Association Between Lower Serum Free T4 and Greater Mood Instability and Depression in Lithium-Maintained Bipolar Patients
Abstract
OBJECTIVE: This investigation evaluated the relationship between changes in thyroid indices and mood stability during lithium and carbamazepine prophylaxis for bipolar disorder. METHOD: In the first 2 years, 30 patients with bipolar mood disorder were randomly assigned to 1 year of lithium and then 1 year of carbamazepine, or vice versa; in the third year, they received lithium plus carbamazepine. By stepwise regression analysis, the degree and timing of lithium- and carbamazepine-induced thyroid changes and their subsequent relationship to long-term mood stability were evaluated. RESULTS: During the lithium phase, there was a significant inverse relationship between morbidity and mean serum level of free T4, i.e., a lower mean serum level of free T4 was associated with more affective episodes and greater severity of depression as shown by the Beck Depression Inventory. During the carbamazepine phase, there was an inverse relationship between mean level of total T4 and global severity rating. During the combination phase, no relationships between thyroid indices and clinical outcome were significant. CONCLUSIONS: In the lithium phase, a low level of free T4 was associated with more affective episodes and greater severity of depression. Whether this mood instability is causally related to low free T4 levels and whether it can be attenuated with T4 replacement remain to be studied in a controlled setting.
The clinical literature on thyroid physiology in mood disorders is rich (1–3). The examination of specific iodothyronines has occurred primarily at three time points in the course of illness: 1) acute major depression, 2) recovery from an index episode, and 3) longer-term prophylaxis or relapse prevention.
Prospective studies of thyroid changes specifically associated with commencement of lithium treatment have most notably documented decreases in serum T3, T4, or protein-bound iodine 2 weeks to 6 months after commencement of treatment and normalization of these iodothyronines 3–12 months posttreatment (4–9). Commensurate with these relative decreases, thyroid-stimulating hormone (TSH) was noted to increase 10 days to 6 months after treatment onset (5, 8, 9–10) and generally remain elevated, although Maarbjerg et al. (9) reported TSH normalization after 12 months. However, the potential relationship between these lithium-induced thyroid changes and prospective mood changes has not been well studied.
In a prospective 2-year study, Kusalic and Engelsmann (11) did not find a baseline thyroid value or acute lithium-induced thyroid change that was predictive of a response to lithium prophylaxis. As well, in a prospective study of lithium withdrawal from euthymic bipolar patients, the elevated TSH and decreased free T4 levels were shown to resolve approximately 3 weeks after lithium discontinuation with no evidence of mood deterioration (12).
Longer-term evaluation of thyroid indices in lithium-maintained mood disorder patients has shown an association between decreased serum T3 levels and relapse (13, 14). Although not statistically significant, findings in a third study by Kirkegaard et al. (15) indicated that the only difference in thyroid measures between patients who experienced relapse within 6 months of antidepressant treatment and those who did not was a lower serum level of T3.
In this investigation we further evaluated the relationship between changes in thyroid indices and mood during lithium and carbamazepine prophylaxis.
METHOD
Fifty-two outpatients with bipolar disorder participated in a randomized, double-blind study comparing the efficacy of 1 year of lithium and 1 year of carbamazepine in the first 2 years and a lithium-carbamazepine combination in the third year (16). Written and oral informed consent were obtained for this study as approved by the NIMH Institutional Review Board. For each treatment phase, the prospective life-chart methodology, which has been shown to be reliable and valid (17), was used to quantify illness variables and was the primary instrument for rating overall prophylactic response by the Clinical Global Impression Scale for Bipolar Illness (CGI) (18). The overall rates of CGI-defined response (moderate or marked improvement) were 33% for lithium, 31% for carbamazepine, and 55% for the combination. Study design, secondary mood rating instruments, and clinical outcome have been described further by Denicoff et al. (16).
The present study was an evaluation of prospective data on thyroid function—TSH, free T4, total T4, T3, thyroxine-binding globulin—obtained during clinical follow-up. Patients receiving thyroid supplementation before randomization (N=13) were not included in the analysis. The time until development of de novo hypothyroidism (grade I or II) was calculated, but patients who were receiving lithium before random assignment to blinded lithium treatment (N=3) and patients who became hypothyroid during the third phase and had received lithium in the second phase (N=2) were not included in the analysis. The final group sizes for the three treatment phases were as follows: lithium, N=30; carbamazepine, N=22; and combination, N=19. Of the euthyroid patients evaluated for this study, 12 were taking lithium and one was taking lithium plus carbamazepine before the study.
