Relation of Familial Schizophrenia to Negative Symptoms But Not to the Deficit Syndrome
Abstract
OBJECTIVE: Although a family history of schizophrenia has been associated with negative symptoms, family history is inconsistently related to the presence of the deficit syndrome.METHOD: The authors assessed family history and the deficit syndrome in 99 patients with DSM-III-R-diagnosed schizophrenia who were assessed during clinical treatment. Of these 99 patients, 45 were assessed both while antipsychotic free and during antipsychotic treatment to index their treatment response.RESULTS: Patients with (N=39) and without (N=60) a family history of schizophrenia had similar proportions of the deficit syndrome. Yet family history and deficit syndrome categorizations identified a group with greater negative symptoms on the Positive and Negative Syndrome Scale. Those with a family history had greater emotional withdrawal, poor rapport, and lack of spontaneity. Groups with and without the deficit syndrome similarly differed in these symptoms but also in affective blunting, motor retardation, and passive or apathetic social withdrawal. The study involving antipsychotic-free and antipsychotic treatment phases showed main medication effects explaining positive, psychopathology, depression, and activation symptoms but not negative symptoms. Only patients without a family history had improved negative symptoms with antipsychotic treatment.CONCLUSIONS: Patients with a family history of schizophrenia had greater and more treatment-resistant negative symptoms than those without a family history. They were not more likely to have the deficit syndrome. The group with a family history had more pathology only in negative symptoms related to psychosocial function. The stable negative symptoms specifically related to the genetic vulnerability to inherit schizophrenia might be those associated with psychosocial functioning.
Patients with schizophrenia with affected relatives are reported to have a high magnitude of negative symptoms that are relatively treatment resistant (1–6). Such findings are consistent with the strong genetic component for negative symptoms demonstrated by twin studies (7–9) and with data showing that relatives of probands with greater negative symptoms have higher morbid risks for illness than the relatives of probands who have greater positive symptoms (10, 11). Negative symptoms are also present in some nonschizophrenic relatives of patients with schizophrenia, as in those with schizotypal personality disorders. These and other data suggest that negative symptoms may be related to an inherited susceptibility to schizophrenia and that certain negative symptoms may be primary manifestations of illness. Clarification of these issues may facilitate the identification of genes providing vulnerability for schizophrenia.
If negative symptoms represent an inherited trait that is related to schizophrenia, then it would be conceivable that patients with a family history of schizophrenia might have higher rates of the deficit syndrome (12). The deficit syndrome is a stable (13) and reliable trait designation (14, 15) of patients who have enduring primary negative symptoms. Nonetheless, patients with and without the deficit syndrome are not reported to differ in family history (16).
We sought to clarify the relationship between family history of schizophrenia, negative symptoms, and the deficit syndrome. We independently categorized a large series of patients with schizophrenia by both family history and the presence of the deficit syndrome to examine if prominent negative symptoms were characteristic of familial schizophrenia. We overcame some methodological limitations of other studies by using rigorous family history and diagnostic assessment methods. We conducted structured symptom ratings during clinically determined medication treatment and, for nearly half the patients, also during both phases of a medication crossover study involving antipsychotic-free and antipsychotic treatment phases. The latter protocol provided an index of treatment response by measuring symptom changes between the antipsychotic-free and antipsychotic treatment conditions.
Method
Study Group
Our study group comprised 99 consecutive, voluntary patients with a DSM-III-R diagnosis of schizophrenia who were admitted to an inpatient schizophrenia research unit and who participated in family history assessments; patients with schizoaffective disorder and those with other nonaffective psychoses were excluded. Subjects were physically healthy, with recent normal physical examinations, and had normal blood and urine laboratory test results, including normal thyroid function. They provided written informed consent for these protocols, which were approved by the facility’s institutional review board. Demographic data included age, sex, education, age at onset, and global assessments of function (DSM-IV, p. 32) for the worst period in the current episode and for the last month. Social status was assessed by using a modified Hollingshead scale that considered only the education and vocational achievement of the proband (17). All data available from the 99 patients were included in these analyses, with different group sizes reflecting missing data.
Diagnosis
The Diagnostic Interview for Genetic Studies, which demonstrates kappa values of 0.80 or higher for individual symptoms and 0.95 for diagnosis (18), was administered by one of three master’s-level clinicians. Diagnoses were based on interviews, past records, and symptom ratings and represented a consensus among clinical and research staff.
Deficit Syndrome
The Schedule for the Deficit Syndrome (14) was used to categorize 96 patients into groups with and without the syndrome. This schedule provides a checklist of symptoms for making the diagnosis. The Schedule for the Deficit Syndrome was completed with information from patient and clinician interviews and also longitudinal information from the family, chart review, and previous records. It rated the deficit syndrome as present if two of the following symptoms were primary and stable: restricted affect, diminished emotional range, poverty of speech, curbing of interests, diminished sense of purpose, and diminished social drive. All patients were evaluated by raters who were trained to reliability (kappa=0.71) by the developers of the Schedule for the Deficit Syndrome (13).
