Methamphetamine-Related Psychiatric Symptoms and Reduced Brain Dopamine Transporters Studied With PET
Abstract
OBJECTIVE: A positron emission tomography (PET) study has suggested that dopamine transporter density of the caudate/putamen is reduced in methamphetamine users. The authors measured nucleus accumbens and prefrontal cortex density, in addition to caudate/putamen density, in methamphetamine users and assessed the relation of these measures to the subjects’ clinical characteristics. METHOD: PET and 2-β-carbomethoxy-3β-(4-[11C] fluorophenyl)tropane, a dopamine transporter ligand, were used to measure dopamine transporter density in 11 male methamphetamine users and nine male comparison subjects who did not use methamphetamine. Psychiatric symptoms in methamphetamine users were evaluated by using the Brief Psychiatric Rating Scale and applying a craving score. RESULTS: The dopamine transporter density in all three of the regions observed was significantly lower in the methamphetamine users than the comparison subjects. The severity of psychiatric symptoms was significantly correlated with the duration of methamphetamine use. The dopamine transporter reduction in the caudate/putamen and nucleus accumbens was significantly associated with the duration of methamphetamine use and closely related to the severity of persistent psychiatric symptoms. CONCLUSIONS: These findings suggest that longer use of methamphetamine may cause more severe psychiatric symptoms and greater reduction of dopamine transporter density in the brain. They also show that the dopamine transporter reduction may be long-lasting, even if methamphetamine use ceases. Further, persistent psychiatric symptoms in methamphetamine users, including psychotic symptoms, may be attributable to the reduction of dopamine transporter density.
Over the past six decades, epidemics of methamphetamine abuse have occurred worldwide (1–4). Chronic methamphetamine users show psychiatric signs, including a psychotic state and an anxiety-disorder-like state, under conditions of intoxication, withdrawal, or both. These psychiatric states are sometimes prolonged as residual symptoms, easily exacerbated by methamphetamine reuse or by psychological stress in some chronic users (3, 5–8). The prolongation of psychosis and susceptibility to relapse in chronic methamphetamine users have often been regarded as manifestations of preexistent schizophrenia (9), although differences in symptoms and morbid risk between subjects with schizophrenia and methamphetamine users with psychosis have also been demonstrated (3).
There are three major dopaminergic systems, the nigrostriatal, mesolimbic, and mesocortical systems, which are related to coordinated movements and mentation (10, 11). Given acutely, methamphetamine increases dopamine levels in the synaptic cleft, mainly by inhibiting action of the dopamine transporter (12, 13). Postmortem study has shown that chronic methamphetamine use results in reduction of dopamine transporter density in the caudate/putamen and nucleus accumbens (14). Positron emission tomography (PET) study has also shown the reduction of dopamine transporter density in the caudate/putamen of chronic methamphetamine users (15), although a history of polydrug use might exert some influence on the PET results.
In this study, we selected subjects who abused only methamphetamine (i.e., monodrug users) and investigated the dopamine transporter density of the brain during a period of abstinence from methamphetamine. We used PET and 2-β-carbomethoxy-3β-(4-[11C] fluorophenyl)tropane ([11C]WIN-35,428), which is a cocaine analogue with high specificity to dopamine transporter (16). In addition to the caudate/putamen and nucleus accumbens, we investigated the prefrontal cortex, which receives terminals of the mesocortical dopaminergic system. Further, we examined the relationship between the dopamine transporter density and clinical characteristics, including psychotic symptoms and craving for methamphetamine, in methamphetamine users.
Method
Subjects
Nine healthy comparison subjects who did not use methamphetamine and 11 methamphetamine users were recruited for this study. All participants were men who showed no signs of parkinsonism or other neurological abnormality. In addition, none of the subjects had a history of seizures, dyskinesia, or coma, which may be observed during intoxication from high-dose methamphetamine. Use of nicotine, alcohol, and other drugs was screened by using the nonpatient version of the Structured Clinical Interview for DSM-IV (SCID) (17). All of the comparison subjects and methamphetamine users showed similar habits of occasional smoking and drinking; no subjects met either the nicotine- or the alcohol-related criteria for abuse. The comparison subjects had no experience of use of any other drugs, and none of the methamphetamine users had used any drugs other than methamphetamine.
The comparison subjects had no history of psychiatric disorders, including substance use disorders, as determined by the SCID (17). Both the comparison subjects and the methamphetamine users were selected so as to be similar in age (mean=26.9 years, SD=4.5, for comparison subjects; mean=27.4 years, SD=5.6, for methamphetamine users) and education levels (mean=11.8 years, SD=0.7, for comparison subjects; mean=11.5 years, SD=1.2, for methamphetamine users). The methamphetamine users were referred from Hamamatsu University Hospital, Seimei Hospital, or Hattori Mental Hospital in Japan.
