Neuroanatomy of Down’s Syndrome: A High-Resolution MRI Study
Abstract
OBJECTIVE: Down’s syndrome, the most common genetic cause of mental retardation, results in characteristic physical and neuropsychological findings, including mental retardation and deficits in language and memory. This study was undertaken to confirm previously reported abnormalities of regional brain volumes in Down’s syndrome by using high-resolution magnetic resonance imaging (MRI), determine whether these volumetric abnormalities are present from childhood, and consider the relationship between neuroanatomic abnormalities and the cognitive profile of Down’s syndrome. METHOD: Sixteen children and young adults with Down’s syndrome (age range=5–23 years) were matched for age and gender with 15 normal comparison subjects. High-resolution MRI scans were quantitatively analyzed for measures of overall and regional brain volumes and by tissue composition. RESULTS: Consistent with prior imaging studies, subjects with Down’s syndrome had smaller overall brain volumes, with disproportionately smaller cerebellar volumes and relatively larger subcortical gray matter volumes. Also noted was relative preservation of parietal lobe gray and temporal lobe white matter in subjects with Down’s syndrome versus comparison subjects. No abnormalities in pattern of brain asymmetry were noted in Down’s syndrome subjects. CONCLUSIONS: The results largely confirm findings of previous studies with respect to overall patterns of brain volumes in Down’s syndrome and also provide new evidence for abnormal volumes of specific regional tissue components. The presence of these abnormalities from an early age suggests that fetal or early postnatal developmental differences may underlie the observed pattern of neuroanatomic abnormalities and contribute to the specific cognitive and developmental deficits seen in individuals with Down’s syndrome.
With an incidence of 1 in 800 live births, Down’s syndrome is the most common genetic cause of mental retardation (1). Down’s syndrome provides a rare opportunity to explore relationships among genetic, structural, and cognitive or developmental abnormalities. In 95% of cases, Down’s syndrome is caused by a full trisomy of chromosome 21 (2). Individuals with Down’s syndrome typically are microcephalic and have characteristic facies, hypotonia, and small stature. Although this physical phenotype is easily recognized, mental retardation of varying degrees is the most consistent feature of Down’s syndrome (3). Cognitive development in Down’s syndrome has been extensively studied, yielding a profile of global delays with disproportionately impaired speech and language (4). Some studies (5–7) also have identified major deficits in both short-term and long-term verbal memory. It is of interest that, compared to the dramatic impairment in these areas, visuospatial processing skills appear to be relatively preserved in individuals with Down’s syndrome (8, 9).
Until recently, our understanding of the structural brain abnormalities in Down’s syndrome was almost exclusively based on autopsy studies. These have consistently shown a lower brain weight and brachycephaly, with a small cerebellum, frontal and temporal lobes, a simplified appearance of the sulci, and a narrow superior temporal gyrus (3, 10, 11). Only in the past several years have improvements in magnetic resonance imaging (MRI) and image processing techniques allowed quantitative explorations of brain structure in living subjects with Down’s syndrome. Consistent with autopsy reports, volumetric neuroimaging studies of adults with Down’s syndrome (12–16) have revealed smaller overall brain volumes, with disproportionately smaller cerebellar, brainstem, frontal lobe, and hippocampal volumes. Basal ganglia volumes, however, have been reported to be normal in MRI volumetric studies of adults with Down’s syndrome (14, 17). Considering the high prevalence of Down’s syndrome, surprisingly few MRI studies of affected children have been published. Jernigan et al. (18) reported similarly smaller overall brain volumes, with disproportionately smaller volumes in frontal, temporal, and cerebellar regions, in a volumetric MRI study of six children with Down’s syndrome. As in the adult studies, volumes of thalamus and lenticular nuclei were noted to be normal. Caution must be exercised in interpreting these studies of brain structure in subjects with Down’s syndrome, as most have employed small numbers of subjects and much of the volumetric data were obtained with relatively low resolution image acquisition techniques (e.g., 5-mm brain slices with between-slice gaps of 2.5 mm), compared to the techniques now available and used in the present study, which allow 1–2-mm resolution and no between-slice gap (12, 13, 18).