Family History
Family assessments were conducted by researchers who were blind to patient information by using the Family Interview for Genetic Studies. (19). At least one family informant provided information about all first- and second-degree family members. All axis I and II diagnoses for which there was sufficient information were made for the relatives. Only the relatives with schizophrenia-related chronic psychoses that were diagnosed with DSM-III-R were considered affected for the purpose of this analysis; these diagnoses are more reliable for family history methods than are spectrum personality diagnoses. Initially, patients with reoccurrence of illness in a first-degree relative (family history 1) and those with reoccurrence in only a second-degree relative (family history 2) were grouped separately. After preliminary analyses (to follow) these separate familial groups of probands were combined into one group with a family history. Those without any familial reoccurrence were assigned to a group without family history.
Symptom Ratings
The Positive and Negative Syndrome Scale (20) and the 24-item Hamilton Depression Rating Scale were available to assess 83 subjects while they received individualized clinical treatment. (The number was reduced from 99 because of staff availability and scheduling.) Likewise, 81 of the 96 patients categorized by criteria for the deficit syndrome participated in the ratings. Forty-five patients also had their symptoms rated in a crossover study that included medication phases with and without antipsychotic drugs. The 30-item Positive and Negative Syndrome Scale data were examined both with the original positive, negative, general psychopathology, and composite subscales and with the more recent, factor-derived positive, negative, dysthymia, activation, and autistic preoccupation factors from the Positive and Negative Syndrome Scale (21).
Antipsychotic-Free and Antipsychotic Treatment Study
Symptom ratings for the 45 patients in the antipsychotic-free and antipsychotic treatment phases of our crossover study were conducted after at least 14 days of an antipsychotic-free state (after a slow medication taper) and after 4 weeks of stable antipsychotic doses. Antipsychotics were either typical or atypical, depending on the protocol being conducted when the patient was studied. Either haloperidol (or its equivalent) was initiated, 5 mg/day, with benztropine, 1 mg twice daily for 1 week, and then increased to 15 mg/day, along with benztropine, 1–2 mg twice a day, on the basis of physician assessment, or an atypical neuroleptic was initiated at low doses and titrated upward on the basis of response and side effects (risperidone up to 6 mg/day, olanzapine up to 20 mg/day, and clozapine at a dose based on blood levels). Lorazepam, 1–2 mg/day i.m. or p.o., was available for severe agitation, anxiety, or insomnia during any treatment condition.
Statistical Analysis
Initially, we performed an analysis of variance (ANOVA) on the data for the family history 1 group, the family history 2 group, and the patients without a family history of schizophrenia. The family history 1 and family history 2 groups did not differ on any demographic or symptom measures and were therefore combined into a single family history group. Sex distribution was tested across the family history categories by using the chi-square statistic. A two-by-two (family history category and sex) ANOVA was used to compare the family history groups on demographic characteristics and clinical measures.
Demographic variables and clinical treatment scores on the Positive and Negative Syndrome Scale for the 45 patients participating in the medication crossover (antipsychotic-free and antipsychotic treatment) research study were compared with those of the entire study group. We then evaluated patient group data separately across the family history and deficit syndrome categorizations. Symptom differences between antipsychotic treatment conditions were analyzed by an ANOVA with repeated measures; family history (or deficit syndrome) category was the main factor, and medication condition (with or without antipsychotic treatment) was the repeated measure. When symptom subscale scores differed significantly across family history or deficit syndrome categories, an additional two-by-two (family history category and sex) ANOVA was performed on the individual items to determine which symptoms contributed to the score differences.
To address the possible interaction of the family history and deficit syndrome categorizations of patients, we performed a two-by-two (presence or absence of family history and presence or absence of deficit syndrome) analysis of covariance. We also examined if the use of typical or atypical antipsychotic medications related to symptom scores on the Positive and Negative Syndrome Scale during antipsychotic treatment. A one-way ANOVA was performed on all demographic variables and scores on the Positive and Negative Syndrome Scale to compare patients taking typical and atypical agents. Statistical significance was set at 0.05. For post hoc tests, we applied Fisher’s least significant difference method for multiple comparisons; we performed tests only after arriving at a significant result for the overall ANOVA by using the pooled variance for all groups. The alpha level for significance was set at a Bonferroni-corrected p≤0.007 for the post hoc analyses involving individual symptom items. Since these analyses were undertaken to examine negative symptoms, statistical tests dealing with other phenomena should be considered exploratory in nature. In all situations where variances were unequal, the data were transformed by natural log, and statistical tests were conducted with the transformed data.