In order to increase the accuracy of the patient profiles, detailed information on methamphetamine use and history of psychiatric symptoms of methamphetamine users was obtained through interviews with the user and his family members as well as by referral to patient medical records. Seven of the users had taken methamphetamine by intravenous injection, and the other four subjects had smoked it (Table 1). The period of methamphetamine use was designated as the duration between initial use and last use. If there were intervals of abstinence longer than 1 month during the duration of methamphetamine use as defined, these intervals were subtracted from the total duration value. The period of abstinence from methamphetamine use was arbitrarily designated as the duration between the day of last use of methamphetamine and the PET examination. Absence of recent use of methamphetamine was ascertained by urinary drug screening tests.
The Brief Psychiatric Rating Scale (BPRS) (18) was performed by a trained research psychiatrist (Y.S.) blind to the PET data. The positive symptom subscale (conceptual disorganization, tension, mannerism and posturing, grandiosity, hostility, suspiciousness, hallucinatory behavior, uncooperativeness, unusual thought and excitement) and the negative symptom subscale (emotional withdrawal, motor retardation, blunted affect, and disorientation) of the BPRS were also used (19). The Subjective Craving Scale for Alcohol Dependence (20) was modified and used for assessment of craving for methamphetamine. The scores ranged from 0 to 5, with higher scores representing stronger craving. These evaluations were performed on the day of the PET examination. After complete description of the study, written informed consent was obtained from all subjects before study entry, in compliance with the procedures of the Ethics Committee of the Hamamatsu University School of Medicine.
PET Study
Before PET, a magnetic resonance imagining (MRI) scan was performed on each subject with a static magnet (0.3 T MRP7000AD, Hitachi, Tokyo). Following a bolus intravenous injection of a 450-MBq dose of [11C]WIN 35,428, a sequence of 38 PET (SHR2400 PET scanner [21], Hamamatsu Photonics K.K., Hamamatsu, Japan) scans was obtained over 90 minutes. Arterial blood samples were drawn at intervals of 10 seconds to 15 minutes after the tracer injection. The blood samples were analyzed with thin layer chromatography (Whatman AL SIL G/UV [Whatman Japan KK, Tokyo], 20 × 20 cm) and BAS1500 (Fuji Photo Film Co., Tokyo) for determination of the unmetabolized tracer level.
Regions of interest—the caudate/putamen, nucleus accumbens, prefrontal cortex, and cerebellar cortex (Figure 1)—were manually drawn on a PET display, and the corresponding MRI images were referred to as anatomical landmarks (22). The time-activity curves of the regions were generated. Using the radioactivity curves, we calculated dopamine transporter binding potential (k3/k4) in the caudate/putamen, nucleus accumbens, and prefrontal cortex on the basis of a three-compartment and four-parameter (K1, k2, k3, and k4) model (23). As an input function, a plasma time-activity curve was used. The level of radioactivity in the cerebellar cortex was used as a reference to determine K1/k2 in the caudate/putamen, nucleus accumbens, and prefrontal cortex (23). This procedure was performed blind to the clinical data.
Statistical Analysis
For comparison of the mean values of the parameters K1, k2, k3, and k4, the ratio K1/k2, and the binding potential (k3/k4) between comparison subjects and methamphetamine users, the Mann-Whitney U test was used. Correlations between the dopamine transporter binding potential and each clinical parameter, including duration of methamphetamine use, duration of abstinence, BPRS score, and craving score, were evaluated by using Kendall’s tau. Statistical significance was set at p<0.05.
Results
The demographic characteristics of methamphetamine users are shown in Table 1. No significant difference in age or education levels was observed between methamphetamine users and comparison subjects. None of the methamphetamine users had a history of psychotic symptoms before the use of methamphetamine, and none showed negative symptoms during the present examination. Eight methamphetamine users (numbers 1, 3–7, 10, and 11) had previously experienced methamphetamine psychosis. Over the 2 weeks preceding the PET examination, three methamphetamine users (numbers 2, 8, and 10) had no psychiatric symptoms; four (numbers 3, 5, 9, and 11) had persistent anxiety-disorder-like, but not psychotic, symptoms; and three (numbers 4, 6, and 7) had persistent psychotic symptoms such as auditory hallucinations and delusions. One methamphetamine user (number 1) began to show psychotic symptoms during the use of methamphetamine and still exhibited the symptoms on the day of the PET examination. None of the methamphetamine users was taking neuroleptics at the time of PET examination, although four (numbers 4–6 and 9) had received neuroleptic treatment previously.