The goal of the present study was to obtain more precise quantitative neuroimaging data in children and young adults with Down’s syndrome and to determine whether the findings of previous imaging studies could be confirmed using newer high-resolution MRI acquisition techniques and an advanced segmentation and image processing protocol. Since we know of no prior studies that include children with Down’s syndrome under age 10, we also sought to determine whether measurable differences are present from earlier in childhood. On the basis of the results of prior neuroimaging studies, we hypothesized smaller overall brain volumes, with disproportionately smaller cerebellar and frontal volumes and selective preservation of subcortical structures. The pattern of deficits in language and memory, combined with evidence for aberrant language localization (19–21),led us to explore the additional a priori hypothesis that the temporal lobes and, specifically, the superior temporal gyri would be significantly smaller in children with Down’s syndrome and would show an abnormal pattern of asymmetry. Additionally, the relative strengths in visuospatial skills in children with Down’s syndrome pointed to the parietal lobe as a potential region of interest (5, 6).
Method
Subjects
Sixteen individuals with Down’s syndrome (11 males and five females, mean age=11.3 years, SD=5.2, range=5.0–23.8) and 15 normal comparison subjects matched for gender and age to within 1 year (mean age=11.9, SD=4.7, range=5.4–23.2) were studied. All subjects with Down’s syndrome were recruited through the Down Syndrome Clinic at the Kennedy Krieger Institute between 1991 and 1997. After complete description of the study to the subjects’ parents, written informed consent was obtained from the parents of all subjects, with oral assent given by subjects when feasible, prior to participation. The diagnosis of Down’s syndrome was confirmed both by clinical examination by one of the authors (G.T.C.), as well as by karyotype. All Down’s syndrome subjects were found to have trisomy of chromosome 21.
Imaging
MRIs of each subject’s brain were acquired with a GE Signa 1.5-T scanner (General Electric, Milwaukee). Coronal images were acquired with a three-dimensional volumetric radio frequency spoiled gradient echo with the following parameters: TR=35 msec, TE=7 msec, flip angle=45°, number of excitations=1, matrix size=256×128, field of view=20–24 cm, slice thickness=1.5 mm, 124 slices. All scans of both Down’s syndrome and comparison subjects were acquired at the Johns Hopkins University School of Medicine/Kennedy Krieger Institute Brain Imaging Center. The spoiled gradient echo image data were imported into the program BrainImage (22) for semiautomated image processing analysis and quantification. These procedures have been previously described and validated (23–26). Data resulting from this analysis are in the form of gray matter and white matter for each of the cerebral lobes, a subcortical region encompassing the basal ganglia and thalamus, and the cerebellum. To specify regional differences, the brain was divided into lobes with a semiautomated stereotactic-based parcellation method (23, 27, 28). Raters who conducted morphometric analyses were blind to the group membership of the subjects.
Manual delineation of the superior temporal gyrus also was used to supplement the semiautomated procedure. The superior temporal gyrus was measured in the rostral-caudal direction from images in the coronal plane that were derived from the original image data set. This coronal data set was oriented orthogonal to the plane defined by a line drawn between the anterior and posterior commissures on midsagittal images. The boundaries of the superior temporal gyrus were defined laterally by the cortical surface and medially by a line connecting the deepest extension of the superior temporal sulcus to the furthest extent of the inferior ramus of the sylvian fissure. The most anterior slice of the superior temporal gyrus measured coincided with the halfway point between the head of the putamen and the anterior commissure. This designation ensured the operational exclusion of medial temporal gyral tissue, which merges with the superior temporal gyrus at the temporal pole. The most posterior slice of the superior temporal gyrus measured coincided with the first slice in which the crus of the fornix was clearly identified laterally from the pulvinar. Interrater reliability for measurement of the volume of this region was determined by two raters using 10 data sets independent of this study and resulted in an intraclass correlation coefficient of 0.96.