Results
Comparing Patients Grouped by Family History
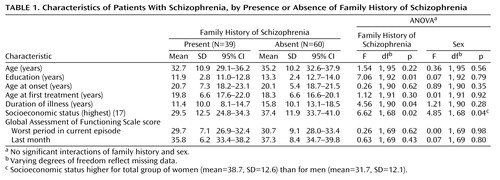
Family history groups did not differ significantly in the average number of relatives considered in making family history assessments (family history 1: mean=35.4, SD=15.5; family history 2: mean=32.8, SD=12.6; family history absent: mean=31.5, SD=10.0) (F=1.13, df=2, 96, p<0.34). The family history 1 and 2 groups were combined for subsequent analyses since they did not differ on any symptom measure or demographic variable or in sex distribution. Demographic characteristics of the patients grouped by family history are in Table 1. The groups with and without family history did not differ in sex distribution (group with family history present: 27 of 63 men, 42.9%; 12 of 36 women, 33.3%) (χ2=0.87, df=1, p=0.35) or ethnicity (group with family history present: 19 of 57 non-Hispanic Caucasians, 33.3%; 20 of 42 minorities, 47.6%) (χ2=2.07, df=1, p=0.15). The groups also did not differ in age, age at onset, age at first treatment, or in global assessments of function during the worst period in the current episode or in the last month. However, the patients with a family history had significantly less education, a lower current socioeconomic status, and a shorter duration of illness. A single significant sex effect was present for socioeconomic status: the women exhibited a higher socioeconomic level. No significant interactions of family history and sex were revealed.
A similar proportion of the groups with and without family history had the deficit syndrome (group with family history present: N=12 of 37, 32.4%; group with family history absent: N=16 of 59, 27.1%) (Pearson χ2=0.31, df=1, p=0.58), but the category assignments for deficit syndrome and family history were unrelated. It was not surprising that all items from the Schedule for the Deficit Syndrome (which were used to make deficit syndrome designations) differed significantly between the groups with and without a deficit syndrome (F values ranged from 16.9 to 61.2, df=1, 93, all p<0.002), and there were no interactions of group and sex. In contrast, the groups with family history and no family history did not differ on any item on the Schedule for the Deficit Syndrome, although a sex effect, with men having a more diminished emotional range and sense of purpose than women, was present.
Comparing Patients Grouped by Deficit Syndrome
Only sex and education differed between the groups with and without the deficit syndrome: those with the deficit syndrome were more likely to be men (23 of 61 men, 37.7%; five of 35 women, 14.3%) (χ2=5.90, df=1, p=0.02) with less education (mean=12.1 years, SD=1.9; mean=13.2, SD=2.5, respectively) (F=3.98, df=1, 89, p=0.05). A significant interaction of deficit syndrome and sex was present for socioeconomic status (F=4.62, df=1, 64, p=0.04), with men with and without the deficit syndrome exhibiting similar socioeconomic status levels (32.1 and 31.5, respectively) and women with and without the deficit syndrome having quite different socioeconomic status (25.0 and 40.9, respectively).
Symptoms Across Groups With and Without Family History
Figure 1 (column 1) presents ANOVA results for patient symptom ratings during individualized clinical treatment for the groups with and without family history. Only negative symptom ratings differed for the groups with and without family history. Those with family history present had higher negative subscale (F=7.89, df=1, 79, p=0.006) and negative factor (F=4.96, df=1, 79, p=0.03) scores and lower composite subscale scores, indicating that those with a family history had greater negative than positive symptoms. A sex effect was present on the positive subscale of the original Positive and Negative Syndrome Scale (with women having greater positive symptoms than men), and there was an interaction of family history group and sex on composite subscale scores (F=4.03, df=1, 79, p=0.05). Women in the groups with and without family history had identical composite subscale scores (2.1), but men had quite different scores (family history present: –9.2; family history absent:–1.1). We examined the individual items of the Positive and Negative Syndrome Scale that composed the subscale or factor scores that differed across the family history groups post hoc and found that the group with family history present had higher scores for emotional withdrawal (F=7.70, df=1, 79, p=0.007), poor rapport (F=14.83, df=1, 79, p<0.001), and lack of spontaneity (F=7.62, df=1, 79, p=0.007) than did those without family history.