Figure 2 shows PET images from a comparison subject and from the methamphetamine user with the lowest dopamine transporter binding potential (number 7). Both images show high accumulation of radioactivity in the caudate/putamen and nucleus accumbens and low accumulation in the prefrontal cortex and cerebellar cortex. The radioactivity in the caudate/putamen and nucleus accumbens was lower in the methamphetamine user than in the comparison subject.
Figure 3 shows representative curves of radioactivity in the brain and in the arterial plasma from a comparison subject and a methamphetamine user. Following a venous injection of the tracer, the radioactivity in the caudate/putamen and nucleus accumbens increased until the end of measurement, whereas that in the prefrontal cortex and cerebellar cortex decreased gradually after reaching a peak within 10 minutes.
Values of dopamine transporter binding potential in the caudate/putamen, nucleus accumbens, and prefrontal cortex are shown in Figure 4. The mean binding potential for the caudate/putamen was 4.40 (SD=0.18) in comparison subjects and 3.51 (SD=0.45) in methamphetamine users. The latter value constitutes a 20.2% difference. In the nucleus accumbens, the mean binding potentials in comparison subjects and methamphetamine users were 4.16 (SD=0.64) and 2.93 (SD=0.77), respectively. The latter value represents a 29.6% difference. The mean binding potential for the prefrontal cortex was 0.18 (SD=0.05) in comparison subjects and 0.12 (SD=0.03) in methamphetamine users. The value of the latter was 33.3% lower. The dopamine transporter binding potentials for the caudate/putamen, nucleus accumbens, and prefrontal cortex were significantly lower in methamphetamine users than the comparison subjects (caudate/putamen: U=1.0, N=20, p<0.001; nucleus accumbens: U=8.5, N=20, p<0.001; prefrontal cortex: U=23.5, N=20, p<0.05, Mann-Whitney U test).
There was a significant correlation between the clinical data for the methamphetamine users and the dopamine transporter binding potential in the caudate/putamen and in the nucleus accumbens (Figure 5), but no correlation was found between the clinical measures and prefrontal cortex dopamine transporter binding potential (data not shown). The dopamine transporter binding potential in the caudate/putamen and nucleus accumbens had a significant negative correlation with the duration of methamphetamine use (caudate/putamen: Kendall’s tau=–0.48, df=10, p<0.05; nucleus accumbens: Kendall’s tau=–0.73, df=10, p<0.01). No significant correlation was observed between the duration of abstinence and the dopamine transporter binding potential for either the caudate/putamen or the nucleus accumbens. The caudate/putamen dopamine transporter binding potential, but not the nucleus accumbens binding potential, showed a significant negative correlation with the total BPRS score (Kendall’s tau=–0.62, df=10, p<0.05). There was a significant negative correlation between the score on the BPRS subscale for positive symptoms and the dopamine transporter binding potential for the caudate/putamen and the nucleus accumbens (caudate/putamen: Kendall’s tau=–0.73, df=10, p<0.01; nucleus accumbens: Kendall’s tau=–0.50, df=10, p<0.05). The subjective craving score was not significantly correlated with the dopamine transporter binding potential for either the caudate/putamen or the nucleus accumbens.
There was a significant correlation between the score on the BPRS subscale for positive symptoms and the duration of methamphetamine use (Kendall’s tau=0.64, df=10, p<0.01) (Figure 6). The subjective craving score showed a significant negative correlation with the duration of abstinence from methamphetamine (Kendall’s tau=–0.50, df=10, p<0.05) but not with the duration of methamphetamine use (Figure 6).
Discussion
In the present PET study, the rank order of the dopamine transporter density represented by binding potential observed in methamphetamine users and comparison subjects who did not use methamphetamine was caudate/putamen, then nucleus accumbens, then prefrontal cortex. The finding of low dopamine transporter density in the prefrontal cortex was compatible with the results of a previous PET study demonstrating very low density of dopamine D2 receptors in the human prefrontal cortex (24). The present results also show that in the caudate/putamen, nucleus accumbens, and prefrontal cortex, the dopamine transporter density of methamphetamine users was significantly lower than that of comparison subjects. Therefore, we suggest that in the human brain, methamphetamine causes reduction of the dopamine transporter density in the mesolimbic, mesocortical, and nigrostriatal dopaminergic systems, although the number of subjects was small in this study.
Our finding that dopamine transporter density was lower in the caudate/putamen and nucleus accumbens of the methamphetamine users may be in agreement with the results of previous postmortem and PET studies (14, 15). With respect to the postmortem study (14), however, it is unclear whether polydrug users were included as subjects. In addition, the cause of death was not clear in the postmortem study: in the case of overdose, several conditions would have to be considered in order to understand the results. In the PET study (15), users of multiple drugs, including cocaine, cannabis, and LSD, were included as subjects, although these substances are known to cause psychosis and to influence neural transmission in the brain (25).