During the study, adjunctive treatment with an antidepressant and/or an antipsychotic or benzodiazepine was used as clinically indicated. All affective episodes in patients for whom adjunctive treatment was instituted met the DSM-III-R criteria for major depression or mania. The mean percentage of time in each treatment phase during which antidepressants were given was 16.27% (SD=25.32) for lithium monotherapy, 10.46% (SD=24.83) for carbamazepine monotherapy, and 10.09% (SD=21.46) for the lithium-carbamazepine combination. The mean percentage of time in each treatment phase during which an antipsychotic or benzodiazepine was given was 9.05% (SD=21.65) for lithium monotherapy, 14.99% (SD=15.78) for carbamazepine monotherapy, and 9.70% (SD=20.93) for the lithium-carbamazepine combination. According to analysis of variance (ANOVA), the percentage of time during which either antidepressant or antipsychotic/benzodiazepine treatment was received did not differ among the three treatment phases (F=0.87, df=2, 32, p=0.41, N=17).
The mean numbers of depressive, manic, and total affective episodes in each treatment phase were as follows—lithium monotherapy: 1.76 depressive (SD=2.21), 3.58 manic (SD=3.08), and 5.28 total (SD=3.78) episodes; carbamazepine monotherapy: 1.95 depressive (SD=3.53), 4.50 manic (SD=3.79), and 6.45 total (SD=6.61) episodes; lithium-carbamazepine combination: 1.13 depressive (SD=1.60), 2.96 manic (SD=3.15), and 4.09 total episodes (SD=3.91). ANOVAs indicated no significant difference among the three treatment phases in the number of depressive (F=1.40, df=2, 32, p=0.26, N=17), manic (F=1.35, df=2, 32, p=0.27, N=17), or total episodes (F=2.06, df=2, 32, p=0.15, N=17). During the 3-year study period 13 hospitalizations occurred for mania or depression (eight during the lithium phase, two during the carbamazepine phase, and three during combination treatment).
Serum TSH, free T4, T3, and thyroxine-binding globulin were measured at each monthly visit. The assays for TSH and thyroxine-binding globulin were conducted by means of a chemiluminescent immunoassay. The free T4 assay was conducted by radioimmunoassay. The T3 and total T4 assays were conducted by microparticle enzyme immunoassay.
The McNemar chi-square test was used for evaluating the de novo incidence of thyroid abnormality according to treatment phase. Frequency of thyroid abnormality according to gender, cycling, and response status was evaluated with Fisher’s exact test. At the time of de novo subclinical hypothyroidism, the Pearson correlation was used to assess the relationship between TSH and mood ratings. Repeated measures ANOVA was used to assess differences in mean thyroid hormone levels between treatment groups. Survival curve analysis and correlations between thyroid indices at the time of recovery from an episode (defined as euthymia by the life-chart methodology) and length of time to the beginning of the next episode during the lithium phase were evaluated by using the nonparametric Spearman correlation coefficient.
We examined the relationship between illness variables and thyroid indices. Ten potentially relevant illness measures were identified through analysis of the clinician-determined life chart and dealt with cycling frequency (i.e., total number of episodes per year, manic episodes per year, depressive episodes per year, and switches per year), duration of illness (i.e., total time ill, percentage of time manic, percentage of time depressed), and severity of illness (i.e., total average severity, mania severity, and depression severity according to the life chart). In order to reduce the number of predictors relative to the number of subjects, the relationships among illness variables were assessed by Pearson correlation. A correlation value of r>0.6 was used to group the variables that were highly interrelated, leaving three illness variables with no significant relationship to each other: total number of episodes per year, average mania severity, and average depression severity. An exploratory stepwise multiple regression analysis was then performed to evaluate the relationships between these three illness variables and the mean thyroid indices during each treatment phase. A similar exploratory stepwise multiple regression analysis was used in each treatment phase to evaluate the relationships of the mean thyroid indices and mean mood ratings on the Hamilton Rating Scale for Depression (19), Beck Depression Inventory (20), Young Mania Rating Scale (21), Spielberger State-Trait Anxiety Inventory (22), and Global Assessment Scale (GAS) (23).