Symptoms Across Groups With and Without the Deficit Syndrome
As seen in Figure 1 (column 2), patients with the deficit syndrome also exhibited greater negative subscale and factor scores on the Positive and Negative Syndrome Scale (F=23.40, df=1, 76, p<0.001; F=18.77, df=1, 76, p<0.001, respectively) and lower composite subscale scores (F=18.94, df=1, 76, p<0.001) than patients without the deficit syndrome. Unlike the family history groups, the groups with and without the deficit syndrome differed in dysthymia ratings, with the group with the deficit syndrome having lower dysthymia scores (F=9.17, df=1, 76, p=0.003). Significant sex effects were present for positive and composite subscale scores (with women having greater symptoms than men) (F=6.14, df=1, 76, p=0.02; F=4.97, df=1, 76, p=0.03). There were no significant or marginal interactions of deficit syndrome and sex. The negative subscale and factor items on the Positive and Negative Syndrome Scale that contributed to the previous differences (using an alpha set at p<0.007 for significance) were all higher in the group with the deficit syndrome and consisted of blunted affect (F=22.21, df=1, 76, p<0.001), emotional withdrawal (F=11.84, df=1, 76, p<0.001), poor rapport (F=11.96, df=1, 79, p=0.001), passive or apathetic social withdrawal (F=8.08, df=1, 76, p<0.006), lack of spontaneity (F=18.83, df=1, 76, p<0.001), and motor retardation (F=18.75, df=1, 76, p<0.001). The differences in dysthymia scale scores were accounted for by lower guilt feelings (F=9.14, df=1, 76, p<0.003) and depression (F=9.77, df=1, 76, p<0.003) in the patients with the deficit syndrome, as would be expected.
Thus, both deficit syndrome and family history categorizations identified groups with greater emotional withdrawal, poor rapport, and lack of spontaneity. However, only the deficit syndrome group included patients with more affective blunting, motor retardation, and passive or apathetic social withdrawal.
Symptoms of Patients Studied Both With and Without Antipsychotic Medication
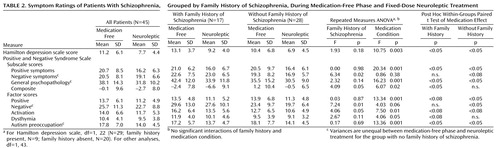
The 45 patients participating in the antipsychotic-free and antipsychotic treatment protocol did not differ from the larger group in demographic characteristics, symptoms, or proportion of group with family history: 43.6% of the patients with family history (17 of 39) and 46.7% of the patients with no family history (28 of 60). The median number of antipsychotic-free (median=21, range=7 to >30) and antipsychotic treatment (median=34, range=19 to >50) days for symptom scores on the Positive and Negative Syndrome Scale did not differ between patients with and without family history. Symptom ratings during the antipsychotic-free and antipsychotic treatment phases (medication conditions) were analyzed by a repeated measures ANOVA for patients grouped by family history and no family history (Table 2) and then for patients with the deficit syndrome and no deficit syndrome (Table 3) separately.
Family History Group and Medication Crossover Study
Family history group and medication condition affected subscale scores on the Positive and Negative Syndrome Scale, but there were no interactions of family history group and medication condition. For family history groupings, the group with family history exhibited greater negative symptom (negative subscale: F=6.34, df=1, 43, p<0.02; negative factor: F=7.24, df=1, 43, p<0.01), lower composite subscale (F=4.09, df=1, 43, p<0.05), and higher activation factor (F=4.06, df=1, 43, p<0.05) scores than the group without family history (Table 2). For medication condition, all scores on the Positive and Negative Syndrome Scale, except for those on the negative subscale, showed significant decreases from medication-free to active treatment phases, consistent with a global improvement in symptoms during antipsychotic treatment. Post hoc tests revealed a similar group response for patients with and without family history across the medication conditions except for the negative symptom ratings. Although there was not a significant interaction in the repeated measures ANOVA, post hoc tests revealed that negative subscale scores for patients with family history did not improve during active treatment, whereas those for patients without family history did. The variances on a few symptom measures were unequal between medication-free and treatment phases for the group without family history but not for the group with family history.
Deficit Syndrome Group and Medication Crossover Study
Repeated measures ANOVAs for groups with and without the deficit syndrome across medication conditions (Table 3) yielded similar results for scores on both negative symptom measures and the composite subscale of the Positive and Negative Syndrome Scale. Furthermore, there were no significant interactions for deficit syndrome group and medication condition. The patients with the deficit syndrome exhibited higher negative symptom scores (negative subscale: F=6.78, df=1, 43, p<0.01; negative factor: F=4.32, df=1, 43, p<0.05) and lower composite subscale scores (F=7.30, df=1, 43, p<0.01) than patients without the deficit syndrome. Again, for medication condition, all scores on the Positive and Negative Syndrome Scale except the negative subscale decreased significantly from medication-free to active treatment phases. Post hoc tests also revealed patterns across the medication condition for the groups with and without the deficit syndrome similar to those for the groups with and without family history. The variances on a few symptom measures were unequal between the medication-free and treatment phases for the groups with and without the deficit syndrome.
