Link, Google Scholar
2. Wehr TA, Goodwin FK: Can antidepressants cause mania and worsen the course of affective illness? Am J Psychiatry 1987; 144:1403-1411Link, Google Scholar
3. Hlastala SA, Frank E, Mallinger AG, Thase ME, Ritenour AM, Kupfer DJ: Bipolar depression: an underestimated treatment challenge. Depress Anxiety 1997; 5:73-83Crossref, Medline, Google Scholar
4. Mallinger AG, Frank E, Barwell MM, Thase ME, Kupfer DJ: Effectiveness of traditional antidepressants is suboptimal in the depressed phase of bipolar disorder, in Report of the 39th Annual Meeting of the New Clinical Drug Evaluation Unit Program. Bethesda, Md, National Institute of Mental Health, NCDEU, 1999Google Scholar
5. Zornberg GL, Pope HGJ: Treatment of depression in bipolar disorder: new directions for research. J Clin Psychiatry 1993; 13:397-408Google Scholar
6. Asher R: Myxoedematous madness. Br Med J 1949; 2:555-562Crossref, Medline, Google Scholar
7. Whybrow PC, Prange AJ, Treadway CR: Mental changes accompanying thyroid gland dysfunction. Arch Gen Psychiatry 1969; 20:48-63Crossref, Medline, Google Scholar
8. Wenzel KW, Meinhold H, Raffenberg M, Adlkofer F, Schleusener H: Classification of hypothyroidism in evaluating patients after radioiodine therapy by serum cholesterol, T3 uptake, total T4, FT4 index, total T3, basal TSH and TRH test. Eur J Clin Invest 1974; 4:141-148Medline, Google Scholar
9. Arem R, Escalante D: Subclinical hypothyroidism: epidemiology, diagnosis, and significance. Adv Intern Med 1996; 41:213-250Medline, Google Scholar
10. Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, Evans JG, Young E, Bird T, Smith PA: The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf) 1977; 7:481-493Crossref, Medline, Google Scholar
11. Esposito S, Prange AJ Jr, Golden RN: The thyroid axis and mood disorders: overview and future prospects. Psychopharmacol Bull 1997; 33:205-217Medline, Google Scholar
12. Haggerty JJ Jr, Stern RA, Mason GA, Beckwith J, Morey CE, Prange AJ Jr: Subclinical hypothyroidism: a modifiable risk factor for depression? Am J Psychiatry 1993; 150:508-510Link, Google Scholar
13. Joffe RT, Levitt AJ: The thyroid and depression, in The Thyroid Axis and Psychiatric Illness. Edited by Joffe RT, Levitt AJ. Washington, DC, American Psychiatric Press, 1993, pp 195-254Google Scholar
14. Gold MS, Pottash AL, Extein I: Hypothyroidism and depression: evidence from complete thyroid function evaluation. JAMA 1981; 245:1919-1922Crossref, Medline, Google Scholar
15. Targum SD, Sullivan AC, Byrnes SM: Compensatory pituitary-thyroid mechanisms in major depressive disorder. Psychiatry Res 1982; 6:85-96Crossref, Medline, Google Scholar
16. Tunbridge WM, Brewis M, French JM, Appleton D, Bird T, Clark F, Evered DC, Evans JG, Hall R, Smith P, Stephenson J, Young E: Natural history of autoimmune thyroiditis. Br Med J (Clin Res Ed) 1981; 282:258-262Crossref, Medline, Google Scholar
17. Howland RH: Thyroid dysfunction in refractory depression: implications for pathophysiology and treatment. J Clin Psychiatry 1993; 54:47-54Medline, Google Scholar
18. Prange AJ, Haggerty JJ, Browne JL: Marginal hypothyroidism in mental illness: preliminary assessments of prevalence and significance, in Neuropsychopharmacology, vol 1. Edited by Bunney WE Jr, Hippius H, Laakmann G, Schmauss M. Berlin, Springer-Verlag, 1990, pp 352-361Google Scholar
19. Frye MA, Denicoff KD, Bryan AL, Smith-Jackson EE, Ali SO, Luckenbaugh D, Leverich GS, Post RM: Association between lower mean serum free T4 and greater mood instability and depression in lithium-maintained bipolar patients. Am J Psychiatry 1999; 156:1909-1914Abstract, Google Scholar
20. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria: rationale and reliability. Arch Gen Psychiatry 1978; 35:773-782Crossref, Medline, Google Scholar
21. Spitzer RL, Endicott J: Schedule for Affective Disorders and Schizophrenia (SADS), 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
22. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
23. Raskin A, Schulterbrandt J, Reatig N, McKeon JJ: Replication of factors of psychopathology in interview, ward behavior and self-report ratings of hospitalized depressives. J Nerv Ment Dis 1969; 148:87-98Crossref, Medline, Google Scholar
24. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62Crossref, Medline, Google Scholar
25. Bech P, Bolwig TG, Kramp P, Rafaelsen OJ: The Bech-Rafaelsen Mania Scale and the Hamilton Depression Scale. Acta Psychiatr Scand 1979; 59:420-430Crossref, Medline, Google Scholar
26. Frank E, Kupfer DJ, Ehlers CL, Carter C, Frankel D: Interpersonal and social rhythm therapy for bipolar disorder: integrating interpersonal and behavioral approaches. Behavior Therapist 1994; 17:143-149Google Scholar
27. Cox DR, Oakes D: Analysis of Survival Data. London, Chapman & Hall, 1984Google Scholar
28. Bagchi N, Brown TR, Mack RE: Studies on the mechanism of inhibition of thyroid function by lithium. Biochim Biophys Acta 1978; 542:163-169Crossref, Medline, Google Scholar