To control for the effects of antidepressants and antipsychotics or benzodiazepines on thyroid hormone level, we performed a second set of regression analyses, forcing the percentage of time with antidepressant or antipsychotic/benzodiazepine treatment into the equation at the first step. The mean thyroid hormone levels served as dependent variables, while the illness variables and mood ratings functioned as independent variables. Similar results were obtained from the first and second stepwise multiple regressions, and only the latter (i.e., with antidepressant and antipsychotic/benzodiazepine treatment controlled) are reported. Unstandardized beta weights are reported.
RESULTS
As presented in table 1, the incidence of de novo hypothyroidism (grade I or II) differed significantly between lithium and carbamazepine monotherapy. At the time of the first elevated TSH level, TSH significantly correlated with the anxiety rating on the Spielberger State-Trait Anxiety Inventory (r=0.59, N=15, p<0.05) but not with the score on the Hamilton depression scale (r=0.49, N=15, p<0.10). There was no difference by gender, cycling status, or CGI response/nonresponse in de novo hypothyroid state (table 1). The latency to TSH elevation was 56.5 days (SD=47) for lithium (mean dose=1247 mg/day, SD=262) and 88.0 days (SD=21) for the combination (mean lithium dose=1179 mg/day, SD=103; mean carbamazepine dose=614 mg/day, SD=186).
The levels of the thyroid measures are presented in table 2. According to repeated measures ANOVA, the mean levels of TSH and thyroxine-binding globulin were significantly lower in the carbamazepine phase than in the lithium phase, and the levels during combination treatment were intermediate between those with carbamazepine only and lithium only. Total T4, free T4, and T3 all showed lower mean levels with carbamazepine than with lithium and the lowest levels during treatment with the lithium-carbamazepine combination. During lithium monotherapy the mean serum drug level was 0.83 mmol/liter (SD=0.13), during carbamazepine monotherapy the mean drug level was 7.99 mg/liter (SD=1.36), and during combination treatment the mean level of lithium was 0.82 mmol/liter (SD=0.17) and the mean level of carbamazepine was 7.91 mg/liter (SD=1.21). The lithium and carbamazepine doses and blood levels did not correlate with the mean thyroid values during any treatment phase. In addition, there was no relationship, according to either survival curve analysis or correlation, between any mean thyroid value just after an episode (i.e., recovery) and length of time to the beginning of the next episode (i.e., relapse).
Stepwise multiple regression analysis during the lithium phase revealed a highly significant relationship between the number of episodes per year and the mean serum level of free T4, i.e., a lower serum level of free T4 was associated with more affective episodes (Figure 1). The next order of entry showed a significant positive relationship between mean level of free T4 and severity of mania, as measured by the life-chart methodology (R2=0.40, beta=0.35, t=2.76, df=4, 25, p<0.05). A separate regression showed a significant inverse relationship between mean free T4 and severity of depression as measured by the Beck inventory, i.e., a lower mean free T4 level was associated with a higher self-rating of depression (Figure 1). No other clinical measures were entered as significant in the stepwise regression analyses for the other thyroid indices (i.e., T3, TSH, thyroxine-binding globulin, and total T4).
Stepwise multiple regression analysis during the carbamazepine phase revealed an inverse relationship between mean total T4 and severity of illness as indicated by the GAS, i.e., a higher T4 level was associated with a lower GAS score (R2=0.29, beta=0.05, t=–2.50, df=3, 18, p=0.02). No relationships between any other thyroid indices and measures of clinical outcome were identified as significant for the combination phase.
DISCUSSION
This longitudinal study suggests a time-dependent development of lithium-induced hypothyroidism (grade I or II) of approximately 2 months. As in previous studies (24, 25), the combination of lithium and carbamazepine treatment in this study provided some degree of protection against subclinical hypothyroidism, in terms of both the percentage incidence and the time until development of a high TSH level. Although a high TSH level did not predict treatment response, it did correlate with anxiety severity, consistent with a previous finding of a greater prevalence of concurrent panic disorder and relative antidepressant inefficacy in subclinically hypothyroid depressed patients than in euthyroid depressed patients (26).