We conducted ANOVAs to look at measures that showed a significant main effect for the groups with and without family history and/or the groups with and without the deficit syndrome and examined if there were interactions between the groups. Table 4 is a summary of findings for the family history and deficit syndrome categorizations and addresses any interaction of these groups. There were only two significant interactions that would not survive a Bonferroni correction. For the poverty of speech item on the Schedule for the Deficit Syndrome, both the groups with family history and the deficit syndrome exhibited the most variant (highest) scores. For the autistic preoccupation factor on the Positive and Negative Syndrome Scale, the scores for patients with family history were similar across the deficit syndrome parameter, whereas the scores for patients without family history and with the deficit syndrome were much higher than those for the patients without family history and without the deficit syndrome.
Typical Versus Atypical Antipsychotics in the Medication Crossover Study
We examined possible differences in symptom scores on the Positive and Negative Syndrome Scale for subjects receiving typical versus atypical antipsychotics in the medication condition of the crossover study. Scores were compared for groups with and without family history (and then for groups with and without the deficit syndrome) across typical or atypical antipsychotic treatment arms by repeated measures ANOVAs (data not shown). The use of atypical versus typical agents was unrelated to family history grouping. Typical antipsychotic agents were used among 64.1% (25 of 39) of the patients with family history present and 75.0% (45 of 60) of the patients with family history absent; the type of antipsychotic agent used had no effect on the data. The results were also unchanged if only patients receiving atypical agents were analyzed, although the group size was reduced. In addition, there was no interaction of antipsychotic type and family history group for any subscale scores on the Positive and Negative Syndrome Scale. For example, mean negative subscale scores for the group with family history during antipsychotic-free and typical antipsychotic treatment conditions were 21.9 (SD=7.0) and 22.2 (SD=6.7), respectively, whereas, for the group with family history receiving atypical antipsychotics, mean negative subscale scores were 23.8 (SD=8.9) and 24.2 (SD=6.5) (repeated measures ANOVA; effect of antipsychotic type: F=0.35, df=1, 14, p=0.56; effect of medication condition: F=0.00, df=1, 14, p=0.99). Likewise, mean negative subscale scores for the group without family history while antipsychotic free and during the typical antipsychotic treatment condition were 18.9 (SD=9.3) and 16.9 (SD=6.2); those for the group with no family history receiving atypical antipsychotics during the treatment condition were 20.4 (SD=3.9) and 16.6 (SD=3.8) (repeated measures ANOVA; effect of antipsychotic type: F=0.05, df=1, 25, p=0.82; effect of medication condition: F=0.29, df=1, 25, p=0.59.).
Discussion
The patients with schizophrenia and a family history of the illness were not more likely to have the deficit syndrome, although the presence of either family history or the deficit syndrome identified patients with greater negative symptoms. The family history characteristic actually better separated the patients into groups with improving and unchanging negative symptoms between the antipsychotic-free and treatment phases of the study. Indeed, only the group without family history showed improving negative symptoms with antipsychotic treatment. Neither patient group, when classified by the deficit syndrome, showed significant negative symptom differences between the treatment phases.
We next examined differences between the family history and deficit syndrome groupings that might explain why both categorizations were related to global negative symptoms but were unrelated to one another. We looked at the relationship between the family history and deficit syndrome categorizations, both of which are unchanging (trait) characteristics, and individual items on the Positive and Negative Syndrome Scale that assess clinical state symptom measures. Since there were so few interaction terms between the groupings to explain observed phenomena, it is feasible that the family history and deficit syndrome designations capture different dimensions of negative symptoms.
There were three negative symptoms that differed between groups with and without the deficit syndrome that did not significantly differ between the patients when they were grouped by family history: affective blunting, motor retardation, and passive or apathetic social withdrawal. The first symptom, blunted affect, is related to two of the enduring and primary criteria on the Schedule for the Deficit Syndrome that define the deficit syndrome: restricted affect and diminished emotional range. Also, the passive or apathetic social withdrawal symptom may phenomenologically contribute to another three of four remaining criteria on the Schedule for the Deficit Syndrome: curbing of interests, diminished sense of purpose, and diminished social drive. These state symptom differences between the groups with and without the deficit syndrome are consistent with the requisite criteria for the deficit syndrome.
The three other items on the Positive and Negative Syndrome Scale that significantly differed between the groups with and without the deficit syndrome comprised the only symptoms that distinguished the groups with and without family history: emotional withdrawal, poor rapport, and lack of spontaneity. If these state measures differ from the primary and stable criteria on the Schedule for the Deficit Syndrome, it might explain why the familial patients did not have increased rates of the deficit syndrome, despite having high and medication-resistant negative symptoms. Two of the three symptom items differing only between groups with and without the deficit syndrome (affective blunting and motor retardation) reflect psychomotor performance more than social functioning. In contrast, the limited set of symptoms that distinguished the familial groups are related to interpersonal functioning and are remarkably similar to the criteria for DSM-IV axis II schizoid personality disorder, which has demonstrated a genetic relationship to schizophrenia. It should be noted that there is significant overlap among types of negative symptoms and that this study does not in itself contain any empiric evidence to support the distinction between the types of negative symptoms we found differentially characterizing the deficit syndrome and family history groupings.