Data Analyses
All volumetric data met the necessary criteria for employment of parametric statistical analyses, including normality and heteroscedasticity. Analysis of variance (ANOVA) was first used to compare total brain volumes between groups. The data were then analyzed by multivariate analysis of covariance (MANCOVA) to determine group differences in profiles of regional brain volumes. Follow-up ANCOVAs of each brain region were used to assess the combined left and right volumes and used total brain volume as a covariate. The regions reaching a significance threshold p=0.05, two-tailed, were analyzed by tissue composition (volumes of gray and white matter). As for the parietal lobes, as well as the temporal lobes and the superior temporal gyri, our a priori hypotheses led us to further analyze these by component, despite their analyses not reaching the threshold for significance. Brain asymmetry was assessed by repeated measures ANOVA of cerebral tissue in each lobe and the superior temporal gyrus by using diagnostic category as a between-subject factor and side (left versus right) as a within-subject factor. The interaction effect of group by side was used to determine group differences in asymmetry.
Results
Volumes in Down’s Syndrome
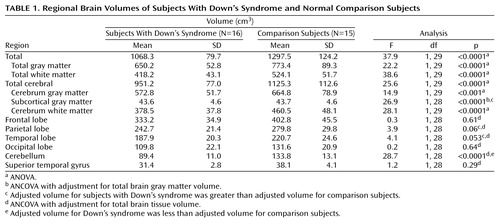
As shown in Table 1 and Figure 1, mean total brain volume was 18% smaller in the subjects with Down’s syndrome than in the comparison subjects (F=37.9, df=1, 29, p<0.0001). To investigate whether the subjects with Down’s syndrome have an atypical pattern of cerebral morphology, a MANCOVA was computed with group as a main effect (Down’s syndrome group versus comparison group), and total brain volume was entered as a covariate to statistically control for differences in overall brain size. Dependent variables consisted of the combined left and right volumes for each of the four lobes of the brain and the cerebellum. A Wilks’s lambda of 0.21 (F=19.0, df=5, 24, p<0.0001) indicated a unique pattern of cerebral morphological variation that distinguishes individuals with Down’s syndrome from comparison subjects.
Follow-up ANCOVAs were used to further investigate possible regional differences. With control for differences in total brain tissue volume, the Down’s syndrome group showed a disproportionately smaller cerebellar volume than the comparison group (F=28.7, df=1, 28, p<0.0001). Differences that approached significance were noted for temporal (F=4.1, df=1, 28, p=0.053) and parietal (F=3.9, df=1, 28, p=0.06) lobe volumes; both regions were relatively larger in the Down’s syndrome group after adjustment for overall brain volume. No group differences were noted when comparing adjusted volumes of frontal (F=0.3, df=1, 28, p=0.61) and occipital (F=0.2, df=1, 28, p=0.64) lobes. Superior temporal gyrus tissue volumes were not significantly different between groups when corrected for total brain tissue volumes (F=1.2, df=1, 28, p=0.29).
Cerebral Tissue Composition
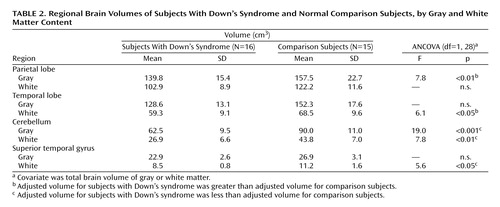
On the basis of our initial findings, segmentation by gray and white matter composition was conducted to further explore the significant between-group difference in cerebellar volume, as well as the likelihood of larger adjusted volumes in parietal and temporal lobes (Table 2). After adjustment for total brain gray and white matter, cerebellar volumes in the Down’s syndrome group were significantly smaller for both gray matter (F=19.0, df=1, 28, p<0.001) and white matter (F=7.8, df=1, 28, p<0.01). Parietal lobe gray matter volumes were relatively larger in the Down’s syndrome group when adjusted for total brain gray matter volume (F=7.8, df=1, 28, p<0.01) (Figure 2, top panel). Adjusted volumes for both parietal lobe white matter (F=0.03, df=1, 28, p=0.87) and temporal lobe gray matter (F=0.0001, df=1, 28, p=0.99) were not significantly different between groups, but temporal lobe white matter volumes were larger in the Down’s syndrome group when adjusted for total brain white matter volume (F=6.1, df=1, 28, p<0.05).