The high TSH level, and not the serum lithium level, has been shown to significantly correlate with verbal learning and memory deficits in euthymic lithium-maintained bipolar patients with subclinical hypothyroidism (27). Furthermore, recent data (28) suggest an inverse correlation between peripheral TSH and absolute global cerebral blood flow and glucose metabolic rate in patients with depression. Although the treatment of subclinical hypothyroidism remains controversial, as Kleiner et al. discuss in their comprehensive review (29), the anxiety, cognitive deficits, and relative perfusion and metabolic decreases associated with a high TSH level would certainly support the clinical utility of treating lithium-induced subclinical hypothyroid states.
During lithium prophylaxis, a lower mean level of serum free T4 was associated with mood instability (i.e., greater number of affective episodes) and more severe depression as shown by the Beck inventory, suggesting that greater lithium-related decrements in free T4, although within the normal range, appear to be detrimental to mood prophylaxis for bipolar patients. This study is limited by the exploratory aspect of multiple regression models, and there is a chance that these observations are false positive results. Nonetheless, these findings are partially consistent with those from the longitudinal studies of Baumgartner et al. (13) and Hatterer et al. (14), who found an association between decreased iodothyronine and mood instability, but the iodothyronine was T3, not T4, as we report on here. The positive relationship between mean free T4 and severity of mania, as measured by the life-chart method, suggests a bidirectional relationship of this thyroid index to mood, i.e., a low T4 level is associated with depression and more episodes, and a high free T4 level is associated with mania.
In the context of clinical management of lithium-maintained bipolar patients, these data suggest that in addition to the monitoring of serum TSH, as much attention, or more, should be paid to the measurement of free T4. Although the mean free T4 level was within normal limits, the finding of greater mood stability (i.e., less cycling and subjective depression) with a higher mean free T4 level bears a striking resemblance to previous clinical reports of hypermetabolic T4 for the treatment of rapid-cycling bipolar illness (30–32) and non-rapid-cycling refractory depression (33) and, in general, the association of therapeutic benefit (i.e., acute antidepressant response and prophylaxis) with higher nonhypermetabolic circulating levels of T4 for some patients (34). Transient hyperthyroxinemia associated with discontinuation of chronic lithium treatment has been reported (35), and its relationship to mood improvement has been noted in some clinical observations (P.K. Gillman, 1996 unpublished findings), but not others (12), and remains to be further studied.
All of these observations over long periods (i.e., greater than 6 months) stand in direct contrast to the large body of literature, albeit limited by different study designs and diagnostic heterogeneity, suggesting that acute iodothyronine increases are associated with acute major depression and that decreases are associated with recovery from an index episode (1–3). Furthermore, the acute increase in thyroid indices, primarily T4, has been reported to be greater in patients who ultimately respond to antidepressant treatment (36) or T3 augmentation (37), and the relative decrease in thyroid indices, primarily T4 but also T3, has been greater in patients who respond to short-term therapy with various somatic treatments (1–3, 38–40).
Conceptually, it is possible that the iodothyronine decrements associated with recovery from an affective episode at some point pose some risk to long-term stability (i.e., relapse). There are reports of induction of the thyroid deiodinase enzyme by psychotropic agents such as lithium and carbamazepine (41), which may contribute to the differential assessment of thyroid indices across the longitudinal course of illness. However, this present study, in addition to a study by Baumgartner et al. (42), did not show a correlation between any thyroid value just after an episode (i.e., recovery, presumably with lower levels of T4 and T3) and length of time to the beginning of the next episode (i.e., relapse). Clearly, the neurobiology associated with this differential iodothyronine economy and the course of affective illness remains to be further explored.
The bipolar pharmacopoeia has fortunately increased to include carbamazepine and valproate. Although not as robust as the lithium data, the inverse relationship between GAS rating and total T4 during carbamazepine prophylaxis again stands in contrast to the acute thyroid decreases associated with the acute carbamazepine response (38). Furthermore, in contrast to lithium, carbamazepine has been shown to decrease circulating iodothyronines without increasing TSH (i.e., hypothyroidism) or changing the resting metabolic rate (43). This T4-GAS relationship is not consistent with carbamazepine’s induction of cytochrome p450 3A4 (44) and 5′ type II deiodinase enzyme (42), which would tend to decrease circulating T4 levels (45). Some (45), but not all (46), epilepsy studies do show decreases in circulating iodothyronines with acute treatment with valproate; to our knowledge, this association has not been assessed in relation to mood stability in bipolar patients.