Although negative symptoms differentiated both the family history and deficit syndrome groupings, only medication condition accounted for the improvement in positive symptoms and global improvement. This independence of positive symptom magnitude and response from that of negative symptoms is in keeping with the idea that positive and negative symptoms are distinct domains of illness (22), as supported by their different relationships to neural regions, cognitive function, and longitudinal courses (23–25).
These data do not address the validity of the deficit concept but rather explore how it differs from the increased stable negative symptoms we observed in familial schizophrenia. This study has limitations, particularly in that its duration was too short to reveal differences in the stability of negative symptoms in the groups with and without family history. There are also potential confounds to assessing and interpreting negative symptoms that we have tried to address. First, negative symptoms can be related to overall illness severity, but our deficit syndrome and family history groups had similar total scores on the Brief Psychiatric Rating Scale and similar global assessments of function. Second, negative symptoms can be reactions to positive symptoms, but none of the patient groups differed in positive symptoms. Third, negative symptoms can be difficult to distinguish from (or can be related to) anxiety and depression, but none of the relevant scores on the Positive and Negative Syndrome Scale or the Hamilton depression scale suggested the presence of these comorbid conditions. Indeed, the patients without the deficit syndrome actually had higher dysthymia factor scores than patients with the deficit syndrome. Also, we excluded patients with schizoaffective disorder from the study. Fourth, typical and atypical antipsychotics may arguably differ in their efficacy in treating negative symptoms and inducing motor side effects. But our patient groups did not differ in the proportions taking atypical and typical antipsychotics, and there was no interaction of antipsychotic type with the family history or deficit syndrome groups. Fifth, antipsychotic side effects such as akinesia can resemble negative symptoms. However, we used benztropine for extrapyramidal symptoms and lorazepam for akinesia on an individualized, clinically determined basis, and the use of these medications did not differ among the groups. Sixth, although demographic characteristics have been related to negative symptoms, the patients with and without the deficit syndrome and family history did not differ in age, age at onset, or ethnicity, and we included sex as a factor in all analyses. Illness chronicity also seems unlikely to account for the greater negative symptoms, since patients with family history actually had a significantly shorter duration of illness than the patients without family history and patients with and without the deficit syndrome did not differ in illness duration. Although demoralization and environmental deprivation have been related to negative symptoms, all of our patients were studied in the same inpatient unit. Our familial patients did have lower socioeconomic attainment, which may be more a consequence than a cause of higher negative symptoms.
We examined and presented Positive and Negative Syndrome Scale symptom data using the original and the new factor-derived solutions of the Positive and Negative Syndrome Scale items (22) so the comparability of these data to other published results could be examined. The factor-derived solution has enhanced properties in separating mood items from other psychopathology and an enhanced distribution of negative-type phenomena among its negative, activation, and autistic preoccupation factors. Items from the Positive and Negative Syndrome Scale on lack of insight, judgment, and preoccupation measures were removed from the scale’s factor pentagonal model (21) because they diffusely loaded with other criteria. It was the use of the factor-derived measures from the Positive and Negative Syndrome Scale that showed the difference in dysthymia scores between the groups with and without the deficit syndrome. In contrast, the groups with and without family history did not differ on any indices of depression. This finding further supports the view that the deficit syndrome categorization was not assessing depressive symptoms.
We recognize that family history research methods can lead to misclassification with respect to genetic causality, especially for nonfamilial patients who have heritable forms of illness. The similar number of relatives considered for diagnosis in the different family history groups suggests that family size did not bias our identification of schizophrenia reoccurrences in the relatives. We used only schizophrenia-related chronic psychosis (schizophrenia, schizoaffective, and psychosis not otherwise specified) to define the affectation status of relatives. It is possible that the proportion of patients with family history would have been higher had we also included personality disorders in the relatives as reoccurrences. Particularly given these caveats, we consider that these differences in negative symptoms between the family history groups provide support for the hypothesis that there are real differences in negative symptoms between the groups with and without family history. The unchanging variance in symptoms between medication-free and treatment conditions only for patients with family history is also consistent with the greater homogeneity found in the familial patients with respect to medication response.
Since we made our family diagnoses from psychiatric information from family informants, we obtained some information about all known relatives (even those who had moved away, died, or committed suicide). Although this method is vulnerable to the underreporting of affective illness, substance abuse, and personality disorders, it is sensitive to the presence of broadly defined psychotic illness in relatives (26), as was used in this study. It is worth noting that the increase in negative symptoms for familial patients was present even though positive symptoms defined illness occurrences in relatives. Our method of combining the patients with a first-degree relative with schizophrenia with those with reoccurrence in second-degree relatives is similar to that in other reports grouping all such patients as familial. It may be of interest that the group with only second-degree relatives with schizophrenia had negative symptom scores that were intermediate to those of the group with first-degree relatives with schizophrenia and the group with no family history.