Although overall adjusted tissue volumes of the superior temporal gyrus did not differ between groups, our a priori hypothesis led us to further segment by gray and white matter composition. No difference between groups in superior temporal gyrus gray matter volume was found (F=0.2, df=1, 28, p=0.63), but significantly smaller superior temporal gyrus white matter was noted in the Down’s syndrome group (F=5.6, df=1, 28, p<0.05). Subcortical gray matter volumes were selectively preserved in Down’s syndrome, with nearly identical unadjusted mean volumes in subjects with Down’s syndrome and comparison subjects (mean=43.6 cm3, SD=4.6, versus mean=43.7 cm3, SD=4.6, respectively) (p=0.94). With correction for overall brain gray matter volume, subcortical gray matter volumes were significantly larger in the Down’s syndrome group (F=26.9, df=1, 28, p<0.0001) (Figure 2, bottom panel).
Brain Symmetry in Down’s Syndrome
Repeated measures ANOVA revealed no significant group-by-side differences in symmetry between the subjects with Down’s syndrome and the comparison subjects for hemispheric, cerebellar, frontal, parietal, temporal, or occipital lobe total tissue volumes. There was also no abnormal pattern of symmetry of the superior temporal gyrus or subcortical region in the subjects with Down’s syndrome.
Discussion
This study is the first we are aware of to specifically evaluate regional brain volumes and tissue composition in Down’s syndrome from early childhood through young adulthood. Our findings indicate that compared to matched developmentally normal subjects, the brains of individuals with Down’s syndrome show 1) overall smaller volumes due to smaller volumes of both cerebral gray and white matter, 2) a disproportionately smaller cerebellar volume, and 3) larger adjusted volumes of subcortical and parietal gray matter and temporal white matter components, with correction for overall brain volumes of gray or white matter, respectively.
Our finding of smaller brain volumes in subjects with Down’s syndrome, including disproportionately smaller volumes of the cerebellum, is consistent with the results of prior pathologic and neuroimaging studies (3, 10, 16, 18). The cerebellar hypoplasia evident in Down’s syndrome has been suggested to be a causal factor for its characteristic hypotonia and motor coordination difficulties, as well as articulatory speech disturbances, found in most children with Down’s syndrome (29). Schmahmann and Sherman (30) showed that patients with cerebellar lesions have impairments in many nonmotor areas, including those involving executive function, verbal fluency, and specific language deficits, including agrammatism. Silveri et al. (31) also reported specific agrammatism resulting from a focal cerebellar lesion. Functional neuroimaging studies, with both positron emission tomography and functional MRI, have provided further evidence for an important role for the cerebellum in language and cognition (32, 33). Taken together, the lesion and functional neuroimaging evidence point to the possibility that the syntactic difficulties seen in individuals with Down’s syndrome may be due to cerebellar hypoplasia and dysfunction (4).
Frontal lobe volumes also were significantly smaller in the subjects with Down’s syndrome, but when they were adjusted for overall brain volume, these smaller volumes were not significant. The frontal lobes have been frequently implicated in the cognitive deficits of Down’s syndrome, including executive dysfunction, inattention, and a tendency toward perseveration. Although our findings do not confirm previous reports of specific “hypofrontality” (18), their proportionately much smaller volumes may still reflect sufficient underdevelopment to account for dysfunction.
Contrary to the absolute smaller cerebellar and frontal lobe volumes found, but consistent with findings from prior studies of both adults and children with Down’s syndrome (14, 17, 18), was the remarkable preservation of subcortical structures, reflected in our study by larger adjusted subcortical gray matter volumes in the Down’s syndrome group. The relatively large size or preservation of these structures in children with Down’s syndrome in the context of significantly smaller overall cerebral volumes suggests that there is a temporal dissociation for the development of cortical versus subcortical regions. The embryologic data supporting this view include studies showing that neither brain volume differences nor neuropathologic abnormalities are seen in fetal Down’s syndrome brains until the third trimester, well after the majority of basal ganglia development is complete, but while dendritic arborization, synaptogenesis, and laminar organization continue in the cerebral cortex (11, 34–36). One interpretation of relatively larger subcortical volumes is that the basal ganglia are relatively normal due to their having developed extensively before the onset of major abnormalities. On the other hand, relatively larger size also may reflect insufficient programmed cell death, resulting in excessive cell numbers and large but dysfunctional basal ganglia and thalami.