Further exploration of the association between thyroid function and affective illness based on the episode presentation (i.e., acute phase, after acute or episode recovery, and long-term prophylaxis) and clarification of the transitional period from acute recovery (relative thyroid decreases) to greater prophylactic stability (relative thyroid increases) for lithium and for anticonvulsant mood stabilizers are encouraged. Whether the mood instability associated with lower free T4 levels is causally related and whether it can be attenuated with T4 replacement remain to be studied in a controlled setting.
Presented in part at the 2nd International Conference on Bipolar Disorder, June 19–21, 1997, Pittsburgh. Received Oct. 29, 1998; revision received April 27, 1999; accepted June 4, 1999. From the Biological Psychiatry Branch, NIMH. Address reprint requests to Dr. Post, Biological Psychiatry Branch, NIMH, Room 3N 212, Bldg. 10, NIH, Bethesda, MD 20892. Supported in part by the Ted and Vada Stanley Foundation. The authors thank Russell T. Joffe, M.D., who reviewed an earlier version of the manuscript.
 |
 |
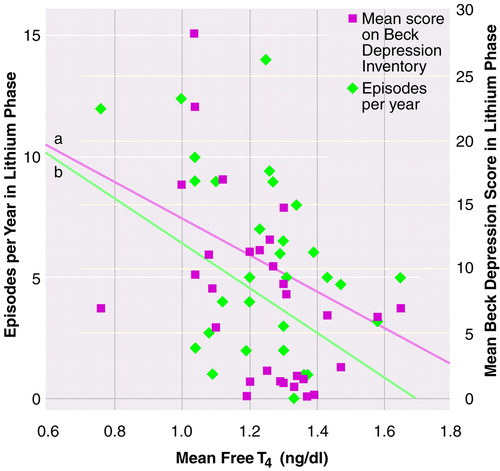
FIGURE 1. Association of Mean Free T4 Level With Affective Episodes per Yeara and Depression Ratingsb for 30 Patients With Bipolar Disorder During Lithium Phase of Lithium-Carbamazepine Study
aSignificant association between free T4 and number of affective episodes per year, according to multiple stepwise regression controlling for antidepressant and neuroleptic use (R2=0.40, beta=–0.02, t=–3.37, df=4, 25, p<0.01).
bSignificant association between free T4 and depression score, according to multiple stepwise regression controlling for antidepressant and neuroleptic use (R2=0.29, beta=–0.01, t=–2.84, df=3, 26, p<0.01).
1. Kirkegaard C, Faber J: The role of thyroid hormones in depression. Eur J Endocrinol 1998; 138:1–9Crossref, Medline, Google Scholar
2. Joffe RT, Sokolov STH: Thyroid hormones, the brain, and affective disorders. Crit Rev Neurobiol 1994; 8:45–63Medline, Google Scholar
3. Bauer MS, Whybrow PC: Thyroid hormones and the central nervous system in affective illness: interactions that may have clinical significance. Integrative Psychiatry 1988; 6:75–85Google Scholar
4. Villeneuve A, Gautier J, Jus A, Perron D: The effect of lithium on thyroid in man. Int J Clin Pharmacol 1974; 9:75–80Medline, Google Scholar
5. Emerson CH, Dyson WL, Utiger RD: Serum thyrotropin and thyroxine concentrations in patients receiving lithium carbonate. J Clin Endocrinol Metab 1973; 36:338–346Crossref, Medline, Google Scholar
6. Myers DH, Carter RA, Burns BH, Armond A, Hussain SB, Chengapa VK: A prospective study of the effects of lithium on thyroid function and on the prevalence of antithyroid antibodies. Psychol Med 1985; 15:55–61Crossref, Medline, Google Scholar
7. Fyrö B, Petterson U, Sedvall G: Time course for the effect of lithium on thyroid function in men and women. Acta Psychiatr Scand 1973; 49:230–236Crossref, Medline, Google Scholar
8. Smigan L, Wahlin A, Jacobsson L, von Knorring L: Lithium therapy and thyroid function tests: a prospective study. Neuropsychobiology 1984; 11:39–43Crossref, Medline, Google Scholar
9. Sofuoglu S, Tutus A, Gönül AS, Bastünrk M, Köse K, Esel E: Thyroidal hormone profile in bipolar patients who were in short- and long-term lithium treatment. Eur Neuropsychopharmacol 1997; 7(suppl 2):S140–S141Google Scholar