The present data add to the literature suggesting that negative symptoms are related to the genetic vulnerability for developing schizophrenia, particularly those symptoms concerning emotional and interpersonal functioning. The data are also consistent with the idea that negative symptoms in familial schizophrenia may differ in etiology and treatment response from those in other patients with schizophrenia (10, 27, 28). Negative symptoms account for the most debilitating aspects of schizophrenia. Their excess may explain the lower education and socioeconomic status of our familial patients, although the burden of psychiatric illness in the family for some patients in the familial group could also have affected these measures.
If family history does contribute some variance in negative symptom response, this might be worth considering in evaluating the efficacy of medication treatment for these symptoms. Further research in this area to refine assessments of the dimensions of negative symptoms may facilitate both genetic and pharmacological research. It may be useful to redefine a version of the deficit syndrome with different terms for its primary features, which could be examined in serious genetic studies using twin and adoption methods.
 |
 |
 |
 |
Received Aug. 26, 1999; revision received Nov. 16, 1999; accepted Nov. 17, 1999. From the Schizophrenia Research Unit, Department of Clinical Psychobiology, New York State Psychiatric Institute; and the Department of Psychiatry, Columbia University, New York. Address reprint requests to Dr. Malaspina, New York State Psychiatric Institute, 722 West 168th St., New York, NY 10032; [email protected] (e-mail). Supported by the G. Harold and Leila Y. Mathers Charitable Foundation and NIMH grant MH-50727.
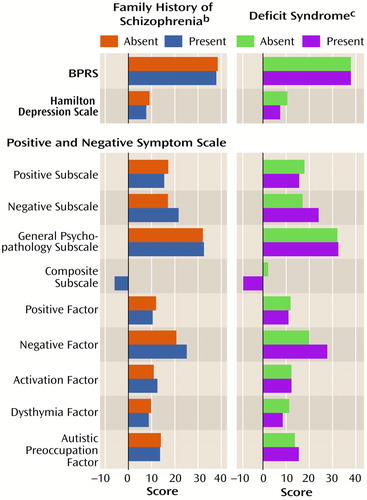
Figure 1. Symptom Ratings of 99 Patients With Schizophrenia, Grouped by Presence or Absence of Family History of Schizophrenia and Deficit Syndrome, During Clinical Treatmenta
aScores were compared by using two-by-two ANOVAs for the interactions of 1) family history and sex and 2) deficit syndrome status and sex.
bSignificant differences between groups with and without family history for negative subscale, composite subscale, and negative factor scores on the Positive and Negative Syndrome Scale. There were main effects for sex on the positive subscale and composite subscale scores on the Positive and Negative Syndrome Scale. The composite subscale score also showed an interaction between sex and group (men with a family history had lower scores than men without a family history, and women with a family history had higher scores than women without a family history). There was no other interaction between group and sex. For scores on the Hamilton depression scale, df=1, 52; for other analyses, df=1, 79.
cSignificant differences between groups with and without the deficit syndrome for composite subscale, negative factor, and dysthymia factor scores on the Positive and Negative Syndrome Scale. There were main effects of sex for the positive subscale and composite subscale scores on the Positive and Negative Syndrome Scale; for both, women scored higher than men. There were no significant or marginal interactions between deficit syndrome status and sex. For scores on the Hamilton depression scale, df=1, 50; for other analyses, df=1, 76.