An intriguing finding in our study is the striking preservation of parietal lobe gray matter. This confirms and further specifies the finding by Jernigan et al. (18) in six young Down’s syndrome subjects of preserved posterior (parietal and occipital) cortical gray matter. Our finding is particularly interesting in light of the neuropsychological profile of Down’s syndrome subjects, which reveals deficits predominantly in language skills (including verbal memory), with relative strengths in visuospatial processing, including visuospatial short-term memory (5, 6, 8). Evidence that the parietal lobes are important for visuospatial skills comes from both lesion studies (37, 38) and from functional neuroimaging studies showing bilateral parietal activations in short-term visuospatial memory tasks (39). The selective preservation of parietal lobe gray matter in Down’s syndrome is consistent with the observed relative strength in visuospatial skills.
In regard to our investigation of structures potentially involved in the language deficits of Down’s syndrome, we found no neuroimaging evidence for our hypothesis of disproportionately smaller overall temporal lobe volumes. In fact, we found larger corrected volumes of the temporal lobes that approached significance. Further segmentation by tissue type revealed this large size to be due to significantly larger corrected temporal lobe white matter volumes in the Down’s syndrome group. It is unclear whether the adjusted larger temporal lobe white matter volumes observed in this study could be related to cognitive deficits. The possibility of an association between larger regional volumes and cognitive dysfunction is supported by studies showing larger parahippocampal gyrus volumes in adults with Down’s syndrome (13, 14) and an inverse relationship between parahippocampal gyrus volumes and IQ in subjects with Down’s syndrome (14). Selective smaller hippocampal volumes shown in MRI studies of adults with Down’s syndrome (13–15) have highlighted the possibility that important temporal lobe subregional volume abnormalities in either direction may be present throughout development and may contribute to language and memory deficits.
Our expectation of finding smaller volumes of the superior temporal gyrus, on the basis of both functional MRI reports of significant activation of the superior temporal gyrus in auditory and language processing in normal adults (40–42) and of the predominant language deficits in children with Down’s syndrome, was not supported by our results. We found no significant difference in adjusted superior temporal gyrus total tissue volumes between the Down’s syndrome and comparison groups, although the corrected superior temporal gyrus white matter volume was significantly smaller in the Down’s syndrome group. It is possible that a selectively smaller white matter volume in this region could contribute to both the observed language deficits and the frequently reported narrowness of the superior temporal gyrus in individuals with Down’s syndrome.
We also found no volumetric evidence for an abnormal pattern of asymmetry of the brain (including the temporal lobes) in Down’s syndrome. This is in contrast to the finding of abnormal rightward temporal asymmetry by Jernigan et al. (18) in children with Down’s syndrome. Neuropsychological studies of individuals with Down’s syndrome have indicated a pattern of cognitive deficits like that seen in patients with left-hemisphere brain damage (9). Furthermore, dichotic listening studies have suggested abnormal language lateralization in subjects with Down’s syndrome (19–21). Although our results cannot explain these findings on a volumetric basis, functional neuroimaging studies may provide support for lateralized dysfunction.
The limitations of our study include our small group size and its limited age range, which did not include children under age 5. Future studies comparing children without Down’s syndrome but with similar levels of cognitive impairment would be valuable and potentially helpful in critically evaluating whether our findings are specific to subjects with Down’s syndrome. Evidence for syndrome-specific regional brain abnormalities has come from quantitative neuroimaging studies (18, 43, 44). These have shown that other genetic causes of mental retardation, including fragile X and Williams syndromes, also have unique patterns of structural abnormalities.
Our results largely confirm the findings of previous studies with respect to overall patterns of brain volumes in individuals with Down’s syndrome and also provide new evidence for abnormal volumes of specific regional tissue components. Larger studies are needed to further investigate the parietal lobe gray matter abnormality identified, as well as to specifically evaluate hippocampal and parahippocampal volumes in children with Down’s syndrome. This report represents a starting point for larger longitudinal studies of the neuroanatomic abnormalities of Down’s syndrome throughout childhood.
 |
 |
Received Oct. 17, 2000; revision received March 6, 2001; accepted March 7, 2001. From the Department of Neurology, University of California, San Francisco; the Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, Calif.; and the Department of Pediatrics, Division of Neurology and Developmental Medicine, Kennedy Krieger Institute, Johns Hopkins University School of Medicine, Baltimore. Address reprint requests to Dr. Pinter, Division of Pediatric Neurology, CH-49, Children’s Hospital & Regional Medical Center, 4800 Sand Point Way, NE, Seattle, WA 98105; [email protected] (e-mail). Supported by NIH grants NS-01692 to Dr. Pinter, RR-00052 to Dr. Capone, and HD-31715 to Dr. Reiss. Supported in part by grants from the M.I.N.D. Institute, the Packard Foundation, and the Sinclair Fund to Dr. Reiss.