In order to evaluate the specific effects of methamphetamine on the dopamine transporter density associated with psychiatric symptoms, we selected methamphetamine users who had abused only methamphetamine (i.e., not polydrug users). Some of the methamphetamine users had a history of treatment with neuroleptics for a psychiatric state, but previous studies have reported that neuroleptics have no effect on dopamine transporter density or affinity (26, 27). In addition, the methamphetamine users in this study took the substance intravenously or by smoking. This difference in the method of methamphetamine intake might contribute to the correlation between the dopamine transporter change and the clinical measures, although methamphetamine possesses similar pharmacological characteristics whether administered intravenously or by inhalation (28, 29). A larger study group and a more homogenous population would be needed to completely eliminate these factors.
In contrast to the dopamine transporter density for the caudate/putamen and nucleus accumbens, which was significantly correlated with some clinical characteristics, the prefrontal cortex dopamine transporter density did not correlate with any of the clinical markers, although it is generally accepted that the dopaminergic neurons in the prefrontal cortex are actively involved in the genesis and expression of a psychotic state (10, 11). In addition, studies suggest the importance of cerebellar function for psychotic disorders (30) and the presence of dopaminergic axons in certain lobules of the cerebellar vermis in primates (31). However, in the present study, which used a ligand of [11C]WIN 35,428, the dopamine transporter density in the cerebellar vermis was not quantitatively measured. To clarify the role of dopamine transporter in the prefrontal cortex and the cerebellar vermis for methamphetamine-related psychiatric symptoms, a larger study will be needed, along with the development of a new tracer with a higher specific-to-nonspecific binding ratio that allows low binding site density to be detected.
The dopamine transporter density for both the caudate/putamen and the nucleus accumbens decreased with increasing duration of methamphetamine use, suggesting that, in the habitual methamphetamine users in this study, the reduction of dopamine transporter density was dependent on the duration of methamphetamine use. Although the methamphetamine-induced change in dopamine transporter density appears to be influenced by the pattern and rate of methamphetamine use (32), our findings did not help to clarify this issue. However, the methamphetamine users in this study used methamphetamine recreationally and had no history of toxic or high-dose methamphetamine use. Since methamphetamine-induced dopamine transporter reduction is known to be dose-dependent (32, 33), the duration of methamphetamine use, as a determining factor of the reduction in dopamine transporter density observed in habitual methamphetamine users, may represent this dose dependency.
The severity of positive symptoms increased with the duration of methamphetamine use. Detoxification from methamphetamine in all users was confirmed by regular urine drug screening tests, including a test on the day of the PET examination, to establish that the psychiatric symptoms evaluated in this study were residual rather than acute symptoms induced by methamphetamine use. The present observations suggest that longer use of methamphetamine induces greater reduction in dopamine transporter density, which in turn is associated with a higher occurrence of residual psychiatric symptoms.
The dopamine transporter density of the caudate/putamen decreased with increasing total score and positive subscale score on the BPRS. The dopamine transporter density of the nucleus accumbens had no apparent correlation with the BPRS total score but decreased with increasing scores on the positive subscale. This finding suggests that the reduction in dopamine transporter density of both the caudate/putamen and nucleus accumbens may actively participate in the pathogenesis of residual psychiatric symptoms in methamphetamine users.
We found no significant correlation between the duration of abstinence, which lasted in our subjects from 1 week to 1.5 years, and dopamine transporter density in either the caudate/putamen or the nucleus accumbens. The previous PET study (15) showed a significant reduction in the caudate/putamen dopamine transporter density in chronic methamphetamine users, some of whom had been abstinent for more than 3 years. These findings suggest that lasting reduction of brain dopamine transporter density could occur after habitual methamphetamine use.
In terms of craving for methamphetamine, a significant correlation was found with the duration of methamphetamine abstinence. However, no correlation was observed between the craving for methamphetamine and the duration of methamphetamine use. In addition, craving was not associated with dopamine transporter density in the nucleus accumbens, although it has been hypothesized that this structure is related to drug reward (34). These findings are not inconsistent with our contention that craving for methamphetamine may be associated with reversible changes in the brain, such as changes in postsynaptic dopamine receptors, which are restored over time (35, 36).
In a previous study using the same PET scanner and tracer as used here, the striatal dopamine transporter density in unmedicated patients with Parkinson’s disease showed a pronounced reduction of about 80% (23). Methamphetamine users in this study, who had no symptoms of parkinsonism or other neurological abnormalities, showed a small reduction of caudate/putamen dopamine transporter of roughly 20%. Since striatal dopamine transporter declines with age (37, 38), chronic methamphetamine users may have a greater risk for developing Parkinson’s disease as they age. Although small, the reduction in caudate/putamen dopamine transporter in the methamphetamine users in our current study was clearly associated with the development of psychiatric symptoms. In the case of methamphetamine users, as distinct from patients with Parkinson’s disease, the small reduction in caudate/putamen dopamine transporter appears to have a predictive value for the development of psychiatric signs. However, in order to strengthen the specificity and sensitivity of the caudate/putamen dopamine transporter reduction as a predictor for psychiatric symptoms, it will be necessary to evaluate another brain area as a negative control after a new tracer more highly specific to the dopamine transporter becomes available.