1. Vazquez-Barquero JL, Cuesta Nunez MJ, Herrera Castanedo S, Diez Manrique JF, Pardo G, Dunn G: Sociodemographic and clinical variables as predictors of the diagnostic characteristics of first episodes of schizophrenia. Acta Psychiatr Scand 1996; 94:149 –155Crossref, Medline, Google Scholar
2. Schexnayder LW, Hirschowitz J, Sautter FJ, Garver DL: Predictors of response to lithium in patients with psychosis. Am J Psychiatry 1995; 152:1511–1513Google Scholar
3. Bartko G, Frecska E, Horvath S, Zador G, Arato M: Predicting neuroleptic response from a combination of multilevel variables in acute schizophrenic patients. Acta Psychiatr Scand 1990; 82:408–412Crossref, Medline, Google Scholar
4. Silverman JM, Mohs RC, Davidson M, Losonczy MF, Keefe RSE, Breitner JCS, Sorokin JE, Davis KL: Familial schizophrenia and treatment response. Am J Psychiatry 1987; 144:1271–1276Google Scholar
5. Keefe RSE, Mohs RC, Losonczy MF, Davidson M, Silverman JM, Kendler KS, Horvath TB, Nora R, Davis KL: Characteristics of very poor outcome schizophrenia. Am J Psychiatry 1987; 144:889–895Link, Google Scholar
6. Verdoux H, van Os J, Sham P, Jones P, Gilvarry K, Murray R: Does familiality predispose to both emergence and persistence of psychosis? a follow-up study. Br J Psychiatry 1996; 168:620–626; correction, 169:116Crossref, Medline, Google Scholar
7. Berenbaum H, Oltmanns TF, Gottesman II: A twin study perspective on positive and negative symptoms of schizophrenia, in Positive and Negative Symptoms of Psychosis: Description, Research, and Future Directions. Edited by Harvey PD, Walker EE. Hillsdale, NY, Lawrence Erlbaum Associates, 1987, pp 50 –67Google Scholar
8. Farmer AE, McGuffin P, Gottesman II: Searching for the split in schizophrenia: a twin study perspective. Psychiatry Res 1984; 13:109 –118Crossref, Medline, Google Scholar
9. Dworkin RH, Lenzenweger MF, Moldin SO, Skillings GF, Levick SE: A multidimensional approach to the genetics of schizophrenia. Am J Psychiatry 1988; 145:1077–1083Google Scholar
10. Kay SR, Opler LA, Fiszbein A: Significance of positive and negative symptoms in chronic schizophrenia. Br J Psychiatry 1986; 149:439–448Crossref, Medline, Google Scholar
11. McGuffin P, Owen M: Molecular genetics of schizophrenia: an overview and forward view. Eur Arch Psychiatry Clin Neurosci 1991; 240:169–173Crossref, Medline, Google Scholar
12. Carpenter WT Jr, Heinrichs DW, Wagman AMI: Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry 1988; 145:578–583Link, Google Scholar
13. Amador XF, Kirkpatrick B, Buchanan RW, Carpenter WT, Marcinko L, Yale SA: Stability of the diagnosis of deficit syndrome in schizophrenia. Am J Psychiatry 1999; 156:637–639Link, Google Scholar
14. Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT Jr: The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res 1989; 30:119–123Crossref, Medline, Google Scholar
15. Fenton WS, McGlashan TH: Testing systems for assessment of negative symptoms in schizophrenia. Arch Gen Psychiatry 1992; 49:179 –184Crossref, Medline, Google Scholar
16. Fenton WS, McGlashan TH: Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry 1994; 151:351–356Link, Google Scholar
17. Hollingshead AB: Four-Factor Index of Social Status. New Haven, Conn, Yale University, Department of Sociology, 1975Google Scholar
18. Nurnberger JI Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T (NIMH Genetics Initiative): Diagnostic Interview for Genetic Studies: rationale, unique features, and training. Arch Gen Psychiatry 1994; 51:849–859Crossref, Medline, Google Scholar
19. NIMH Schizophrenia and Bipolar Disorder Genetics Initiative: Family Interview for Genetic Studies. Bethesda, Md, National Institute of Mental Health, Molecular Genetics Initiative, 1992Google Scholar
20. Kay SR, Opler LA, Lindenmayer JP: The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry 1989; 155:59–67Crossref, Google Scholar
21. White L, Harvey PD, Opler L, Lindenmayer JP (PANSS Study Group): Empirical assessment of the factorial structure of clinical symptoms in schizophrenia: a multisite, multimodel evaluation of the factorial structure of the Positive and Negative Syndrome Scale. Psychopathology 1997; 30:263–274Crossref, Medline, Google Scholar
22. Strauss JS, Carpenter WT, Bartko JJ: Speculations on the processes that underlie schizophrenia symptoms and signs. Schizophr Bull 1974; 11:61–75Crossref, Medline, Google Scholar
23. Crow TJ: The two-syndrome concept: origins and current status. Schizophr Bull 1985; 11:471–486Crossref, Medline, Google Scholar
24. McGuffin P, Farmer AE, Gottesman II, Murray RM, Reveley AM: Twin concordance for operationally defined schizophrenia: confirmation of familiality and heritability. Arch Gen Psychiatry 1984; 41:541–545Crossref, Medline, Google Scholar
25. Arndt S, Andreasen NC, Flaum M, Miller D, Nopoulos P: A longitudinal study of symptom dimensions in schizophrenia: prediction and patterns of change. Arch Gen Psychiatry 1995; 52:352–360Crossref, Medline, Google Scholar
26. Andreasen NC, Rice J, Endicott J, Reich T, Coryell W: The family history approach to diagnosis: how useful is it? Arch Gen Psychiatry 1986; 43:421–429Google Scholar
27. Dworkin RH, Lenzenweger MF: Symptoms and the genetics of schizophrenia: implications for diagnosis. Am J Psychiatry 1984; 141:1541–1546Google Scholar
28. Tsuang MT, Winokur G, Rowe RR: Morbidity risks of schizophrenia and affective disorders among first degree relatives of patients with schizophrenia, mania, depression, and surgical conditions. Br J Psychiatry 1980; 137:497–504Crossref, Medline, Google Scholar