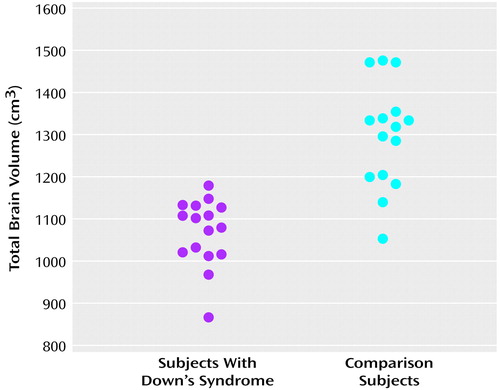
Figure 1. Total Brain Volumes of 16 Subjects With Down’s Syndrome and 15 Normal Comparison Subjects
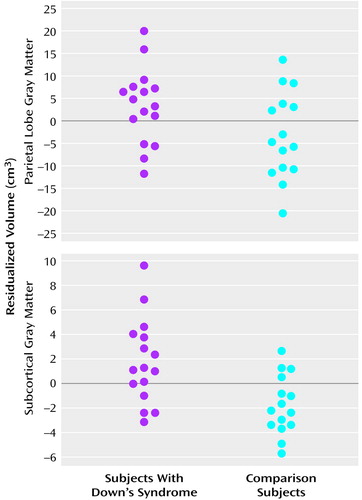
Figure 2. Residualized Volumesa of Parietal Lobe Gray Matter and Subcortical Gray Matter of 16 Subjects With Down’s Syndrome and 15 Normal Comparison Subjects
aAfter the statistical contribution of total brain gray matter was removed.
1. Nadel L: Down syndrome in cognitive neuroscience perspective, in Neurodevelopmental Disorders. Edited by Tager-Flusberg H. Cambridge, Mass, MIT Press, 1999, pp 197-222Google Scholar
2. Antonarakis SE (Down Syndrome Collaborative Group): Parental origin of the extra chromosome in trisomy 21 as indicated by analysis of DNA polymorphisms. N Engl J Med 1991; 324:872-876Crossref, Medline, Google Scholar
3. Coyle JT, Oster-Granite ML, Gearhart JD: The neurobiologic consequences of Down syndrome. Brain Res Bull 1986; 16:773-787Crossref, Medline, Google Scholar
4. Fowler A: Language abilities in children with Down syndrome: evidence for a specific syntactic delay, in Down Syndrome: A Developmental Perspective. Edited by Cicchetti D, Beeghly M. Cambridge, UK, Cambridge University Press, 1990, pp 302-328Google Scholar
5. Wang PP, Bellugi U: Evidence from two genetic syndromes for a dissociation between verbal and visual-spatial short-term memory. J Clin Exp Neuropsychol 1994; 16:317-322Crossref, Medline, Google Scholar
6. Jarrold C, Baddeley AD, Hewes AK: Genetically dissociated components of working memory: evidence from Down’s and Williams syndrome. Neuropsychologia 1999; 37:637-651Crossref, Medline, Google Scholar
7. Carlesimo GA, Marotta L, Vicari S: Long-term memory in mental retardation: evidence for a specific impairment in subjects with Down’s syndrome. Neuropsychologia 1997; 35:71-79Crossref, Medline, Google Scholar
8. Silverstein AB, Legutki G, Friedman SL, Takayama DL: Performance of Down syndrome individuals on the Stanford-Binet Intelligence Scale. Am J Ment Defic 1982; 86:548-551Medline, Google Scholar
9. Wang PP, Doherty S, Rourke SB, Bellugi U: Unique profile of visuo-perceptual skills in a genetic syndrome. Brain Cogn 1995; 29:54-65Crossref, Medline, Google Scholar
10. Becker L, Mito T, Takashima S, Onodera K: Growth and development of the brain in Down syndrome, in The Morphogenesis of Down Syndrome. Edited by Epstein C. New York, Wiley-Liss, 1991, pp 133-152Google Scholar
11. Wisniewski KE: Down syndrome children often have brain with maturation delay, retardation of growth, and cortical dysgenesis. Am J Med Genet Suppl 1990; 7:274-281Medline, Google Scholar
12. Weis S, Weber G, Neuhold A, Rett A: Down syndrome: MR quantification of brain structures and comparison with normal control subjects. AJNR Am J Neuroradiol 1991; 12:1207-1211Medline, Google Scholar
13. Kesslak JP, Nagata SF, Lott I, Nalcioglu O: Magnetic resonance imaging analysis of age-related changes in the brains of individuals with Down’s syndrome. Neurology 1994; 44:1039-1045Crossref, Medline, Google Scholar