Several reports have indicated the possible involvement of the caudate/putamen in cognitive and personality disorders (39, 40). In particular, a previous PET study (41) showed an association between low dopamine transporter density and detached personality. Whether chronic use of methamphetamine influences or enhances such a personality trait is not known, but research into this question may provide further insight into the possible associations between caudate/putamen dopamine transporter density and psychiatric symptoms.
Oxidative dopamine products such as the dopamine quinones, which are increasingly formed under the condition of high concentration of intracellular dopamine (42), are known to modify the proteins at the sites of cysteinyl residues. Dopamine transporter, which contains several cysteinyl residues, is one of the proteins at risk for this modification (43), and methamphetamine is known to increase intracellular dopamine level by inhibiting vesicular transporter and monoamine oxidase B activity (44). Such modification may partly explain the reduction of dopamine transporter density observed here, since the dopamine nerve terminals of chronic methamphetamine users are exposed to excessive dopamine levels for long periods of time.
In summary, our results clearly show parallelism between reduction of dopamine transporter density in the brain, which is associated with the duration of methamphetamine use, and the symptoms of a chronic psychotic state in methamphetamine users. Therefore, chronic methamphetamine use may contribute to the production of prolonged psychosis in methamphetamine users. However, dopamine transporter is only one part of the dopaminergic system, and we examined only one aspect of the dynamics of dopamine transmission. Indeed, in a previous animal study (32), methamphetamine was reported to produce long-term reductions in serotonin transporter density, in addition to the decrease in dopamine transporter density. Therefore, changes in serotonin neurons, which have been implicated in many psychiatric symptoms (45–47), might have occurred in the methamphetamine users in the current study and might thus have influenced our results, including those on clinical symptoms. We also cannot rule out the possibility of preexisting condition of dopamine transporter reduction in methamphetamine users. Clearly, further work will be needed to define the causal mechanism of methamphetamine-associated psychosis.
 |
Received April 11, 2000; revisions received Oct. 6, 2000, and Jan. 29, 2001; accepted Feb. 1, 2001. From the Department of Psychiatry and Neurology, Hamamatsu University School of Medicine; the Positron Medical Center, Hamamatsu Medical Center, Hamakita, Japan; and the Central Research Laboratory, Hamamatsu Photonics K.K., Hamakita, Japan. Address reprint requests to Dr. Sekine, Department of Psychiatry and Neurology, Hamamatsu University School of Medicine, 3600 Handa-cho Hamamatsu, 431-3192 Shizuoka, Japan; [email protected] (e-mail). Supported by a Scientific Research grant from the Ministry of Health and Welfare of Japan and a Target-Oriented Research and Development grant from the Ministry of Science and Technology of Japan. The authors thank Shuji Nobezawa and Toshihiko Kanno for technical support.
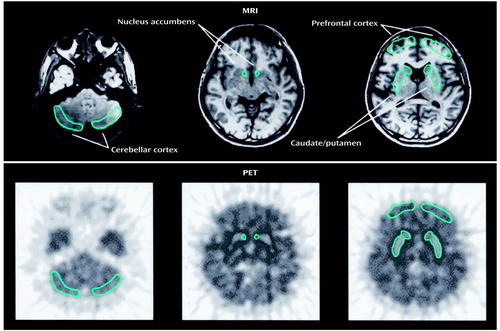
Figure 1. Brain Regions of Interest for a PET Study of 11 Methamphetamine Users and Nine Comparison Subjectsa
aRegions of interest are drawn bilaterally on the MRI and on the PET images. Regions placed on the PET images are based on those in the MRI images.
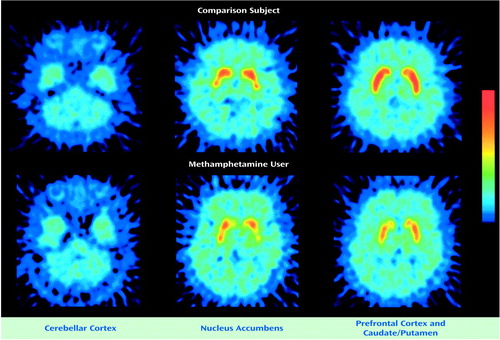
Figure 2. PET Brain Images From a Representative Comparison Subject and Methamphetamine User After Tracer Injectiona
aThese images were constructed between 60 to 90 minutes after the tracer injection ([11C]WIN-35,428) and normalized to the cerebellar radioactivity. Radioactivity levels in the nucleus accumbens and caudate/putamen were lower in the methamphetamine user than in the comparison subject. The color bar indicates the normalized count from 0 to 90 Bq/ml.