10. Lombardi G, Panza N, Biondi B, DiLorenzo L, Lupoli G, Muscettola G, Carella C, Bellastella A: Effect of lithium treatment on hypothalamic-pituitary-thyroid axis: a longitudinal study. J Endocrinol Invest 1993; 16:259–263Crossref, Medline, Google Scholar
11. Kusalic M, Engelsmann F: Predictors of lithium treatment responsiveness in bipolar patients: a two year prospective study. Neuropsychobiology 1998; 37:146–149Crossref, Medline, Google Scholar
12. Gönül AS, Tutus A, Sofuoglu S, Esel E, Köse K, Aslan SS, Bastünrk M: Effects of lithium treatment on thyroid functions in remitted bipolar patients (abstract). Eur Neuropsychopharmacol 1996; 6(suppl):P12Google Scholar
13. Baumgartner A, von Stuckrad M, Müller-Oerlinghausen B, Gräf KJ, Kürten I: The hypothalamic-pituitary-thyroid axis in patients maintained on lithium prophylaxis for years: high triiodothyronine serum concentrations are correlated to prophylactic efficacy. J Affect Disord 1995; 34:211–218Crossref, Medline, Google Scholar
14. Hatterer JA, Kocsis JH, Stokes PE: Thyroid function in patients maintained on lithium. Psychiatry Res 1988; 26:249–257Crossref, Medline, Google Scholar
15. Kirkegaard C, Norlem N, Lauridsen UB, Bjorum N, Christiansen C: Prognostic value of thyrotropin releasing hormone stimulation test in endogenous depression. Acta Psychiatr Scand 1975; 52:170–177Crossref, Medline, Google Scholar
16. Denicoff KD, Smith-Jackson EE, Disney ER, Ali SO, Leverich GS, Post RM: Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997; 58:470–478Crossref, Medline, Google Scholar
17. Denicoff KD, Smith-Jackson EE, Disney ER, Suddath RL, Leverich GS, Post RM: Preliminary evidence of the reliability and validity of the prospective life-chart methodology (LCM-p). J Psychiatry Res 1997; 5:593–603Crossref, Google Scholar
18. Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W: Modification of the Clinical Global Impression (CGI) scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res 1997; 73:159–171Crossref, Medline, Google Scholar
19. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
20. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
21. Young RC, Biggs JT, Ziegler VE, Meyer DA: A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
22. Spielberger CD, Gorsuch RL, Lushene RD: STAI Manual. Palo Alto, Calif, Consulting Psychologists Press, 1970Google Scholar
23. Endicott J, Spitzer RL, Fleiss JL, Cohen J: The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976; 33:766–771Crossref, Medline, Google Scholar
24. Kramlinger KG, Post RM: Addition of lithium carbonate to carbamazepine: hematological and thyroid effects. Am J Psychiatry 1990; 147:615–620Link, Google Scholar
25. Bocchetta A, Cherchi A, Loviselli A, Mossa P, Velluzzi F, Derai R, DelZompo M: Six year follow up of thyroid function during lithium treatment. Acta Psychiatr Scand 1996; 94:45–48Crossref, Medline, Google Scholar
26. Joffe RT, Levitt AJ: Major depression and subclinical (grade 2) hypothyroidism. Psychoneuroendocrinology 1992; 17:215–221Crossref, Medline, Google Scholar
27. Prohaska ML, Stern RA, Nevels CT, Mason GA, Prange AJ: The relationship between thyroid status and neuropsychological performance in psychiatric outpatients maintained on lithium. Neuropsychiatry Neuropsychol Behav Neurol 1996; 9:30–34Google Scholar
28. Marangell LB, Ketter TA, George MS, Pazzaglia PJ, Callahan AM, Parekh P, Andreason PJ, Horowitz B, Herscovitch P, Post RM: Inverse relationship of peripheral thyrotropin-stimulating hormone levels to brain activity in mood disorders. Am J Psychiatry 1997; 154:224–230Link, Google Scholar