14. Raz N, Torres IJ, Briggs SD, Spencer WD, Thornton AE, Loken WJ, Gunning FM, McQuain JD, Driesen NR, Acker JD: Selective neuroanatomic abnormalities in Down’s syndrome and their cognitive correlates: evidence from MRI morphometry. Neurology 1995; 45:356-366Crossref, Medline, Google Scholar
15. Aylward EH, Li Q, Honeycutt NA, Warren AC, Pulsifer MB, Barta PE, Chan MD, Smith PD, Jerram M, Pearlson GD: MRI volumes of the hippocampus and amygdala in adults with Down’s syndrome with and without dementia. Am J Psychiatry 1999; 156:564-568Abstract, Google Scholar
16. Aylward EH, Habbak R, Warren AC, Pulsifer MB, Barta PE, Jerram M, Pearlson GD: Cerebellar volume in adults with Down syndrome. Arch Neurol 1997; 54:209-212Crossref, Medline, Google Scholar
17. Aylward EH, Li Q, Habbak QR, Warren A, Pulsifer MB, Barta PE, Jerram M, Pearlson G: Basal ganglia volume in adults with Down syndrome. Psychiatry Res 1997; 74:73-82Crossref, Medline, Google Scholar
18. Jernigan TL, Bellugi U, Sowell E, Doherty S, Hesselink JR: Cerebral morphologic distinctions between Williams and Down syndromes. Arch Neurol 1993; 50:186-191Crossref, Medline, Google Scholar
19. Hartley XY: Lateralisation of speech stimuli in young Down’s syndrome children. Cortex 1981; 17:241-248Crossref, Medline, Google Scholar
20. Hartley XY: Receptive language processing and ear advantage of Down’s syndrome children. J Ment Defic Res 1985; 29(part 2):197-205Google Scholar
21. Giencke S, Lewandowski L: Anomalous dominance in Down syndrome young adults. Cortex 1989; 25:93-102Crossref, Medline, Google Scholar
22. Reiss A: BrainImage. Stanford, Calif, Stanford University, 2000Google Scholar
23. Kaplan DM, Liu AM, Abrams MT, Warsofsky IS, Kates WR, White CD, Kaufmann WE, Reiss AL: Application of an automated parcellation method to the analysis of pediatric brain volumes. Psychiatry Res 1997; 76:15-27Crossref, Medline, Google Scholar
24. Subramanium B, Hennessey JG, Rubin MA, Beach LS, Reiss AL: Software and methods for quantitative imaging in neuroscience: the Kennedy Krieger Institute Human Brain Project, in Neuroinformatics: An Overview of the Human Brain Project. Edited by Koslow S, Huerta M. Mahwah, NJ, Lawrence Erlbaum Associates, 1997, pp 335-360Google Scholar
25. Reiss AL, Hennessey JG, Rubin M, Beach L, Abrams MT, Warsofsky IS, Liu AM, Links JM: Reliability and validity of an algorithm for fuzzy tissue segmentation of MRI. J Comput Assist Tomogr 1998; 22:471-479Crossref, Medline, Google Scholar
26. Kates WR, Warsofsky IS, Patwardhan A, Abrams MT, Liu AM, Naidu S, Kaufmann WE, Reiss AL: Automated Talairach atlas-based parcellation and measurement of cerebral lobes in children. Psychiatry Res 1999; 91:11-30Crossref, Medline, Google Scholar
27. Andreasen NC, Rajarethinam R, Cizadlo T, Arndt S, Swayze VW II, Flashman LA, O’Leary DS, Ehrhardt JC, Yuh WTC: Automatic atlas-based volume estimation of human brain regions from MR images. J Comp Assist Tomogr 1996; 20:98-106Crossref, Medline, Google Scholar
28. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. Stuttgart, Germany, Georg Thieme, 1988Google Scholar