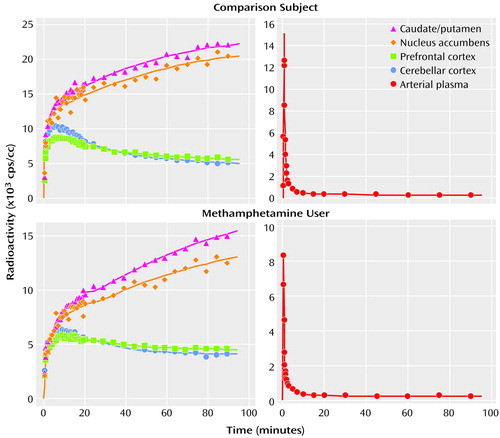
Figure 3. Radioactivity in the Brain and the Arterial Plasma of a Representative Comparison Subject and Methamphetamine User After Tracer Injectiona
aThe tracer was [11C]WIN-35,428; the radioactivity in each region is the averaged value of the left and right sides of the region.
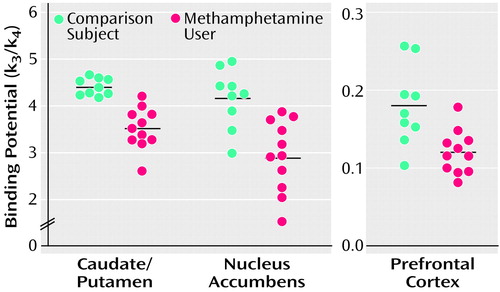
Figure 4. Dopamine Transporter Binding Potential in the Brains of 11 Methamphetamine Users and Nine Comparison Subjectsa
aThe mean binding potentials for the caudate/putamen, nucleus accumbens, and prefrontal cortex were significantly lower in methamphetamine users than in comparison subjects (p<0.05, Mann-Whitney U test). The horizontal lines represent the mean values.
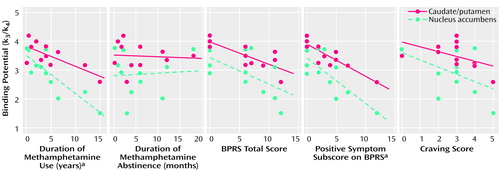
Figure 5. Correlations Between Clinical Variables and Dopamine Transporter Binding Potential in the Caudate/Putamen and Nucleus Accumbens of 11 Methamphetamine Users
aSignificant correlation (p<0.05, Kendall’s tau).
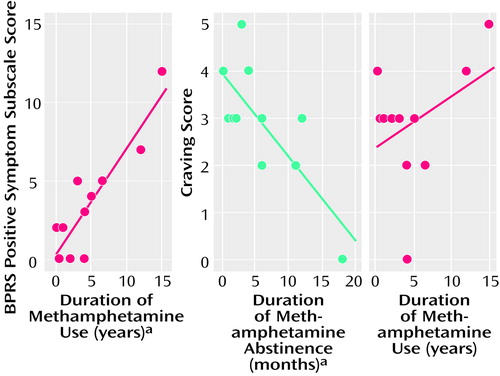
Figure 6. Correlations Between Clinical Variables for 11 Methamphetamine Users
aSignificant correlation (p<0.05, Kendall’s tau).