29. Kleiner J, Altshuler L, Hendrick V, Hershman JM: Lithium-induced subclinical hypothyroidism: review of the literature and guidelines for treatment. J Clin Psychiatry 1999; 60:249-255Crossref, Medline, Google Scholar
30. Mori M, Tajima K, Oda Y, Matsui I, Mashita K, Tarui S: Inhibitory effect of lithium on the release of thyroid hormones from thyrotropin-stimulated mouse thyroids in a perifusion system. Endocrinology 1989; 124:1365-1369Crossref, Medline, Google Scholar
31. Spaulding SW, Burrow GN, Bermudez F, Himmelhoch JM: The inhibitory effect of lithium on thyroid hormone release in both euthyroid and thyrotoxic patients. J Clin Endocrinol Metab 1972; 35:905-911Crossref, Medline, Google Scholar
32. Urabe M, Hershman JM, Pang XP, Murakami S, Sugawara M: Effect of lithium on function and growth of thyroid cells in vitro. Endocrinology 1991; 129:807-814Crossref, Medline, Google Scholar
33. Emerson CH, Dysno WL, Utiger RD: Serum thyrotropin and thyroxine concentrations in patients receiving lithium carbonate. J Clin Endocrinol Metab 1973; 36:338-346Crossref, Medline, Google Scholar
34. Fyro B, Petterson U, Sedvall G: Time course for the effect of lithium on thyroid function in men and women. Acta Psychiatr Scand 1973; 49:230-236Crossref, Medline, Google Scholar
35. Myers DH, Carter RA, Burns BH, Armond A, Hussain SB, Chengapa VK: A prospective study of the effects of lithium on thyroid function and on the prevalence of antithyroid antibodies. Psychol Med 1985; 15:55-61Crossref, Medline, Google Scholar
36. Smigan L, Wahlin A, Jacobsson L, von Knorring L: Lithium therapy and thyroid function tests: a prospective study. Neuropsychobiology 1984; 11:39-43Crossref, Medline, Google Scholar
37. Sofuoglu S, Tutus A, Gonul AS, Basturk M, Kose K, Esel E: Thyroidal hormone profile in bipolar patients who were in short and long-term lithium treatment (abstract). Eur Neuropsychopharmacol 1997; 7(suppl 2):S194Google Scholar
38. Whybrow PC, Prange AJ Jr: A hypothesis of thyroid-catecholamine-receptor interaction: its relevance to affective illness. Arch Gen Psychiatry 1981; 38:106-113Crossref, Medline, Google Scholar
39. Bauer MS, Whybrow PC: Thyroid hormones and the central nervous system in affective illness: interactions that may have clinical significance. Integrative Psychiatry 1988; 6:75-100Google Scholar
40. Haggerty JJ Jr, Prange AJ Jr: Borderline hypothyroidism and depression. Annu Rev Med 1995; 46:37-46Crossref, Medline, Google Scholar
41. Dratman MB: Cerebral versus peripheral regulation and utilization of thyroid hormones, in The Thyroid Axis and Psychiatric Illness. Edited by Joffe RT, Levitt AJ. Washington, DC, American Psychiatric Press, 1993, pp 3-94Google Scholar
42. Kraus RP, Phoenix E, Edmonds MW, Nicholson IR, Chandarana P, Tokmakejian S: Exaggerated TSH response to TRH in depressed patients with “normal” baseline TSH. J Clin Psychiatry 1997; 58:266-270Crossref, Medline, Google Scholar
43. Joffe RT, Roy-Byrne PP, Uhde TW, Post RM: Thyroid function and affective illness: a reappraisal. Biol Psychiatry 1984; 19:1685-1691Medline, Google Scholar
44. Prange AJ Jr, Wilson IC, Rabon AM, Lipton MA: Enhancement of imipramine antidepressant activity by thyroid hormone. Am J Psychiatry 1969; 126:457-469Link, Google Scholar
45. Aronson R, Offman HJ, Joffe RT, Naylor CD: Triiodothyronine augmentation in the treatment of refractory depression: a meta-analysis. Arch Gen Psychiatry 1996; 53:842-848Crossref, Medline, Google Scholar
46. Stern RA, Nevels CT, Shelhorse ME, Prohaska ML, Mason GA, Prange AJ Jr: Antidepressant and memory effects of combined thyroid hormone treatment and electroconvulsive therapy: preliminary findings. Biol Psychiatry 1991; 30:623-627Crossref, Medline, Google Scholar
47. Stancer HC, Persad E: Treatment of intractable rapid-cycling manic-depressive disorder with levothyroxine: clinical observations. Arch Gen Psychiatry 1982; 39:311-312Crossref, Medline, Google Scholar
48. Bauer MS, Whybrow PC, Winokur A: Rapid cycling bipolar affective disorder, I: association with grade I hypothyroidism. Arch Gen Psychiatry 1990; 47:427-432Crossref, Medline, Google Scholar
49. Thase ME, Kupfer DJ, Jarrett DB: Treatment of imipramine-resistant recurrent depression, I: an open clinical trial of adjunctive l-triiodothyronine. J Clin Psychiatry 1989; 50:385-388Medline, Google Scholar
50. Gorman JM, Hatterer JA: The role of thyroid hormone in refractory depression, in Refractory Depression: Current Strategies and Future Directions. Edited by Nolen WA, Zohar J, Roose SP, Amsterdam JD. New York, John Wiley & Sons, 1994, pp 121-128Google Scholar
51. Nierenberg AA, White K: What next? a review of pharmacologic strategies for treatment resistant depression. Psychopharmacol Bull 1990; 26:429-460Medline, Google Scholar
52. Sachs GS: Treatment-resistant bipolar depression. Psychiatr Clin North Am 1996; 19:215-236Crossref, Medline, Google Scholar