29. Frith U, Frith CD: Specific motor disabilities in Down’s syndrome. J Child Psychol Psychiatry 1974; 15:293-301Crossref, Medline, Google Scholar
30. Schmahmann JD, Sherman JC: The cerebellar cognitive affective syndrome. Brain 1998; 121(part 4):561-579Google Scholar
31. Silveri MC, Leggio MG, Molinari M: The cerebellum contributes to linguistic production: a case of agrammatic speech following a right cerebellar lesion. Neurology 1994; 44:2047-2050Crossref, Medline, Google Scholar
32. Ackermann H, Wildgruber D, Daum I, Grodd W: Does the cerebellum contribute to cognitive aspects of speech production? a functional magnetic resonance imaging (fMRI) study in humans. Neurosci Lett 1998; 247:187-190Crossref, Medline, Google Scholar
33. Desmond JE, Gabrieli JD, Glover GH: Dissociation of frontal and cerebellar activity in a cognitive task: evidence for a distinction between selection and search. Neuroimage 1998; 7(4, part 1):368-376Google Scholar
34. Schmidt-Sidor B, Wisniewski KE, Shepard TH, Sersen EA: Brain growth in Down syndrome subjects 15 to 22 weeks of gestational age and birth to 60 months. Clin Neuropathol 1990; 9:181-190Medline, Google Scholar
35. Golden JA, Hyman BT: Development of the superior temporal neocortex is anomalous in trisomy 21. J Neuropathol Exp Neurol 1994; 53:513-520Crossref, Medline, Google Scholar
36. Takashima S, Becker LE, Armstrong DL, Chan F: Abnormal neuronal development in the visual cortex of the human fetus and infant with Down’s syndrome: a quantitative and qualitative Golgi study. Brain Res 1981; 225:1-21Crossref, Medline, Google Scholar
37. Piercy M, Hecaen H, Ajuriaguerra J: Constructional apraxia associated with unilateral cerebral lesions: left and right sided cases compared. Brain 1960; 83:225-242Crossref, Medline, Google Scholar
38. Black FW, Bernard BA: Constructional apraxia as a function of lesion locus and size in patients with focal brain damage. Cortex 1984; 20:111-120Crossref, Medline, Google Scholar
39. Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA: Spatial working memory in humans as revealed by PET. Nature 1993; 363:623-625Crossref, Medline, Google Scholar
40. Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R: The anatomy of phonological and semantic processing in normal subjects. Brain 1992; 115(part 6):1753-1768Google Scholar
41. Price C, Wise R, Ramsay S, Friston K, Howard D, Patterson K, Frackowiak R: Regional response differences within the human auditory cortex when listening to words. Neurosci Lett 1992; 146:179-182Crossref, Medline, Google Scholar
42. Schlosser MJ, Aoyagi N, Fulbright RK, Gore JC, McCarthy G: Functional MRI studies of auditory comprehension. Hum Brain Mapp 1998; 6:1-13Crossref, Medline, Google Scholar
43. Reiss AL, Eliez S, Schmitt JE, Straus E, Lai Z, Jones W, Bellugi U: IV: Neuroanatomy of Williams syndrome: a high-resolution MRI study. J Cog Neurosci 2000; 12 (suppl 1):65-73Google Scholar
44. Reiss AL, Eliez S, Schmitt JE, Patwardhan A, Haberecht M: Brain imaging in neurogenetic conditions: realizing the potential of behavioral neurogenetics research. Ment Retard Dev Disabil Res Rev 2000; 6:186-197Crossref, Medline, Google Scholar