1. Baberg HT, Nelesen RA, Dimsdale JE: Amphetamine use: return of an old scourge in a consultation psychiatry setting. Am J Psychiatry 1996; 153:789-793Link, Google Scholar
2. Murray JB: Psychophysiological aspects of amphetamine- methamphetamine abuse. J Psychol 1998; 132:227-237Crossref, Medline, Google Scholar
3. Konuma K, Hirai S, Kasahara M: Use and abuse of amphetamines in Japan, in Amphetamine and Its Analogs. Edited by Cho AK, Segal DS. San Diego, Academic Press, 1994, pp 459-478Google Scholar
4. Shaw KP: Human methamphetamine-related fatalities in Taiwan during 1991-1996. J Forensic Sci 1999; 44:27-31Medline, Google Scholar
5. Sato M, Numach Y, Hamamura T: Relapse of paranoid psychotic state in methamphetamine model of schizophrenia. Schizophr Bull 1992; 18:115-122Crossref, Medline, Google Scholar
6. Iwanami A, Sugiyama A, Kuroki N, Toda S, Kato N, Nakatani Y, Horita N, Kaneko T: Patients with methamphetamine psychosis admitted to a psychiatric hospital in Japan: a preliminary report. Acta Psychiatr Scand 1994; 89:428-432Crossref, Medline, Google Scholar
7. Iyo M, Sekine Y, Matsunaga T, Tsukamoto T, Takei N, Mori N: Methamphetamine-associated obsessional symptoms and effective risperidone treatment: a case report. J Clin Psychiatry 1999; 60:337-338Crossref, Medline, Google Scholar
8. Buffenstein A, Heaster J, Ko P: Chronic psychotic illness from methamphetamine (letter). Am J Psychiatry 1999; 156:662Link, Google Scholar
9. Angrist B: Amphetamine psychosis: clinical variations of the syndrome, in Amphetamine and Its Analogs. Edited by Cho AK, Segal DS. San Diego, Academic Press, 1994, pp 387-414Google Scholar
10. Wolters EC: Dopaminomimetic psychosis in Parkinson’s disease patients: diagnosis and treatment. Neurology 1999; 52(suppl 3):10-13Google Scholar
11. Davis KL, Kahn RS, Ko G, Davidson M: Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 1991; 148:1474-1486Google Scholar
12. Giros B, Jaber M, Jones SR, Wightman RM, Caron MG: Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996; 379:606-612Crossref, Medline, Google Scholar
13. Rudnick G: Mechanisms of biogenic amine neurotransmitter transporters, in Neurotransmitter Transporters. Edited by Reith MEA. Totowa, NJ, Humana Press, 1997, pp 73-100Google Scholar
14. Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ: Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 1996; 2:699-703Crossref, Medline, Google Scholar
15. McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA: Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci 1998; 18:8417-8422Google Scholar
16. Scheffel U, Boja JW, Kuhar MJ: Cocaine receptors: in vivo labeling with [3H](-)cocaine, [3H]WIN 35,065-2 and [3H]WIN 35,428. Synapse 1989; 4:390-392Crossref, Medline, Google Scholar
17. First MB, Spitzer RL, Gibbon M, Williams JB: Structured Clinical Interview for DSM-IV Axis I Disorders—Non-Patient Edition (SCID-I/NP), version 2.0. New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
18. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799-812Crossref, Google Scholar
19. Mohr P, Horacek J, Motlova L, Libiger J, Czobor P: Prolactin response to d-fenfluramine challenge test as a predictor of treatment response to haloperidol in acute schizophrenia. Schizophr Res 1998; 30:91-99Crossref, Medline, Google Scholar
20. Borg V: Bromocriptine in the prevention of alcohol abuse. Acta Psychiatr Scand 1983; 68:100-110Crossref, Medline, Google Scholar
21. Yamashita T, Uchida H, Okada H, Kurono T, Takemori T, Watanabe M, Shimizu K, Yoshikawa E, Ohmura T, Satoh N, Tanaka E, Nohara N, Tomitani T, Yamamoto M, Murayama H, Endo M: Development of a high resolution PET. IEEE Trans Nucl Sci 1990; 37:594-599Crossref, Google Scholar
22. Iyo M, Namba H, Fukushi K, Shinotoh H, Nagatsuka S, Suhara T, Sudo Y, Suzuki K, Irie T: Measurement of acetylcholinesterase by positron emission tomography in the brains of healthy controls and patients with Alzheimer’s disease. Lancet 1997; 349:1805-1809Google Scholar
23. Ouchi Y, Yoshikawa E, Okada H, Futatsubashi M, Sekine Y, Iyo M, Sakamoto M: Alterations in binding site density of dopamine transporter in the striatum, orbitofrontal cortex, and amygdala in early Parkinson’s disease: compartment analysis for β-CFT binding with positron emission tomography. Ann Neurol 1999; 45:601-610Crossref, Medline, Google Scholar
24. Farde L, Pauli S, Hall H, Eriksson L, Halldin C, Hogberg T, Nilsson L, Sjogren I, Stone-Elander S: Stereoselective binding of 11C-raclopride in living human brain: a search for extrastriatal central D2-dopamine receptors by PET. Psychopharmacology (Berl) 1988; 94:471-478Crossref, Medline, Google Scholar