29. Kleiner J, Altshuler LL, Hendrik V, Hershman JM: Lithium-induced subclinical hypothyroidism: review of the literature and guidelines for treatment. J Clin Psychiatry 1999; 60:249–255Crossref, Medline, Google Scholar
30. Bauer MS, Whybrow PC: Treatment of refractory rapid cycling with high dose levothyroxine: a preliminary study. Arch Gen Psychiatry 1990; 47:435–440Crossref, Medline, Google Scholar
31. Stancer HC, Persad E: Treatment of intractable rapid-cycling manic-depressive disorder with levothyroxine: clinical observations. Arch Gen Psychiatry 1982; 39:311–312Crossref, Medline, Google Scholar
32. Leibow D: l-Thyroxine for rapid-cycling bipolar illness (letter). Am J Psychiatry 1983; 140:1255Medline, Google Scholar
33. Bauer M, Hellweg R, Gräf KJ, Baumgartner A: Treatment of refractory depression with high dose thyroxine. Neuropsychopharmacology 1998; 18:444–455Crossref, Medline, Google Scholar
34. Friend KD, Alter CL: T4 therapy in depression and hypothyroidism. Depression 1995; 2:278–280Crossref, Google Scholar
35. Stratakis CA, Chrousos GP: Transient elevation of serum thyroid hormone levels following lithium discontinuation. Eur J Pediatr 1996; 155:939–941Crossref, Medline, Google Scholar
36. Rao ML, Ruhrmann S, Retey B, Liappis N, Fuger J, Kraemer M, Kasper S, Moller HJ: Low plasma thyroid indices of depressed patients are attenuated by antidepressant drugs and influence treatment outcome. Pharmacopsychiatry 1996; 29:180–186Crossref, Medline, Google Scholar
37. Sokolov STH, Levitt AJ, Joffe RT: Thyroid hormone levels before unsuccessful antidepressant therapy are associated with later response to T3 augmentation. Psychiatry Res 1997; 69:203–206Crossref, Medline, Google Scholar
38. Roy-Byrne PP, Joffe RT, Uhde TW, Post RM: Carbamazepine and thyroid function in affectively ill patients: clinical and theoretical implications. Arch Gen Psychiatry 1984; 41:1150–1153Google Scholar
39. Shelton RC, Win S, Ekhatore N, Loosen PT: The effects of antidepressants on the thyroid axis in depression. Biol Psychiatry 1993; 33:120–126Crossref, Medline, Google Scholar
40. Joffe R, Segal Z, Singer W: Change in thyroid hormone levels following response to cognitive therapy for major depression. Am J Psychiatry 1996; 153:411–413Link, Google Scholar
41. Baumgartner A, Pinna G, Hiedra L, Gaio U, Hessenius C, Campos-Barros A, Eravci M, Prengel H, Thoma R, Meinhold H: Effects of lithium and carbamazepine on thyroid hormone metabolism in rat brain. Neuropsychopharmacology 1997; 16:25–41Crossref, Medline, Google Scholar
42. Baumgartner A, Gräf KJ, Kürten I, Meinhold H: The hypothalamic-pituitary-thyroid axis in psychiatric patients and healthy subjects: parts 1–4. Psychiatry Res 1988; 24:271–332Crossref, Medline, Google Scholar
43. Herman R, Obarzanek E, Mikalauskas KM, Post RM, Jimerson DC: The effects of carbamazepine on resting metabolic rate and thyroid function in depressed patients. Biol Psychiatry 1991; 29:779–788Crossref, Medline, Google Scholar
44. Ketter TA, Flockhart DA, Post RM, Denicoff KD, Pazzaglia PJ, Marangell LB, George MS, Callahan AM: The emerging role of cytochrome P450 3A in psychopharmacology. J Clin Psychopharmacol 1995; 15:387–398Crossref, Medline, Google Scholar
45. Bentsen KD, Gram L, Veje A: Serum thyroid hormones and blood folic acid during monotherapy with carbamazepine or valproate. Acta Neurol Scand 1983; 67:235–241Crossref, Medline, Google Scholar
46. Larkin JG, Macphee GJA, Beastall GH, Brodie MJ: Thyroid hormone concentrations in epileptic patients. Eur J Clin Pharmacol 1989; 36:213–216Crossref, Medline, Google Scholar