25. Poole R, Brabbins C: Drug induced psychosis. Br J Psychiatry 1996; 168:135-138Crossref, Medline, Google Scholar
26. Allard P, Eriksson K, Ross SB, Marcusson JO: Unaltered [3H]GBR-12935 binding after chronic treatment with dopamine active drugs. Psychopharmacology (Berl) 1990; 102:291-294Crossref, Medline, Google Scholar
27. Rivest R, Falardeau P, Di Paolo T: Brain dopamine transporter: gender differences and effect of chronic haloperidol. Brain Res 1995; 692:269-272Crossref, Medline, Google Scholar
28. Meng Y, Dukat M, Bridgen DT, Martin BR, Lichtman AH: Pharmacological effects of methamphetamine and other stimulants via inhalation exposure. Drug Alcohol Depend 1999; 53:111-120Crossref, Medline, Google Scholar
29. Cook CE, Jeffcoat AR, Hill JM, Pugh DE, Patetta PK, Sadler BM, White WR, Perez-Reyes M: Pharmacokinetics of methamphetamine self-administered to human subjects by smoking S-(+)-methamphetamine hydrochloride. Drug Metab Dispos 1993; 21:717-723Medline, Google Scholar
30. Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Boles Ponto LL, Watkins GL, Hichwa RD: Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA 1996; 93:9985-9990Google Scholar
31. Melchitzky DS, Lewis DA: Tyrosine hydroxylase- and dopamine transporter-immunoreactive axons in the primate cerebellum: evidence for a lobular- and laminar-specific dopamine innervation. Neuropsychopharmacology 2000; 22:466-472Crossref, Medline, Google Scholar
32. Villemagne V, Yuan J, Wong DF, Dannals RF, Hatzidimitriou G, Mathews WB, Ravert HT, Musachio J, McCann UD, Ricaurte GA: Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci 1998; 18:419-427Crossref, Medline, Google Scholar
33. Gibb JW, Hanson GR, Johnson M: Neurochemical mechanisms of toxicity, in Amphetamine and Its Analogs. Edited by Cho AK, Segal DS. San Diego, Academic Press, 1994, pp 269-295Google Scholar
34. Wise RA, Hoffman DC: Localization of drug reward mechanisms by intracranial injections. Synapse 1992; 10:247-263Crossref, Medline, Google Scholar
35. Iyo M, Nishio M, Itoh T, Fukuda H, Suzuki K, Yamasaki T, Fukui S, Tateno Y: Dopamine D2 and serotonin S2 receptors in susceptibility to methamphetamine psychosis detected by positron emission tomography. Psychiatry Res Neuroimaging 1993; 50:217-231Crossref, Medline, Google Scholar
36. Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D: Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry 1990; 147:719-724Link, Google Scholar
37. Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley SJ, Hitzemann R, Smith G, Fields SD, Gur R: Dopamine transporters decrease with age. J Nucl Med 1996; 37:554-559Medline, Google Scholar
38. Tissingh G, Booij J, Bergmans P, Winogrodzka A, Janssen AG, van Royen EA, Stoof JC, Wolters EC: Iodine-123-N-omega-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl)tropane SPECT in healthy controls and early-stage, drug-naive Parkinson’s disease. J Nucl Med 1998; 39:1143-1148Google Scholar
39. Caine ED, Shoulson I: Psychiatric syndromes in Huntington’s disease. Am J Psychiatry 1983; 140:728-733Link, Google Scholar
40. Bhatia KP, Marsden CD: The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain 1994; 117:859-876Crossref, Medline, Google Scholar
41. Laakso A, Vilkman H, Kajander J, Bergman J, Haaparanta M, Solin O, Hietala J: Prediction of detached personality in healthy subjects by low dopamine transporter binding. Am J Psychiatry 2000; 157:290-292Link, Google Scholar
42. Hastings TG, Lewis DA, Zigmond MJ: Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci USA 1996; 93:1956-1961Google Scholar
43. Berman SB, Zigmond MJ, Hastings TG: Modification of dopamine transporter function: effect of reactive oxygen species and dopamine. J Neurochem 1996; 67:593-600Crossref, Medline, Google Scholar
44. Kuczenski R, Segal DS: Neurochemistry of amphetamine, in Amphetamine and Its Analogs. Edited by Cho AK, Segal DS. San Diego, Academic Press, 1994, pp 81-113Google Scholar
45. Meltzer CC, Price JC, Mathis CA, Greer PJ, Cantwell MN, Houck PR, Mulsant BH, Ben-Eliezer D, Lopresti B, DeKosky ST, Reynolds CF III: PET imaging of serotonin type 2A receptors in late-life neuropsychiatric disorders. Am J Psychiatry 1999; 156:1871-1878Google Scholar
46. Benedetti F, Serretti A, Colombo C, Campori E, Barbini B, di Bella D, Smeraldi E: Influence of a functional polymorphism within the promoter of the serotonin transporter gene on the effects of total sleep deprivation in bipolar depression. Am J Psychiatry 1999; 156:1450-1452Google Scholar
47. Mann JJ, Malone KM, Diehl DJ, Perel J, Cooper TB, Mintun MA: Demonstration in vivo of reduced serotonin responsivity in the brain of untreated depressed patients. Am J Psychiatry 1996; 153:174-182; correction, 153:588Link, Google Scholar



